Micro/Nano Energy Storage Devices Based on Composite Electrode Materials
Abstract
:1. Introduction
2. Experimental Section
Material Preparation
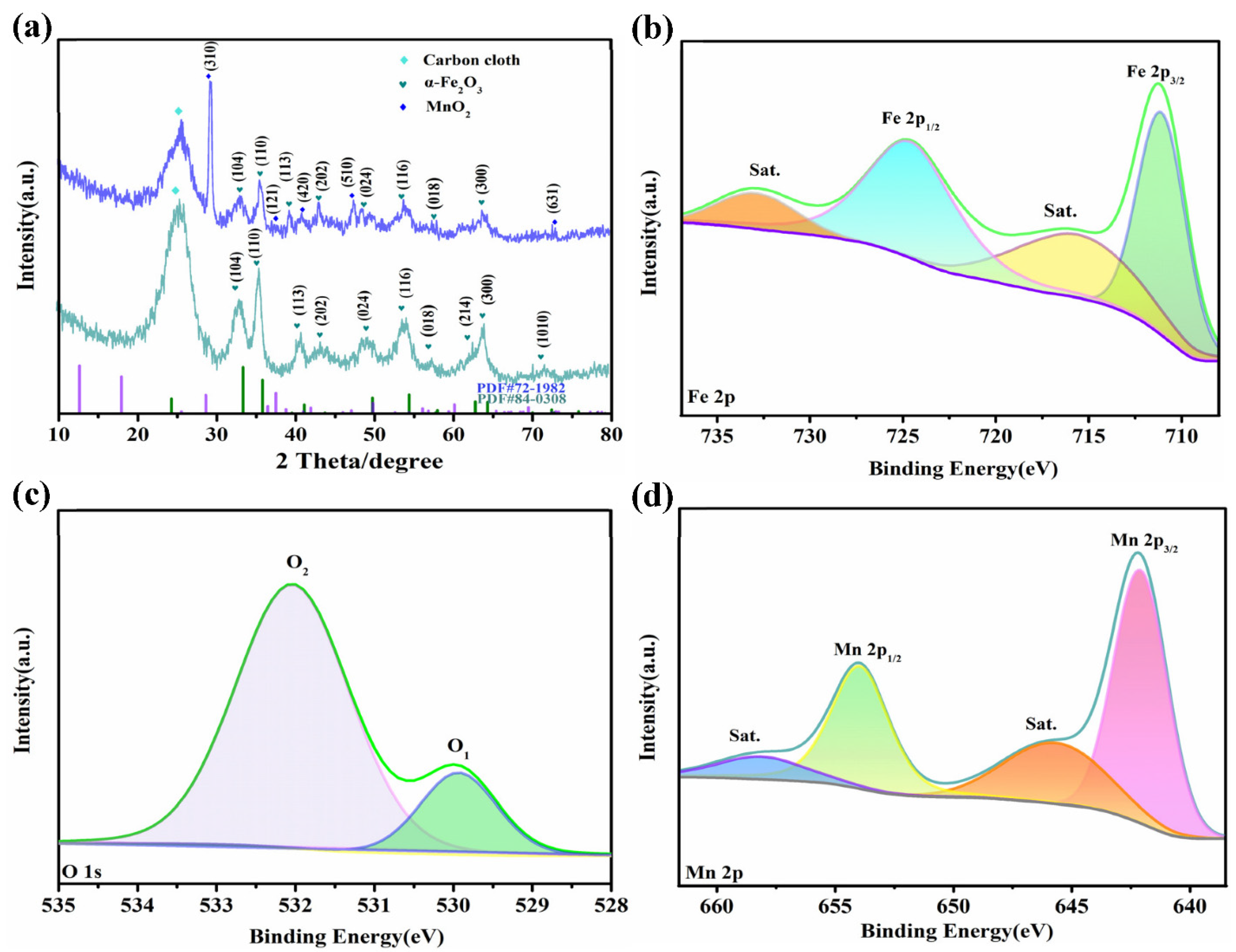
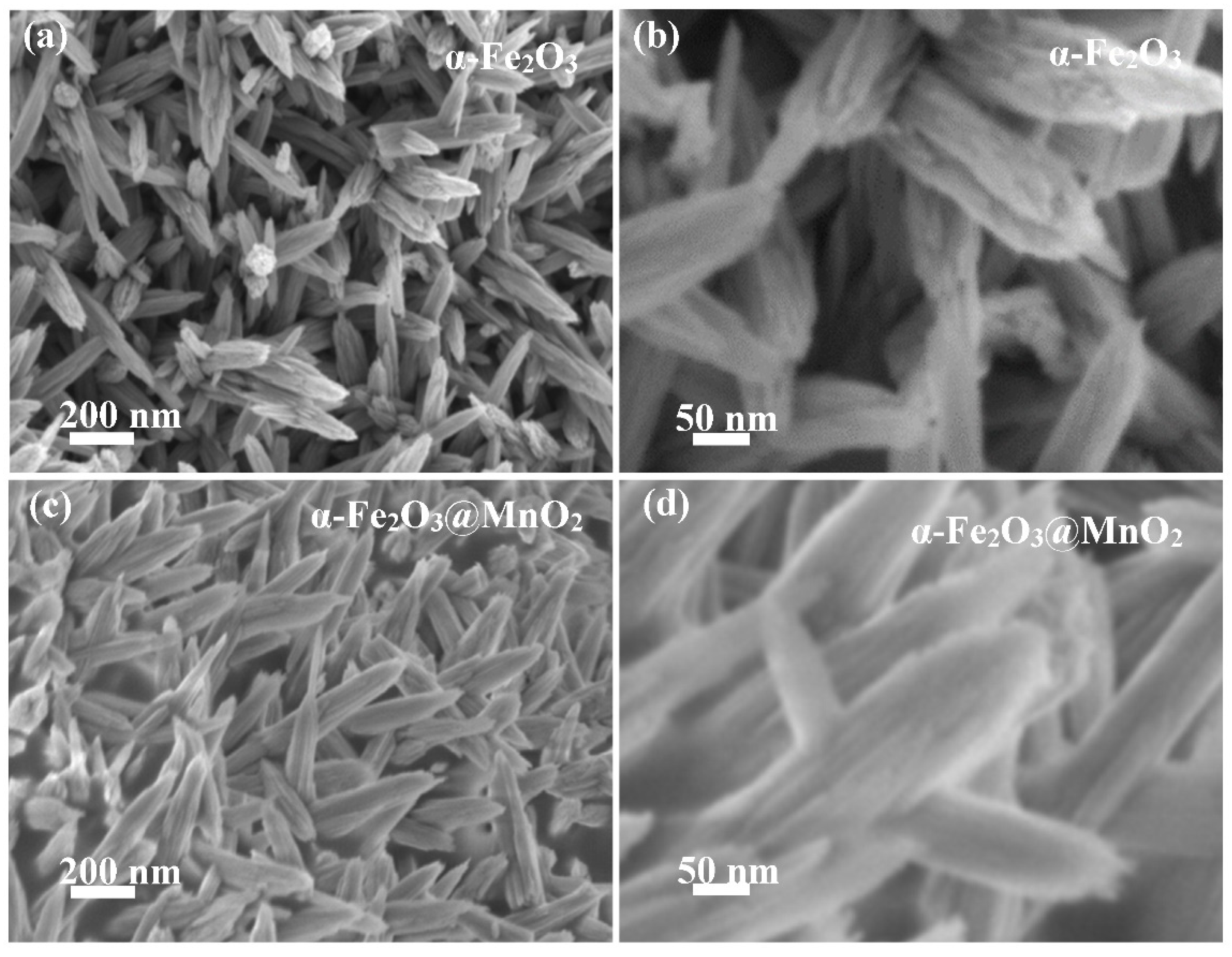
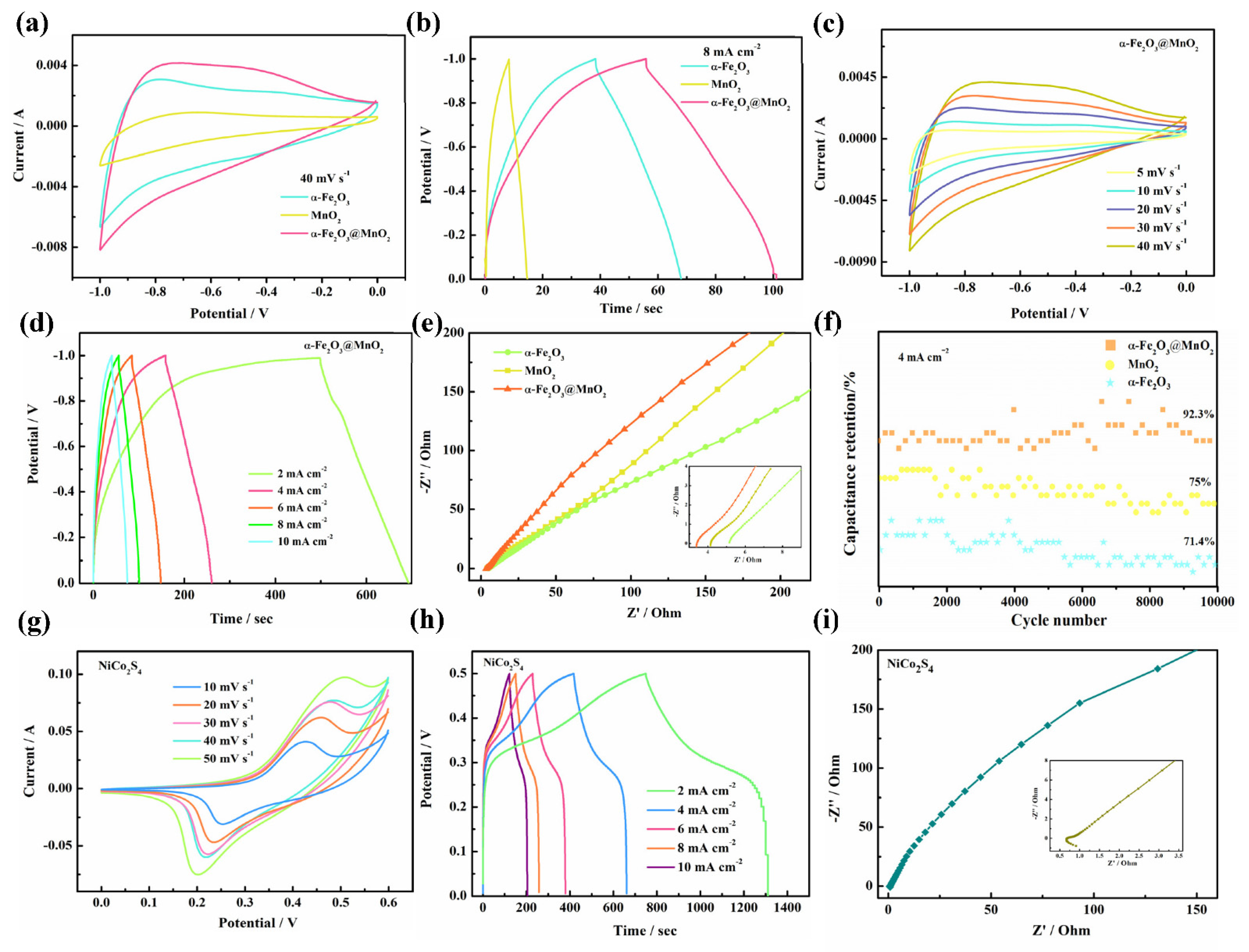
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ye, S.; Ding, C.M.; Liu, M.Y.; Wang, A.Q.; Huang, Q.G.; Li, C. Water oxidation catalysts for artificial photosynthesis. Adv. Mater. 2019, 31, 1902069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X. Hydrogen and sodium ions co-intercalated vanadium dioxide electrode materials with enhanced zinc ion storage capacity. Nano Energy 2021, 86, 106124. [Google Scholar] [CrossRef]
- Cao, X.H.; Tan, C.L.; Zhang, X.; Zhao, W.; Zhang, H. Solution-processed two-dimensional metal dichalcogenide-based nanomaterials for energy storage and conversion. Adv. Mater. 2016, 28, 6167. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.Z.; Zhao, D.P.; Wu, X. Research progress on transition metal oxide based electrode materials for asymmetric hybrid capacitors. Chin. Chem. Lett. 2020, 31, 2177–2188. [Google Scholar] [CrossRef]
- Rakibuddin, M.; Shinde, M.A.; Kim, H. Sol-gel fabrication of NiO and NiO/WO3 based electrochromic device on ITO and flexible substrate. Ceram. Int. 2020, 46, 8631–8639. [Google Scholar] [CrossRef]
- Zheng, X.; Han, Z.C.; Yao, S.Y.; Xiao, H.H.; Chai, F.; Qu, F.Y.; Wu, X. Spinous α-Fe2O3 hierarchical nanostructures anchored on Ni foam for supercapacitors electrodes and visible light driven photocatalysts. Dalton Trans. 2016, 45, 7094–7103. [Google Scholar] [CrossRef]
