PdAg/Ag(111) Surface Alloys: A Highly Efficient Catalyst of Oxygen Reduction Reaction
Abstract
:1. Introduction
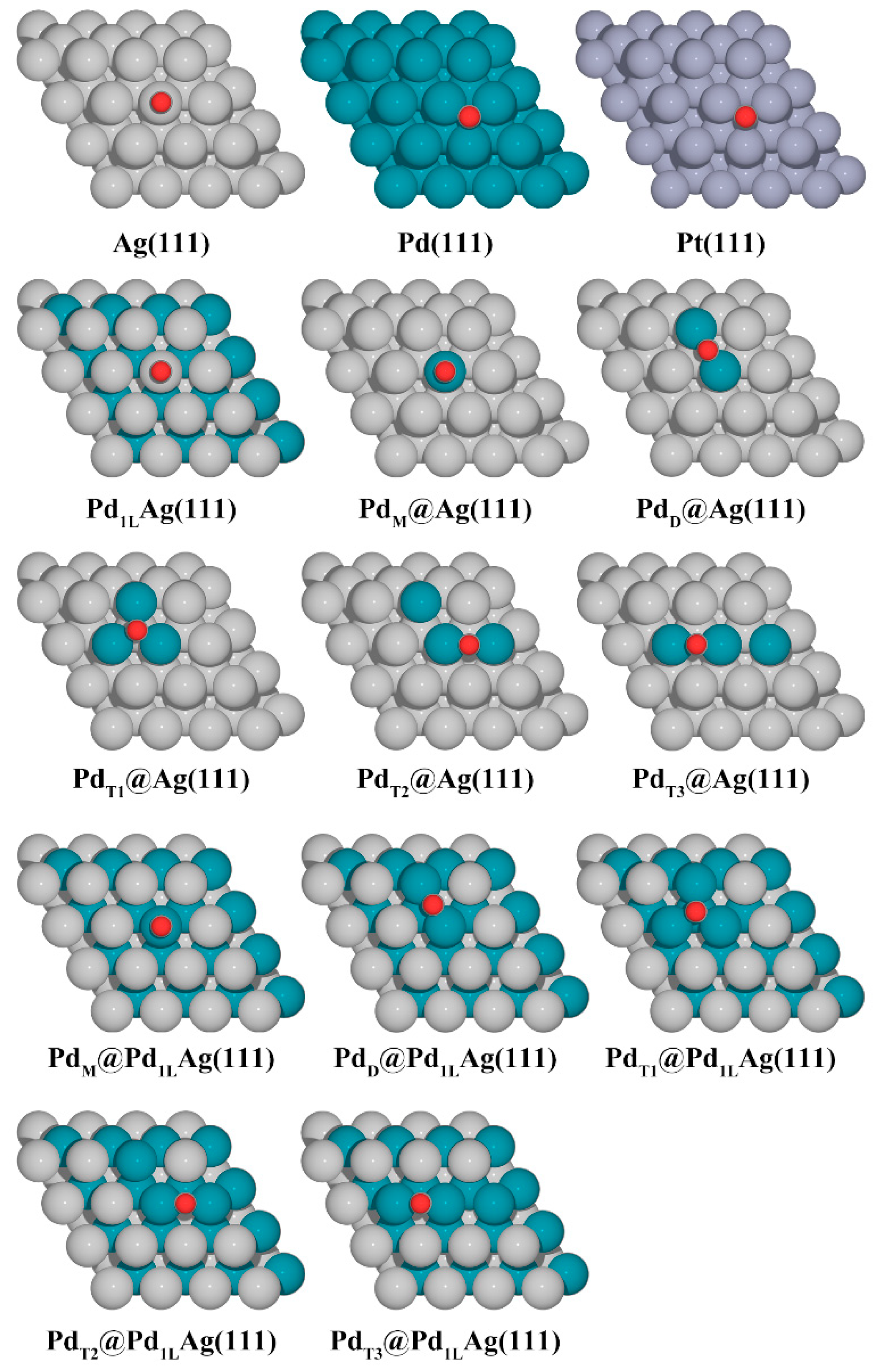
2. Method
2.1. Density Functional Theory Calculations
2.2. Oxygen Reduction Reaction
3. Results and Discussion
3.1. Reaction Profile and Calculated Overpotentials
3.2. Reduced CO Poisoning of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnsson, F.; Kjärstad, J.; Rootzén, J. The threat to climate change mitigation posed by the abundance of fossil fuels. Clim. Policy 2019, 19, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhang, X.; Gu, F.; Zhang, H.; Fan, Y. Does air pollution stimulate electric vehicle sales? Empirical evidence from twenty major cities in China. J. Clean. Prod. 2020, 249, 119372. [Google Scholar] [CrossRef]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-Free Catalysts for Proton Exchange Membrane Fuel Cells: Stability Challenges and Material Solutions. Adv. Mater. 2021, 33, 1908232. [Google Scholar] [CrossRef] [PubMed]
- Stephens, I.E.L.; Rossmeisl, J.; Chorkendorff, I. Toward sustainable fuel cells. Science 2016, 354, 1378–1379. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhao, X.; Su, Y.Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.M.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef]
- Ham, H.C.; Stephens, J.A.; Hwang, G.S.; Han, J.; Nam, S.W.; Lim, T.H. Role of small Pd ensembles in boosting CO oxidation in AuPd alloys. J. Phys. Chem. Lett. 2012, 3, 566–570. [Google Scholar] [CrossRef]
- Papanikolaou, K.G.; Darby, M.T.; Stamatakis, M. CO-Induced Aggregation and Segregation of Highly Dilute Alloys: A Density Functional Theory Study. J. Phys. Chem. C 2019, 123, 9128–9138. [Google Scholar] [CrossRef] [Green Version]
