Role of Zirconia in Oxide-Zeolite Composite for Thiolation of Methanol with Hydrogen Sulfide to Methanethiol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.1.1. Preparation of NaZSM-5 Molecular Sieve
2.1.2. Preparation of ZrO2
2.1.3. Preparation of Composite Catalysts
2.2. Test of Catalysts
2.3. Characterization of Catalysts
3. Results
3.1. Effects of ZrO2 Crystal Phase Structure
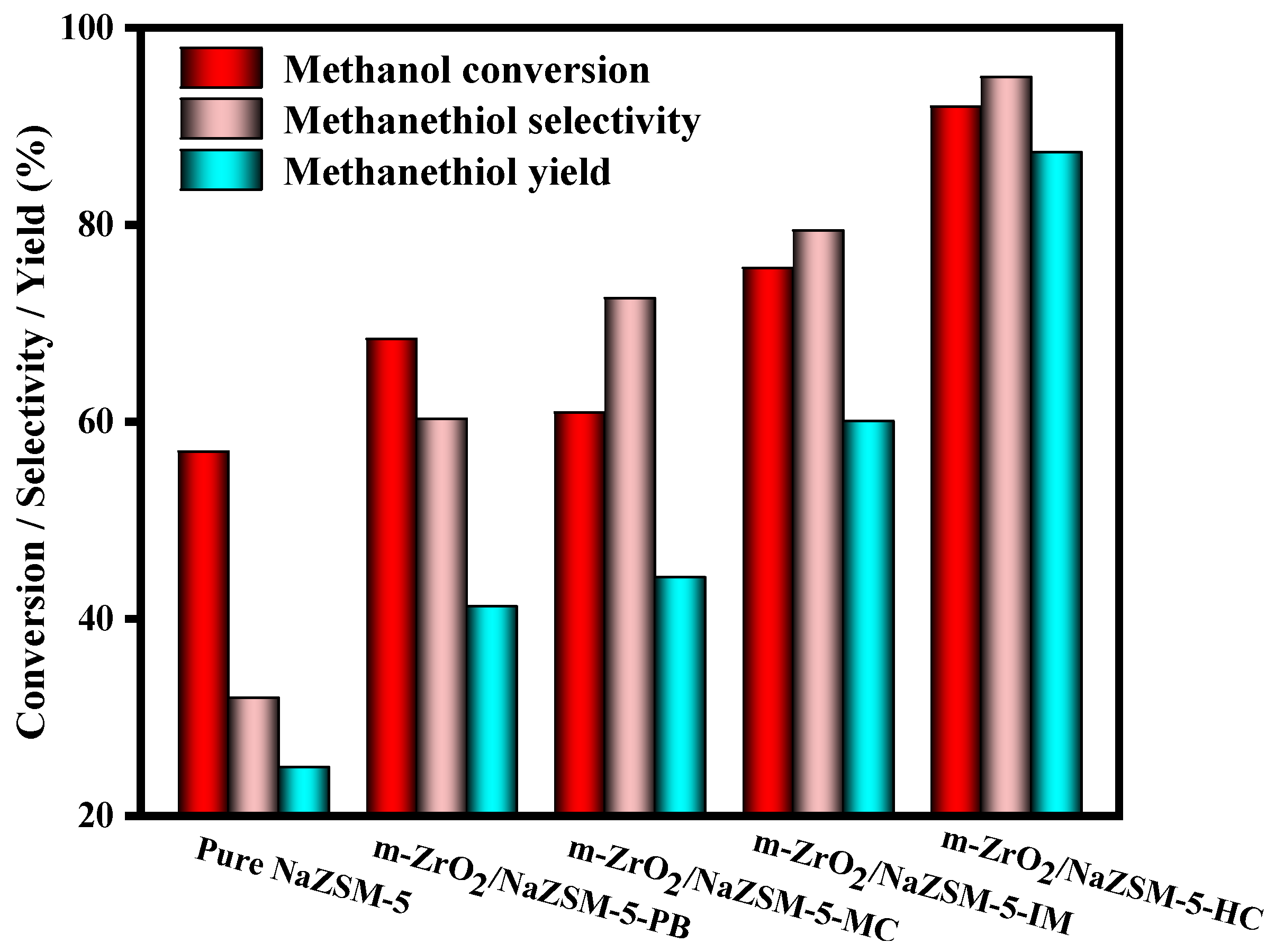
3.2. Effect of the m-ZrO2 Compositing Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, J.; Kumar, D. Production of l-methionine by submerged fermentation: A review. Enzym. Microb. Technol. 2005, 37, 3–18. [Google Scholar] [CrossRef]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taifan, W.; Arvidsson, A.A.; Nelson, E.; Hellman, A.; Baltrusaitis, J. CH4 and H2S reforming to CH3SH and H2 catalyzed by metal-promoted Mo6S8 clusters: A first-principles micro-kinetic study. Catal. Sci. Technol. 2017, 7, 3546–3554. [Google Scholar] [CrossRef]
- Taifan, W.; Baltrusaitis, J. CH4 conversion to value added products: Potential, limitations and extensions of a single step heterogeneous catalysis. Appl. Catal. B Environ. 2016, 198, 525–547. [Google Scholar] [CrossRef]
- Willke, T. Methionine production-a critical review. Appl. Microbiol. Biotechnol. 2014, 98, 9893–9914. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wu, M.; Fang, W.; Yang, Y. Promoting effect of SiO2 on the K2WO4/Al2O3 catalysts for methanethiol synthesis from methanol and H2S. Catal. Commun. 2012, 22, 48–51. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; He, Q.; Peng, S.; Yang, Y.; Li, M. Synthesis of Methanethiol from Methanol and Carbon Disulfide over CoKW/Al2O3 Catalysts: The Possible Reaction Network and Reaction Mechanism. ChemistrySelect 2018, 3, 9663–9671. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zhang, X.; Fang, W.; Yang, Y. Catalytic synthesis of methanethiol from methanol and carbon disulfide over KW/Al2O3 catalysts. Catal. Commun. 