- Zhao, D.P.; Zhang, R.; Dai, M.Z.; Liu, H.Q.; Jian, W.; Bai, F.Q.; Wu, X. Constructing high efficient CoZnxMn2-xO4 electrocatalyst by regulating the electronic structure and surface reconstruction. Small 2022, 18, 107268. [Google Scholar] [CrossRef]
- Liu, H.Q.; Zhao, D.P.; Hu, P.F.; Chen, K.F.; Wu, X.; Xue, D.F. Design strategies toward achieving high performance [email protected] electrode materials, Mater. Today Phys. 2020, 13, 100197. [Google Scholar]
- Rakibuddin, M.; Shinde, M.A.; Kim, H. Facile solegel fabrication of MoS2 bulk, flake and quantum dot for electrochromic device and their enhanced performance with WO3. Electrochim. Acta 2020, 349, 136403. [Google Scholar] [CrossRef]
- Ahmad, K.; Ahmad, M.A.; Song, G.; Kim, H. Design and fabrication of MoSe2/WO3 thin films for the construction of electrochromic devices on indium tin oxide based glass and flexible substrates. Ceram. Int. 2021, 47, 34297–34306. [Google Scholar] [CrossRef]
- Liu, H.Q.; Zhao, D.P.; Liu, Y.; Tong, Y.L.; Wu, X.; Shen, G.Z. NiMoCo layered double hydroxides for electrocatalyst and supercapacitor electrode. Sci. China Mater. 2021, 64, 581–591. [Google Scholar] [CrossRef]
- Xia, T.; Zhao, D.P.; Xia, Q.; Umar, A.; Wu, X. Realizing high performance flexible supercapacitors by electrode modification. RSC Adv. 2021, 11, 39045–39050. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Xia, T.; Dai, M.Z.; Wu, X.; Zhao, Y.F. A facile synthetic protocol of α-Fe2O3@FeS2 nanocrystals for advanced electrochemical capacitor. CrystEngComm 2021, 23, 2432–2438. [Google Scholar] [CrossRef]