- Darby, M.T.; Sykes, E.C.H.; Michaelides, A.; Stamatakis, M. Carbon Monoxide Poisoning Resistance and Structural Stability of Single Atom Alloys. Top. Catal. 2018, 61, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Groß, A. Reactivity of bimetallic systems studied from first principles. Top. Catal. 2006, 37, 29–39. [Google Scholar] [CrossRef]
- Darby, M.T.; Réocreux, R.; Sykes, E.C.H.; Michaelides, A.; Stamatakis, M. Elucidating the Stability and Reactivity of Surface Intermediates on Single-Atom Alloy Catalysts. ACS Catal. 2018, 8, 5038–5050. [Google Scholar] [CrossRef]
- Mao, J.; Yin, J.; Pei, J.; Wang, D.; Li, Y. Single atom alloy: An emerging atomic site material for catalytic applications. Nano Today 2020, 34, 100917. [Google Scholar] [CrossRef]
- Hannagan, R.T.; Giannakakis, G.; Réocreux, R.; Schumann, J.; Finzel, J.; Wang, Y.; Michaelides, A.; Deshlahra, P.; Christopher, P.; Flytzani-Stephanopoulos, M.; et al. First-Principles design of a single-atom-alloy propane dehydrogenation catalyst. Science 2021, 372, 1444–1447. [Google Scholar] [CrossRef]
- Aich, P.; Wei, H.; Basan, B.; Kropf, A.J.; Schweitzer, N.M.; Marshall, C.L.; Miller, J.T.; Meyer, R. Single-Atom Alloy Pd-Ag Catalyst for Selective Hydrogenation of Acrolein. J. Phys. Chem. C 2015, 119, 18140–18148. [Google Scholar] [CrossRef]
- Ouyang, M.; Papanikolaou, K.G.; Boubnov, A.; Hoffman, A.S.; Giannakakis, G.; Bare, S.R.; Stamatakis, M.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Directing reaction pathways via in situ control of active site geometries in PdAu single-atom alloy catalysts. Nat. Commun. 2021, 12, 1549. [Google Scholar] [CrossRef]
- Hannagan, R.T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom Alloy Catalysis. Chem. Rev. 2020, 120, 12044–12088. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, G.; Zeng, R.; Villarino, A.M.; Disalvo, F.J.; Van Dover, R.B.; Abruña, H.D. Combinatorial Studies of Palladium-Based Oxygen Reduction Electrocatalysts for Alkaline Fuel Cells. J. Am. Chem. Soc. 2020, 142, 3980–3988. [Google Scholar] [CrossRef]