2015, 69, 104–108. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Zhang, Y.; Wei, Y.; Fang, W.; Yang, Y. Thiolation of dimethyl sulfide to methanethiol over WO3/ZrO2 catalysts. J. Mol. Catal. A Chem. 2012, 365, 60–65. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Wu, M.; Fang, W.; Yang, Y. Study on methanethiol synthesis from H2S and dimethyl sulfide over Al2O3 catalysts promoted with phosphorus. Appl. Catal. A Gen. 2012, 431–432, 151–156. [Google Scholar] [CrossRef]
- Koshelev, S.N.; Mashkina, A.V.; Paukshtis, E.A. Stability of catalysts in dimethyl sulfide conversion in the presence of H2S. React. Kinet. Catal. Lett. 1991, 44, 367–373. [Google Scholar] [CrossRef]
- Andersson, R.; Boutonnet, M.; Järås, S. Higher alcohols from syngas using a K/Ni/MoS2 catalyst: Trace sulfur in the product and effect of H2S-containing feed. Fuel 2014, 115, 544–550. [Google Scholar] [CrossRef]
- Zhang, B.; Taylor, S.H.; Hutchings, G.J. Synthesis of methyl mercaptan and thiophene from CO/H2/H2S using α-Al2O3. Catal. Lett. 2003, 91, 3–4. [Google Scholar] [CrossRef]
- Chen, A.; Wang, Q.; Hao, Y.; Fang, W.; Yang, Y. The Promoting Effect of Tellurium on K2MoO4/SiO2 Catalyst for Methanethiol Synthesis from High H2S-Containing Syngas. Catal. Lett. 2007, 118, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Cordova, A.; Blanchard, P.; Lancelot, C.; Frémy, G.; Lamonier, C. Probing the Nature of the Active Phase of Molybdenum-Supported Catalysts for the Direct Synthesis of Methylmercaptan from Syngas and H2S. ACS Catal. 2015, 5, 2966–2981. [Google Scholar] [CrossRef]