- Xia, Q.; Xia, T.; Wu, X. PPy decorated α-Fe2O3 nanosheets as flexible supercapacitor electrode. Rare Met. 2022, 41, 1195–1201. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Li, Z.; Shi, Z.; Zhu, J.; Mei, H. Fe2O3/N doped rGO anode hybridized with NiCo LDH/Co(OH)2 cathode for battery-like supercapacitor. Chem. Eng. J. 2021, 403, 126325. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Chen, P.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.L.; Cao, D.X.; Yan, J. Creating oxygen-vacancies in MoO3-x nanobelts toward high volumetric energydensity asymmetric supercapacitors with long lifespan. Nano Energy 2019, 58, 455–465. [Google Scholar] [CrossRef]
- Feng, J.X.; Ye, S.H.; Lu, X.F.; Tong, Y.X.; Li, G.R. Asymmetric paper supercapacitor based on amorphous porous Mn3O4 negative electrode and Ni(OH)2 positive Electrode: A novel and high-performance flexible electrochemical energy storage device. ACS Appl. Mater. Inter. 2015, 7, 11444–11451. [Google Scholar] [CrossRef]
- Liu, L.; Lang, J.; Zhang, P.; Hu, B.; Yan, X. Facile synthesis of Fe2O3 nano-dots@nitrogen-doped graphene for supercapacitor electrode with ultralong cycle life in KOH electrolyte. ACS Appl. Mater. Inter. 2016, 8, 9335–9344. [Google Scholar] [CrossRef]
- Ready, D.W. Mass Transport and Sintering in Impure Ionic Solids. J. Am. Ceram. Soc. 1966, 49, 366. [Google Scholar] [CrossRef]