- Erikson, H.; Liik, M.; Sarapuu, A.; Kozlova, J.; Sammelselg, V.; Tammeveski, K. Oxygen reduction on electrodeposited Pd coatings on glassy carbon. Electrochim. Acta 2013, 88, 513–518. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Alexeyeva, N.; Tammeveski, K.; Solla-Gullón, J.; Feliu, J.M. Electrochemical reduction of oxygen on palladium nanocubes in acid and alkaline solutions. Electrochim. Acta 2012, 59, 329–335. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Svenum, I.H.; Herron, J.A.; Mavrikakis, M.; Venvik, H.J. Pd3Ag(111) as a Model System for Hydrogen Separation Membranes: Combined Effects of CO Adsorption and Surface Termination on the Activation of Molecular Hydrogen. Top. Catal. 2020, 63, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xiao, W.; Feng, X.; Xiong, Y.; Gong, M.; Shen, T.; Lu, Y.; Abruña, H.D.; Wang, D. Golden Palladium Zinc Ordered Intermetallics as Oxygen Reduction Electrocatalysts. ACS Nano 2019, 13, 5968–5974. [Google Scholar] [CrossRef]
- Slanac, D.A.; Hardin, W.G.; Johnston, K.P.; Stevenson, K.J. Atomic ensemble and electronic effects in Ag-rich AgPd nanoalloy catalysts for oxygen reduction in alkaline media. J. Am. Chem. Soc. 2012, 134, 9812–9819. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Zhu, F.; Tian, R.; Li, L.; Shen, S.; Yan, X.; Zhang, J. Composition-Graded PdxNi1-x Nanospheres with Pt Monolayer Shells as High-Performance Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2017, 7, 5420–5430. [Google Scholar] [CrossRef]
- Betancourt, L.E.; Rojas-Pérez, A.; Orozco, I.; Frenkel, A.I.; Li, Y.; Sasaki, K.; Senanayake, S.D.; Cabrera, C.R. Enhancing ORR Performance of Bimetallic PdAg Electrocatalysts by Designing Interactions between Pd and Ag. ACS Appl. Energy Mater. 2020, 3, 2342–2349. [Google Scholar] [CrossRef]