- Cordova, A.; Blanchard, P.; Salembier, H.; Lancelot, C.; Frémy, G.; Lamonier, C. Direct synthesis of methyl mercaptan from H2/CO/H2S using tungsten based supported catalysts: Investigation of the active phase. Catal. Today 2017, 292, 143–153. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Chen, A.; Fang, W.; Yang, Y. Study on Methanethiol Synthesis from H2S-Rich Syngas Over K2MoO4 Catalyst Supported on Electrolessly Ni-Plated SiO2. Catal. Lett. 2009, 129, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Liu, P.; Xu, Z.; He, S.; Luo, Y. Investigation of the reaction pathway for synthesizing methyl mercaptan (CH3SH) from H2S-containing syngas over K-Mo-type materials. RSC Adv. 2018, 8, 21340–21353. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Luo, Y.; He, D.; Xu, Z.; He, S.; Xie, D.; Mei, Y. An exploration into potassium (K) containing MoS2 active phases and its transformation process over MoS2 based materials for producing methanethiol. Catal. Today 2020, 339, 93–104. [Google Scholar] [CrossRef]
- Zhang, B.; Taylor, S.H.; Hutchings, G.J. Catalytic synthesis of methanethiol from CO/H2/H2S mixtures using α-Al2O3. New J. Chem. 2004, 28, 471–476. [Google Scholar] [CrossRef]
- Gilman, H.; Beaber, N.J. The alkylation of mercaptans by means of sulfonic esters. J. Am. Chem. Soc. 1925, 47, 1449–1451. [Google Scholar] [CrossRef]
- Hauser, C.R.; Kantor, S.W.; Brasen, W.R. Rearrangement of benzyl sulfides to mercaptans and of sulfonium ions to sulfides involving the aromatic ring by alkali amides1,2. J. Am. Chem. Soc. 1953, 75, 2660–2663. [Google Scholar] [CrossRef]
- Raghunath, M.; Viswanathan, C.L. Benzimidazole-2-carbamic acid as a privileged scaffold for antifungal, anthelmintic and antitumor activity: A Review. Int. J. Pharm. Sci. 2014, 6, 17–25. [Google Scholar]
- Scott, C.B.; Dorsey, W.S.; Huffman, H.C. Pilot plant methyl mercaptan from methyl chloride. Ind. Eng. Chem. 1955, 47, 876–877. [Google Scholar] [CrossRef]
- Takagi, K. Synthesis of aromatic thiols from aryl iodides and thiourea by means of nickel catalyst. Chem. Lett. 1985, 14, 1307–1308. [Google Scholar] [CrossRef] [Green Version]