- Liu, T.; Ling, Y.; Yang, Y.; Finn, L.; Collazo, E.; Zhai, T.; Li, Y. Investigation of hematite nanorod–nanoflake morphological transformation and the application of ultrathin nanoflakes for electrochemical devices. Nano Energy 2015, 12, 169–177. [Google Scholar] [CrossRef]
- Racik, M.K.; Manikandan, A.; Mahendiran, M.; Madhavan, J.; Raj, M.V.A.; Mohamed, M.G.; Maiyalagan, T. Hydrothermal synthesis and characterization studies of α-Fe2O3/MnO2 nanocomposites for energy storage supercapacitor application. Ceram. Int. 2020, 46, 6222–6233. [Google Scholar] [CrossRef]
- Sun, Z.P.; Ai, W.; Liu, J.L.; Qi, X.Y.; Wang, Y.L.; Zhu, J.H.; Zhang, H.; Yu, T. Facile fabrication of hierarchical ZnCo2O4/NiO core/shell nanowire arrays with improved lithium-ion battery performance. Nanoscale 2014, 6, 6563. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Jiang, J.; Cheng, C.W.; Li, H.X.; Zhang, J.X.; Gong, H.; Fan, H.J. Co3O4 nanowire@MnO2 ultrathin nanosheet core/shell arrays: A new class of high-performance pseudocapacitive materials. Adv. Mater. 2011, 23, 2076. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q.; Lv, Y.N.; Mao, Y.B. Three-dimensional ZnO@MnO2 core@shell nanostructures for electrochemical energy storage. Chem. Commun. 2013, 49, 4456–4458. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, M.; Wang, T.; Li, F.; Zhang, Y.X. Construction of unique cupric oxide-manganese dioxide core-shell arrays on a copper grid for high-performance supercapacitors. J. Mater. Chem. A 2016, 4, 10786. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, Y.; Yin, B.S.; Zhang, S.W.; Qu, F.Y.; Wu, X. Enhanced electrochemical performance of hybrid SnO2@MOx (M = Ni, Co, Mn) core-shell nanostructures grown on flexible carbon fibers as the supercapacitor electrode materials. J. Mater. Chem. A 2015, 3, 3676. [Google Scholar] [CrossRef]