- Sekol, R.C.; Li, X.; Cohen, P.; Doubek, G.; Carmo, M.; Taylor, A.D. Silver palladium core-shell electrocatalyst supported on MWNTs for ORR in alkaline media. Appl. Catal. B Environ. 2013, 138–139, 285–293. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Tammeveski, K. Oxygen Reduction Reaction on Silver Catalysts in Alkaline Media: A Minireview. ChemElectroChem 2019, 6, 73–86. [Google Scholar] [CrossRef]
- Holewinski, A.; Idrobo, J.C.; Linic, S. High-Performance Ag-Co alloy catalysts for electrochemical oxygen reduction. Nat. Chem. 2014, 6, 828–834. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, N.; An, L.; Li, X.; Xia, D. Synthesis of high dispersed intermetallic Ag4Sn/C and its enhanced oxygen reduction reaction activity. J. Power Sources 2013, 240, 606–611. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Strømsheim, M.D.; Svenum, I.H.; Mahmoodinia, M.; Boix, V.; Knudsen, J.; Venvik, H.J. Segregation dynamics of a Pd-Ag surface during CO oxidation investigated by NAP-XPS. Catal. Today 2022, 384–386, 265–273. [Google Scholar] [CrossRef]
- Van Spronsen, M.A.; Daunmu, K.; O’Connor, C.R.; Egle, T.; Kersell, H.; Oliver-Meseguer, J.; Salmeron, M.B.; Madix, R.J.; Sautet, P.; Friend, C.M. Dynamics of Surface Alloys: Rearrangement of Pd/Ag(111) Induced by CO and O2. J. Phys. Chem. C 2019, 123, 8312–8323. [Google Scholar] [CrossRef]
- Gedara, B.S.A.; Muir, M.; Islam, A.; Liu, D.; Trenary, M. Room Temperature Migration of Ag Atoms to Cover Pd Islands on Ag(111). J. Phys. Chem. C 2021, 125, 27828–27836. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Rassolov, A.V.; Mashkovsky, I.S.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Panafidin, M.A.; Zubavichus, Y.V.; Bukhtiyarov, V.I.; et al. CO-Induced segregation as an efficient tool to control the surface composition and catalytic performance of PdAg3/Al2O3 catalyst. Mendeleev Commun. 2019, 29, 547–549. [Google Scholar] [CrossRef]