- Bermejo-Deval, R.; Walter, R.M.H.; Gutiérrez, O.Y.; Lercher, J.A. On the role of the alkali cations on methanol thiolation. Catal. Sci. Technol. 2017, 7, 4437–4443. [Google Scholar] [CrossRef]
- Pashigreva, A.V.; Kondratieva, E.; Bermejo-Deval, R.; Gutiérrez, O.Y.; Lercher, J.A. Methanol thiolation over Al2O3 and WS2 catalysts modified with cesium. J. Catal. 2017, 345, 308–318. [Google Scholar] [CrossRef]
- Weber-Stockbauer, M.; Gutiérrez, O.Y.; Bermejo-Deval, R.; Lercher, J.A. Cesium induced changes in the acid-base properties of metal oxides and the consequences for methanol thiolation. ACS Catal. 2019, 9, 9245–9252. [Google Scholar] [CrossRef]
- Maugé, F.; Sahibed-Dine, A.; Gaillard, M.; Ziolek, M. Modification of the acidic properties of NaY zeolite by H2S adsorption-an infrared study. J. Catal. 2002, 207, 353–360. [Google Scholar] [CrossRef]
- Plaisance, C.P.; Dooley, K.M. Zeolite and metal oxide catalysts for the production of dimethyl sulfide and methanethiol. Catal. Lett. 2009, 128, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Rep, M.; Palomares, A.E.; Eder-Mirth, G.; van Ommen, J.G.; Rösch, N.; Lercher, J.A. Interaction of methanol with alkali metal exchanged molecular sieves. 1. IR spectroscopic study. J. Phys. Chem. B 2000, 104, 8624–8630. [Google Scholar] [CrossRef]
- Trejda, M.; Kujawa, J.; Ziolek, M. Iron modified MCM-41 materials characterised by methanol oxidation and sulphurisation reactions. Catal. Lett. 2006, 108, 141–146. [Google Scholar] [CrossRef]
- Trejda, M.; Tuel, A.; Kujawa, J.; Kilos, B.; Ziolek, M. Niobium rich SBA-15 materials–preparation, characterisation and catalytic activity. Microporous Mesoporous Mater. 2008, 110, 271–278. [Google Scholar] [CrossRef]
- Trejda, M.; Wojtaszek, A.; Ziolek, M.; Kujawa, J. Various hexagonally ordered mesoporous silicas as supports for chromium species-The effect of support on surface properties. Appl. Catal. A Gen. 2009, 365, 135–140. [Google Scholar] [CrossRef]