- Mamatkulov, M.; Yudanov, I.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Neyman, K.M. Pd Segregation on the Surface of Bimetallic PdAu Nanoparticles Induced by Low Coverage of Adsorbed CO. J. Phys. Chem. C 2019, 123, 8037–8046. [Google Scholar] [CrossRef]
- Farkas, A.P.; Diemant, T.; Bansmann, J.; Behm, R.J. The adsorption of oxygen and coadsorption of CO and oxygen on structurally well-defined PdAg surface alloys. ChemPhysChem 2012, 13, 3516–3525. [Google Scholar] [CrossRef]
- Xiao, B.B.; Jiang, X.B.; Jiang, Q. Density functional theory study of oxygen reduction reaction on Pt/Pd3Al(111) alloy electrocatalyst. Phys. Chem. Chem. Phys. 2016, 18, 14234–14243. [Google Scholar] [CrossRef]
- Farberow, C.A.; Godinez-Garcia, A.; Peng, G.; Perez-Robles, J.F.; Solorza-Feria, O.; Mavrikakis, M. Mechanistic Studies of Oxygen Reduction by Hydrogen on PdAg(110). ACS Catal. 2013, 3, 1622–1632. [Google Scholar] [CrossRef]
- Viswanathan, V.; Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Universality in oxygen reduction electrocatalysis on metal surfaces. ACS Catal. 2012, 2, 1654–1660. [Google Scholar] [CrossRef]
- Mancera, L.A.; Behm, R.J.; Groß, A. Structure and local reactivity of PdAg/Pd(111) surface alloys. Phys. Chem. Chem. Phys. 2013, 15, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Shin, K.; Henkelman, G. Stability of Pt skin intermetallic core catalysts and adsorption properties for the oxygen reduction reaction. J. Phys. Chem. C 2021, 125, 3527–3534. [Google Scholar] [CrossRef]
- Sakong, S.; Mosch, C.; Groß, A. CO adsorption on Cu-Pd alloy surfaces: Ligand versus ensemble effects. Phys. Chem. Chem. Phys. 2007, 9, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Hansen, H.A.; Dickens, C.F.; Nørskov, J.K.; Vegge, T. Functional Independent Scaling Relation for ORR/OER Catalysts. J. Phys. Chem. C 2016, 120, 24910–24916. [Google Scholar] [CrossRef] [Green Version]