- Ziolek, M.; Kujawa, J.; Czyzniewska, J.; Nowak, I.; Aboulayt, A.; Saur, O.; Lavalley, J.C. Effect on the reaction between methanol and hydrogen sulphide of Na or Mo doping on zirconia and alumina. Appl. Catal. A Gen. 1998, 171, 109–115. [Google Scholar] [CrossRef]
- Huang, F.; Cao, J.; Wang, L.; Wang, X.; Liu, F. Enhanced catalytic behavior for methanol to lower olefins over SAPO-34 composited with ZrO2. Chem. Eng. J. 2020, 380, 122626. [Google Scholar] [CrossRef]
- Liu, P.; Cao, J.; Xu, Z.; Yang, C.; Wang, X.; Liu, F. Thiolation of methanol with H2S using core-shell structured ZSM-5@t-ZrO2 catalyst. Chem. Eng. Sci. 2020, 211, 115273. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Liu, F.; Zhao, T.; Wang, X.; Cao, J. High selectivity in methanethiol synthesis over a coated composite comprising ZSM-5 with t-ZrO2. Microporous Mesoporous Mater. 2020, 305, 110358. [Google Scholar] [CrossRef]
- Feng, R.; Yan, X.; Hu, X.; Wang, Y.; Li, Z.; Hou, K.; Lin, J. Hierarchical ZSM-5 zeolite designed by combining desilication and dealumination with related study of n-heptane cracking performance. J. Porous Mater. 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Li, J.; Liu, M.; Guo, X.; Zeng, S.; Xu, S.; Wei, Y.; Liu, Z.; Song, C. Influence of Al coordinates on hierarchical structure and T atoms redistribution during base leaching of ZSM-5. Ind. Eng. Chem. Res. 2018, 57, 15375–15384. [Google Scholar] [CrossRef]
- Li, T.; Roy, K.; Krumeich, F.; Artiglia, L.; Huthwelker, T.; Bokhoven, J.A. Variation of aluminium distribution in small-sized ZSM-5 crystals during desilication. Chem.-A Eur. J. 2019, 25, 15879–15886. [Google Scholar] [CrossRef]
- Ma, Q.; Fu, T.; Wang, Y.; Li, H.; Cui, L.; Li, Z. Development of mesoporous ZSM-5 zeolite with microporosity preservation through induced desilication. J. Mater. Sci. 2020, 55, 11870–11890. [Google Scholar] [CrossRef]
- Peng, P.; Wang, Y.; Zhang, Z.; Qiao, K.; Liu, X.; Yan, Z.; Subhan, F.; Komarneni, S. ZSM-5-based mesostructures by combined alkali dissolution and re-assembly: Process controlling and scale-up. Chem. Eng. J. 2016, 302, 323–333. [Google Scholar] [CrossRef]
- Thl, A.; Min, C.; Bhj, C.; Jhp, B.; Shk, A.; Kbl, A. Prevention of deactivation of HZSM-5 by mixing with NaZSM-5 in catalytic reaction of methylcyclohexane. Catal. Today 2020, 358, 116–121. [Google Scholar]
- Frei, M.S.; Mondelli, C.; Cesarini, A.; Krumeich, F.; Hauert, R.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Role of zirconia in indium cxide-catalyzed CO2 hydrogenation to methanol. ACS Catal. 2020, 10, 1133–1145. [Google Scholar] [CrossRef]
- Rahman, N.J.A.; Ramli, A.; Jumbri, K.; Uemura, Y. Tailoring the surface area and the acid-base properties of ZrO2 for biodiesel production from Nannochloropsis sp. Sci. Rep. 2019, 9, 16223. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, K.; Yamaguchi, T. Acid-base bifunctional catalysis by ZrO2 and its mixed oxides. Catal. Today 1994, 20, 185–197. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Li, Y.; Huang, S.; An, J.; Shi, R.; Pei, Y.; Li, Z.; Ren, J. Effects of surface acid-base properties of ZrO2 on the direct synthesis of DMC from CO2 and methanol: A combined DFT and experimental study. Chem. Eng. Sci. 2021, 229, 116018. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, L.; Ke, C.; Fan, G.; Yang, L.; Li, F. Highly efficient catalytic transfer hydrogenation of furfural over defect-rich amphoteric ZrO2 with abundant surface acid-base sites. Dalton Trans. 2021, 50, 2616–2626. [Google Scholar] [CrossRef]