- Sakong, S.; Mahlberg, D.; Roman, T.; Li, M.; Pandey, M.; Groß, A. Influence of local inhomogeneities and the electrochemical environment on the oxygen reduction reaction on Pt-based electrodes: A DFT study. J. Phys. Chem. C 2020, 124, 27604–27613. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Hansen, H.A.; Viswanathan, V.; Nørskov, J.K. Unifying kinetic and thermodynamic analysis of 2 e− and 4 e− reduction of oxygen on metal surfaces. J. Phys. Chem. C 2014, 118, 6706–6718. [Google Scholar] [CrossRef]
- Hua, M.; Tian, X.; Li, S.; Zhang, X.; Shao, A.; Song, L.; Lin, X. A casting combined quenching strategy to prepare PdAg single atom alloys designed using the cluster expansion combined Monte Carlo method. Phys. Chem. Chem. Phys. 2022, 24, 2251–2264. [Google Scholar] [CrossRef]
- Song, L.; Tian, X.; Shao, A.; Hua, M.; Li, L.; Li, H.; Lin, X. The structure of metallic melts in eutectic alloys based on the Wulff cluster model: Theory meets experiment. Phys. Chem. Chem. Phys. 2021, 23, 3606–3614. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B—Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Schüttler, K.M.; Mancera, L.A.; Diemant, T.; Groß, A.; Behm, R.J. Interaction of CO with PtxAg1-x/Pt(111) surface alloys: More than dilution by Ag atoms. Surf. Sci. 2016, 650, 237–254. [Google Scholar] [CrossRef]
- Larsen, A.H.; Mortensen, J.J.; Blomqvist, J.; Castelli, I.E.; Christensen, R.; Dułak, M.; Friis, J.; Groves, M.N.; Hammer, B.; Hargus, C.; et al. The atomic simulation environment—A Python library for working with atoms. J. Phys. Condens. Matter 2017, 29, 273002. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.X.; Jiang, M.; Lu, Z.; Gao, C.; Chen, Z.W.; Singh, C.V. Two-Dimensional Graphdiyne-Confined Platinum Catalyst for Hydrogen Evolution and Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2021, 13, 47541–47548. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Gunasooriya, G.T.K.K.; Nørskov, J.K. Analysis of Acid-Stable and Active Oxides for the Oxygen Evolution Reaction. ACS Energy Lett. 2020, 5, 3778–3787. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Zamora Zeledón, J.A.; Stevens, M.B.; Gunasooriya, G.T.K.K.; Gallo, A.; Landers, A.T.; Kreider, M.E.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Tuning the electronic structure of Ag-Pd alloys to enhance performance for alkaline oxygen reduction. Nat. Commun. 2021, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.A.; Fischer, J.M.T.A.; Yuan, Q.; Hankel, M.; Searles, D.J. Evaluating the Catalytic Efficiency of Paired, Single-Atom Catalysts for the Oxygen Reduction Reaction. ACS Catal. 2019, 9, 7660–7667. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.A.; Rossmeisl, J.; Nørskov, J.K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni(111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 2008, 10, 3722–3730. [Google Scholar] [CrossRef] [PubMed]
- Darby, M.T.; Stamatakis, M. Single-Atom Alloys for the Electrochemical Oxygen Reduction Reaction. ChemPhysChem 2021, 22, 499–508. [Google Scholar] [CrossRef]
- Santoveña-Uribe, A.; Maya-Cornejo, J.; Bahena, D.; Ledesma, J.; Pérez, R.; Esparza, R. Synthesis and Characterization of AgPd Bimetallic Nanoparticles as Efficient Electrocatalysts for Oxygen Reduction Reaction. Electrocatalysis 2021, 11, 536–545. [Google Scholar] [CrossRef]
- Qiu, X.; Yan, X.; Cen, K.; Zhang, H.; Gao, G.; Wu, L.; Sun, D.; Tang, Y. One-Pot synthesis of Ag-rich AgPd alloy nanoactiniae and their enhanced electrocatalytic activity toward oxygen reduction. J. Energy Chem. 2019, 28, 111–117. [Google Scholar] [CrossRef]

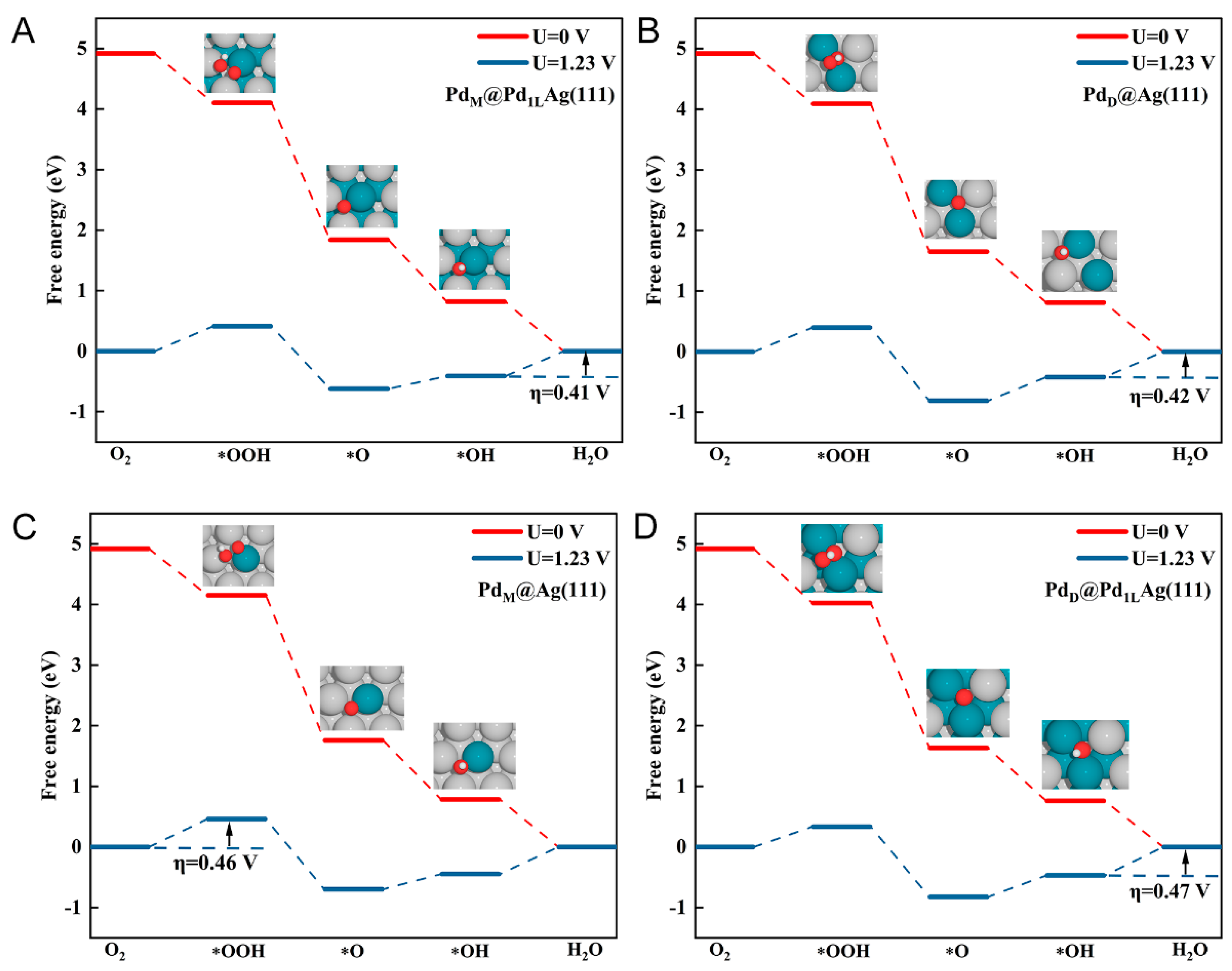
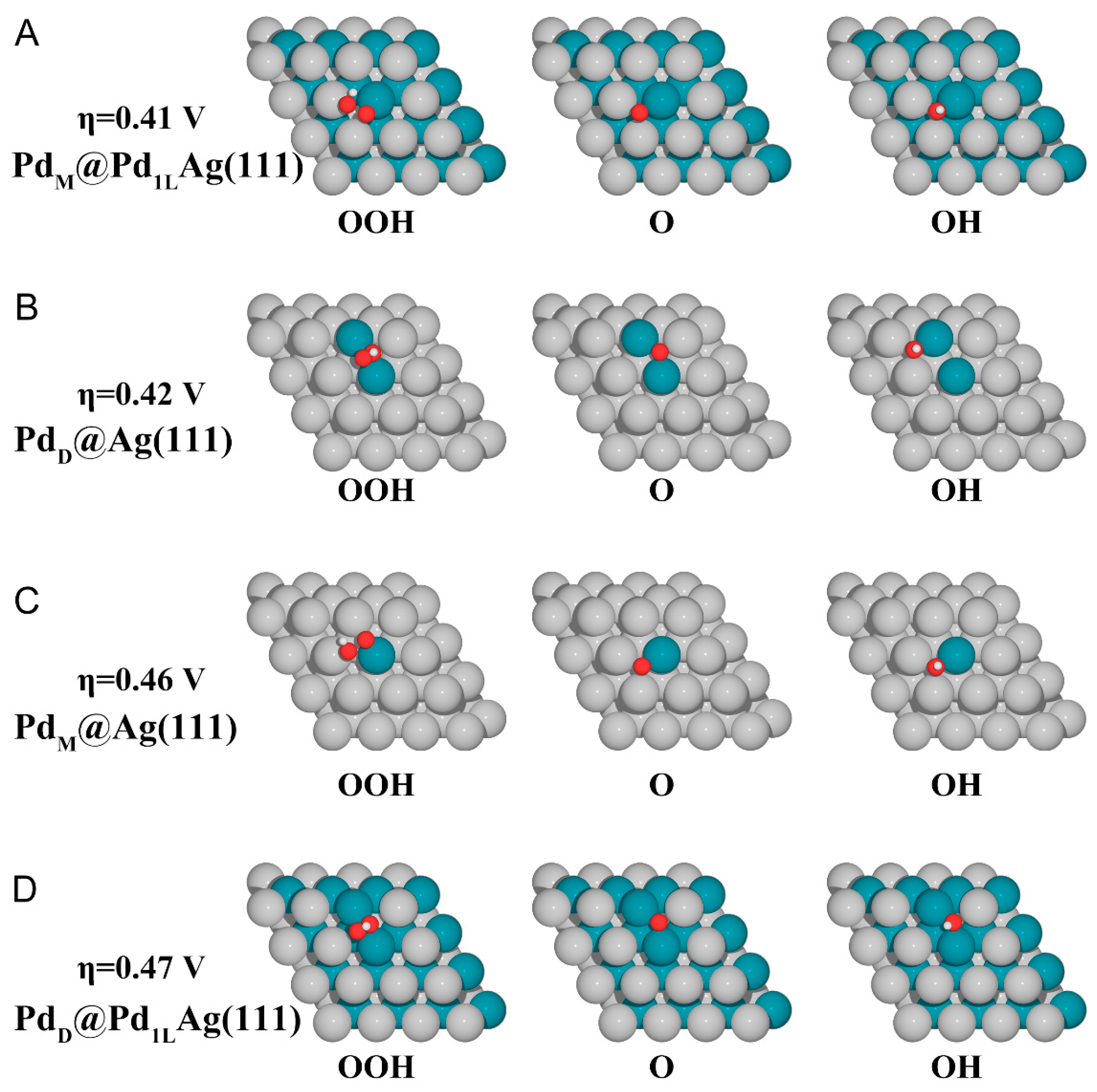
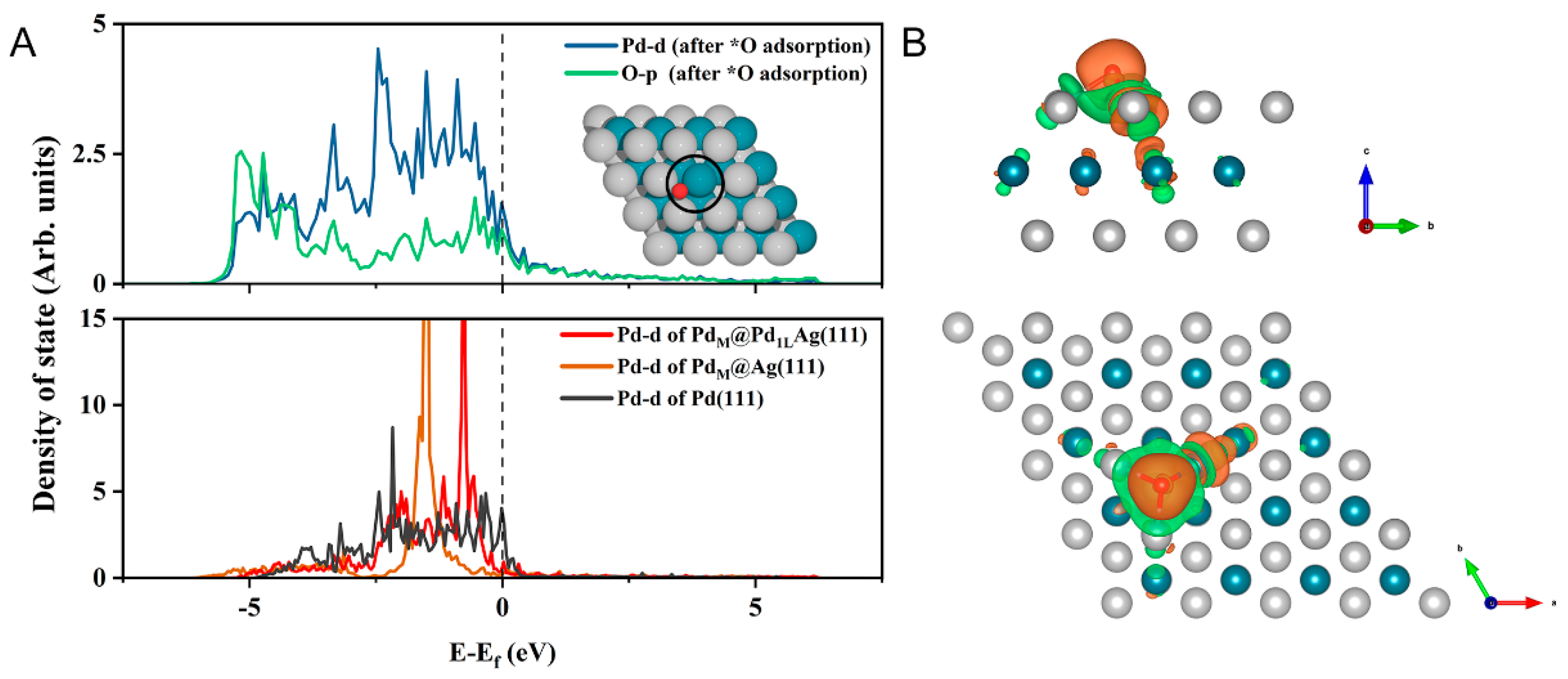
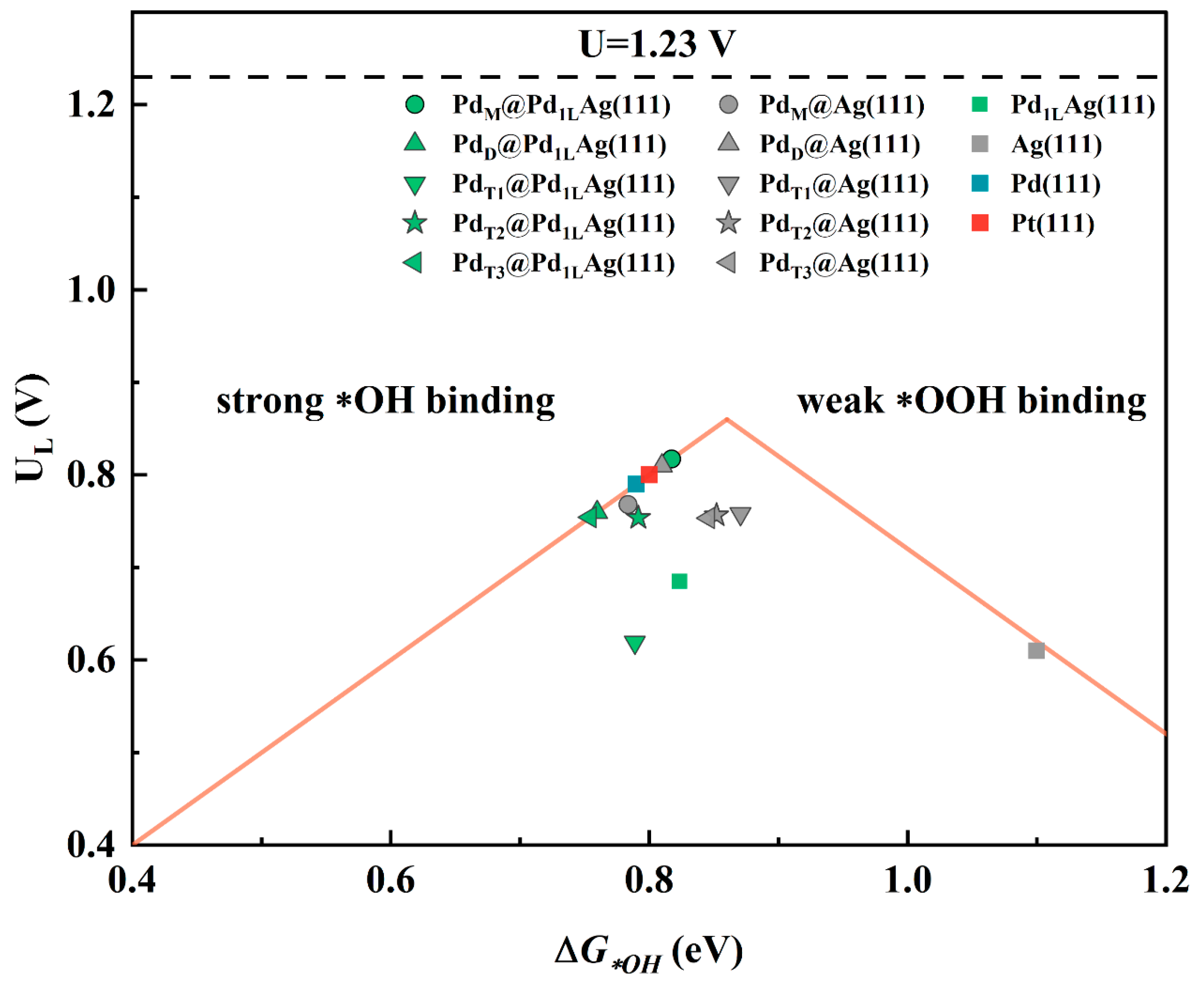

| Active Site Model | Active Site Model | ||
|---|---|---|---|
| PdM@Ag(111) | −0.12 | PdM@Pd1LAg(111) | −0.08 |
| PdD@Ag(111) | −0.11 | PdD@Pd1LAg(111) | −0.06 |
| PdT1@Ag(111) | −0.09 | PdT1@Pd1LAg(111) | −0.04 |
| PdT2@Ag(111) | −0.10 | PdT2@Pd1LAg(111) | −0.04 |
| PdT3@Ag(111) | −0.10 | PdT3@Pd1LAg(111) | −0.04 |
| Pd1LAg(111) | −0.10 |
| Active Site Model | ||||||
|---|---|---|---|---|---|---|
| PdM@Ag(111) | −0.768 | −2.391 | −0.977 | −0.784 | 0.768 | 0.462 |
| PdD@Ag(111) | −0.832 | −2.439 | −0.840 | −0.810 | 0.810 | 0.420 |
| PdT1@Ag(111) | −0.772 | −2.520 | −0.758 | −0.871 | 0.758 | 0.472 |
| PdT2@Ag(111) | −0.756 | −2.464 | −0.847 | −0.852 | 0.756 | 0.474 |
| PdT3@Ag(111) | −0.753 | −2.490 | −0.831 | −0.846 | 0.753 | 0.477 |
| PdM@Pd1LAg(111) | −0.817 | −2.262 | −1.023 | −0.817 | 0.817 | 0.413 |
| PdD@Pd1LAg(111) | −0.895 | −2.390 | −0.875 | −0.760 | 0.760 | 0.470 |
| PdT1@Pd1LAg(111) | −0.937 | −2.575 | −0.619 | −0.789 | 0.619 | 0.611 |
| PdT2@Pd1LAg(111) | −0.870 | −2.505 | −0.753 | −0.792 | 0.753 | 0.477 |
| PdT3@Pd1LAg(111) | −0.878 | −2.420 | −0.868 | −0.754 | 0.754 | 0.476 |
| Pd1LAg(111) | −0.685 | −2.129 | −1.282 | −0.824 | 0.685 | 0.545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, M.; Tian, X.; Li, S.; Lin, X. PdAg/Ag(111) Surface Alloys: A Highly Efficient Catalyst of Oxygen Reduction Reaction. Nanomaterials 2022, 12, 1802. https://doi.org/10.3390/nano12111802
Hua M, Tian X, Li S, Lin X. PdAg/Ag(111) Surface Alloys: A Highly Efficient Catalyst of Oxygen Reduction Reaction. Nanomaterials. 2022; 12(11):1802. https://doi.org/10.3390/nano12111802
Chicago/Turabian StyleHua, Minghao, Xuelei Tian, Shuo Li, and Xiaohang Lin. 2022. "PdAg/Ag(111) Surface Alloys: A Highly Efficient Catalyst of Oxygen Reduction Reaction" Nanomaterials 12, no. 11: 1802. https://doi.org/10.3390/nano12111802