- Petchmark, M.; Ruangpornvisuti, V. Hydrogen adsorption on c-ZrO2(111), t-ZrO2(101), and m-ZrO2(111) surfaces and their oxygen-vacancy defect for hydrogen sensing and storage: A first-principles investigation. Mater. Lett. 2021, 301, 130243. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Wu, R.; Zhao, L.; Wei, X.; Zhao, Y. Effects of zirconia crystal phases on the catalytic decomposition of N2O over Co3O4/ZrO2 catalysts. Appl. Surf. Sci. 2020, 514, 145892. [Google Scholar] [CrossRef]
- Barthomeuf, D. Conjugate acid-base pairs in zeolites. J. Phys. Chem. 1984, 88, 42–45. [Google Scholar] [CrossRef]
- Corma, A.; Martín-Aranda, R.M.; Sánchez, F. Zeolites as base catalysts: Condensation of benzaldehyde derivatives with activated methylenic compounds on Germanium-substituted faujasite. J. Catal. 1990, 126, 192–198. [Google Scholar] [CrossRef]
- Gil, B.; Mokrzycki, Ł.; Sulikowski, B.; Olejniczak, Z.; Walas, S. Desilication of ZSM-5 and ZSM-12 zeolites: Impact on textural, acidic and catalytic properties. Catal. Today 2010, 152, 24–32. [Google Scholar] [CrossRef]
- Qi, T.; Zhou, L.; Li, Y.; Chu, G.W.; Sun, B.C. Tailoring oxygen vacancies in ZSM-5@MnOx catalysts for efficient oxidation of benzyl alcohol. Chem. Eng. Sci. 2021, 241, 116691. [Google Scholar] [CrossRef]
- Su, J.Y.; Park, E.D. Effects of dealumination and desilication of H-ZSM-5 on xylose dehydration. Microporous Mesoporous Mater. 2014, 186, 121–129. [Google Scholar]
- Zhou, F.; Gao, Y.; Wu, G.; Ma, F.; Liu, C. Improved catalytic performance and decreased coke formation in post-treated ZSM-5 zeolites for methanol aromatization. Microporous Mesoporous Mater. 2017, 240, 96–107. [Google Scholar] [CrossRef]


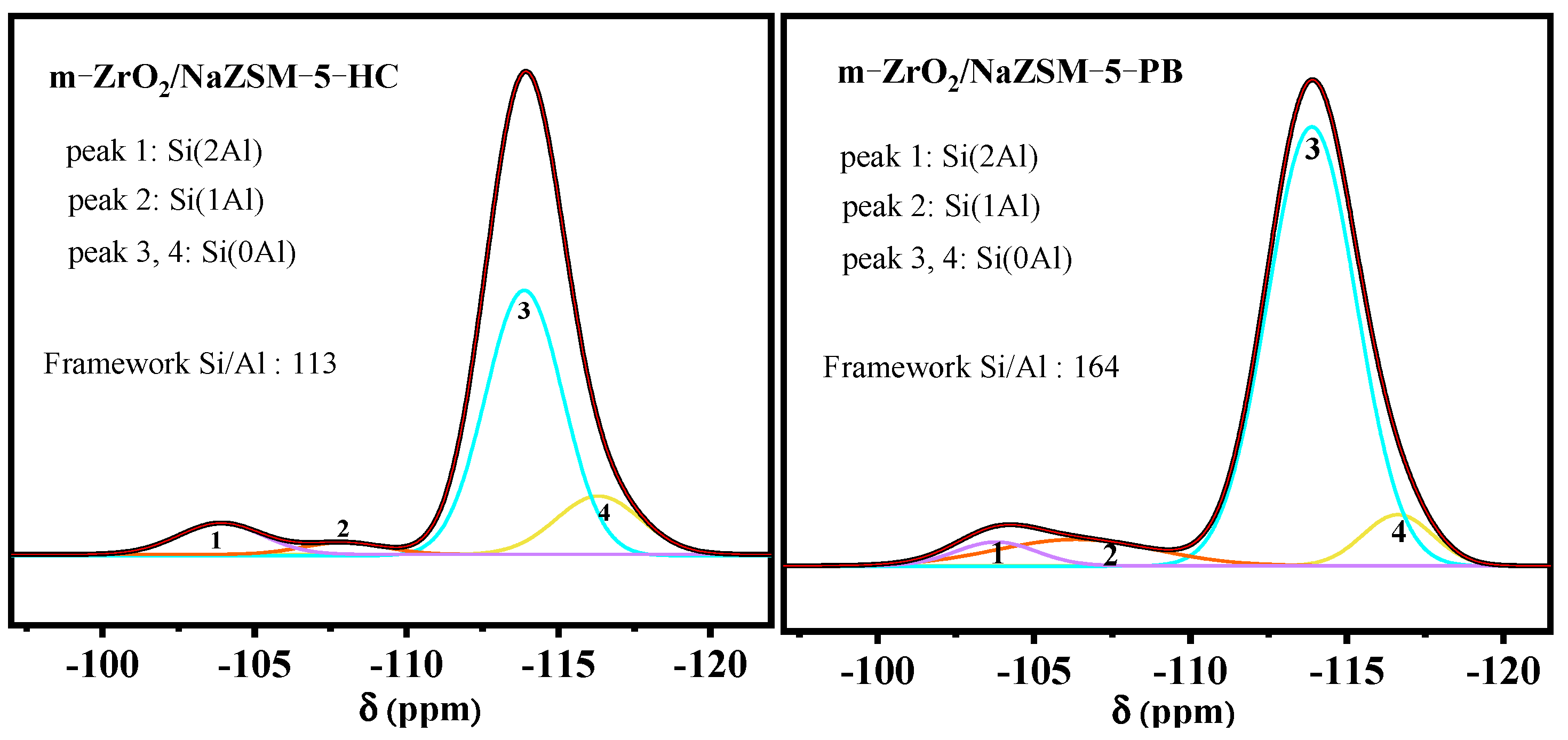

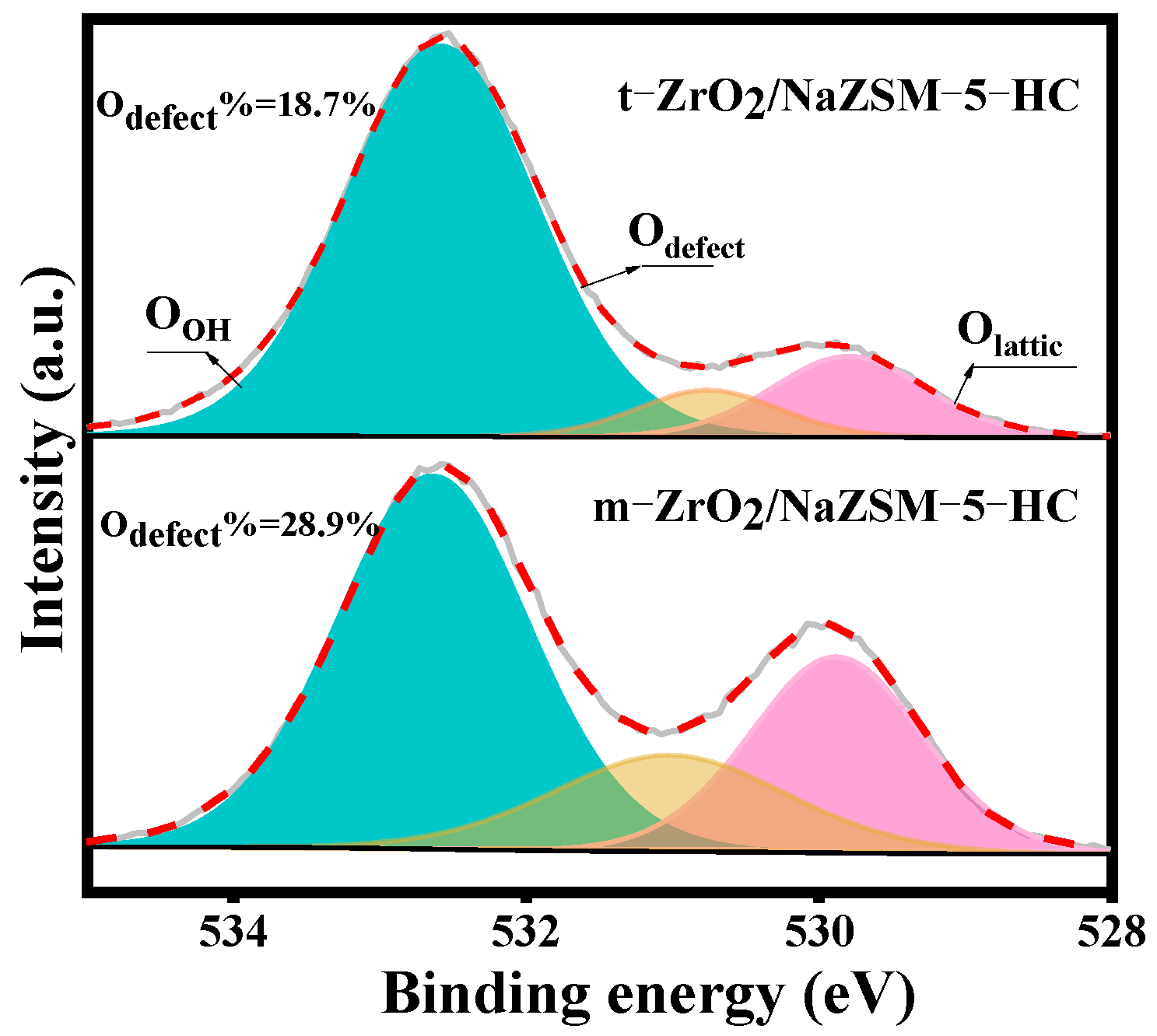
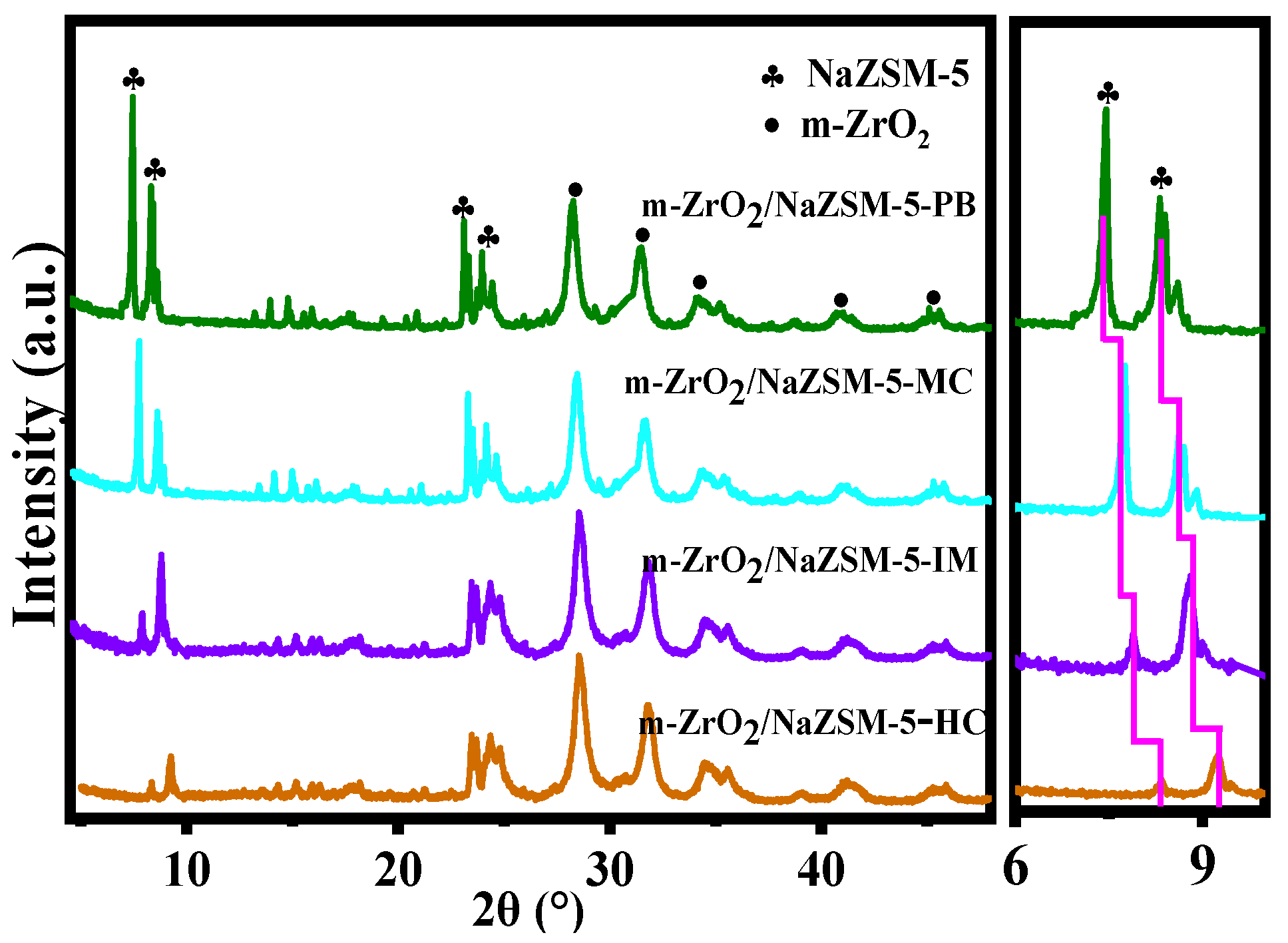
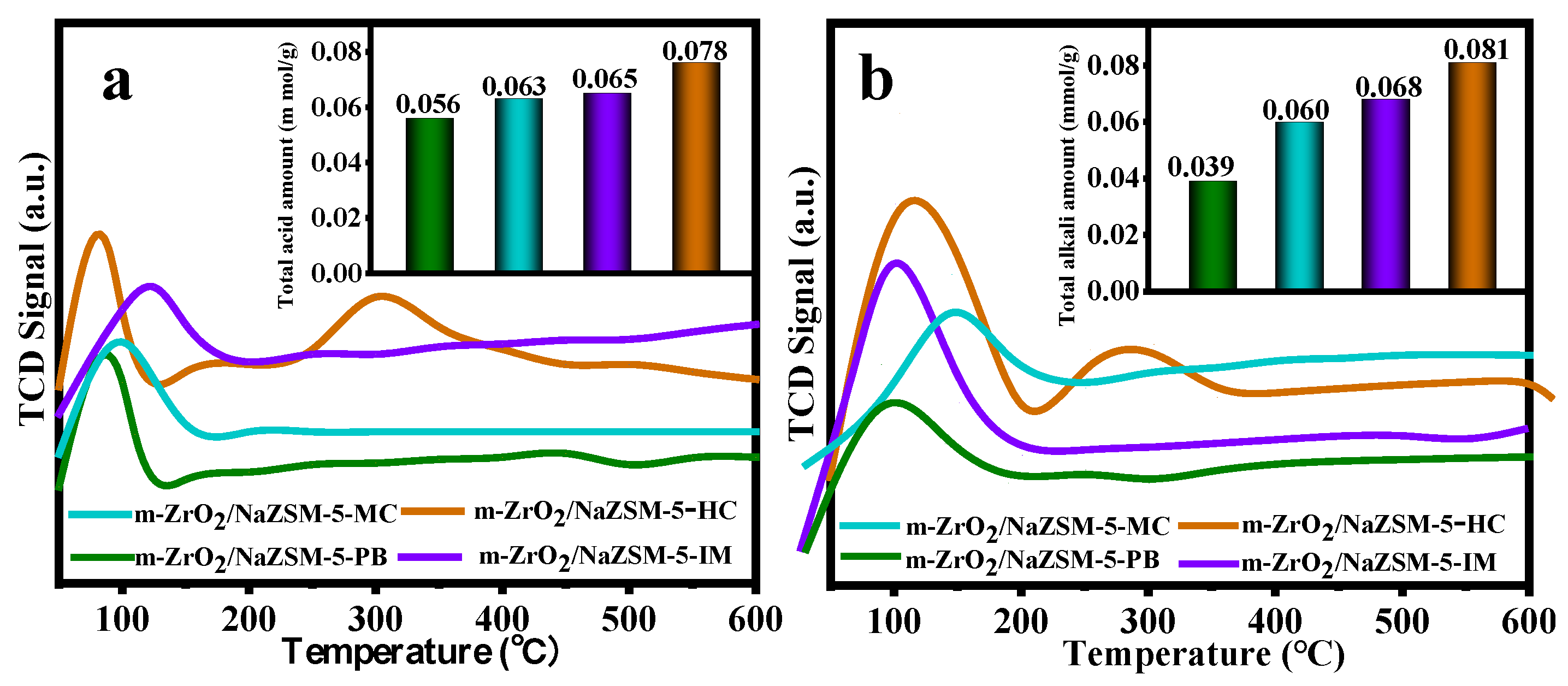
| Samples | Element Content (wt.%) | Bulk Zr/Si Bulk Si/Al | |||||
|---|---|---|---|---|---|---|---|
| Si | Al | Zr | O | Na | |||
| m-ZrO2/NaZSM-5-HC | 17.09 | 0.106 | 36.53 | 43.22 | 0.106 | 2.14 | 161 |
| t-ZrO2/NaZSM-5-HC | 20.42 | 0.117 | 31.83 | 44.58 | 0.115 | 1.56 | 174 |
| NaZSM-5 | 35.89 | 0.20 | - | 60.75 | 0.19 | - | 179 |
| Composites | BET Surface Area (m2/g) | Pore Volume (cm3/g) | ||||
|---|---|---|---|---|---|---|
| SBET | Smic | Smes | Vt | Vmic | Vmes | |
| Pure NaZSM-5 | 365 | 330 | 35 | 0.41 | 0.34 | 0.07 |
| m-ZrO2/NaZSM-5-HC | 360 | 126 | 234 | 0.52 | 0.17 | 0.35 |
| t-ZrO2/NaZSM-5-HC | 239 | 114 | 125 | 0.43 | 0.12 | 0.31 |
| Pure m-ZrO2 | 134 | 40 | 94 | 0.47 | 0.21 | 0.26 |
| Pure t-ZrO2 | 184 | - | 184 | 0.50 | - | 0.50 |
| Catalyst | BET Surface Area (m2/g) a | Volume (cm3/g) a | XRF b Si/Al | ||||
|---|---|---|---|---|---|---|---|
| SBET | Smic | Smes | Vt | Vmic | Vmes | ||
| m-ZrO2/NaZSM-5-PB | 268 | 154 | 114 | 0.42 | 0.28 | 0.14 | 179 |
| m-ZrO2/NaZSM-5-MC | 235 | 152 | 83 | 0.40 | 0.31 | 0.09 | 170 |
| m-ZrO2/NaZSM-5-IM | 276 | 161 | 115 | 0.42 | 0. 30 | 0.12 | 167 |
| m-ZrO2/NaZSM-5-HC | 360 | 126 | 234 | 0.52 | 0.17 | 0.35 | 161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Yao, M.; Ma, J.; Chen, P.; Zhao, T.; Yang, C.; Liu, F.; Cao, J. Role of Zirconia in Oxide-Zeolite Composite for Thiolation of Methanol with Hydrogen Sulfide to Methanethiol. Nanomaterials 2022, 12, 1803. https://doi.org/10.3390/nano12111803
Yang T, Yao M, Ma J, Chen P, Zhao T, Yang C, Liu F, Cao J. Role of Zirconia in Oxide-Zeolite Composite for Thiolation of Methanol with Hydrogen Sulfide to Methanethiol. Nanomaterials. 2022; 12(11):1803. https://doi.org/10.3390/nano12111803
Chicago/Turabian StyleYang, Tinglong, Mengqin Yao, Jun Ma, Peng Chen, Tianxiang Zhao, Chunliang Yang, Fei Liu, and Jianxin Cao. 2022. "Role of Zirconia in Oxide-Zeolite Composite for Thiolation of Methanol with Hydrogen Sulfide to Methanethiol" Nanomaterials 12, no. 11: 1803. https://doi.org/10.3390/nano12111803