- Seol, M.L.; Nam, I.; Ribeiro, E.L.; Segel, B.; Lee, D.; Palma, T.; Wu, H.L.; Mukherjee, D.; Khomami, B.; Hill, C.; et al. All-Printed in-plane supercapacitors by sequential additive manufacturing process. ACS Appl. Energy Mater. 2020, 3, 4965–4973. [Google Scholar] [CrossRef]
- Sarkar, D.; Khan, G.G.; Singh, A.K.; Mandal, K. High-performance pseudocapacitor electrodes based on α-Fe2O3/MnO2 core-shell nanowire eterostructure arrays. J. Phys. Chem. C 2013, 117, 15523. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, T.; Huang, D.X.; Yan, W.; Zhang, Y.Y.; Wan, Q.J.; Yang, N.J. High-energy-density supercapacitors from dual pseudocapacitive nanoelectrodes. ACS Appl. Energy Mater. 2021, 3, 10685–10694. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Wang, W.Q.; Wang, G.C. The perfect matching between the low-cost Fe2O3 nanowire anode and the NiO nanoflake cathode significantly enhances the energy density of asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 6662–6670. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.J.; Yuan, X.C.; Yang, Z.; Zhang, M.; Meng, A.; Li, Q.D. A high-energy density asymmetric supercapacitor based on Fe2O3 nanoneedle arrays and NiCo2O4/Ni(OH)2 hybrid nanosheet arrays grown on sic nanowire networks as free-standing advanced electrodes. Adv. Energy Mater. 2018, 8, 1702787. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.W.; Yang, D.Z.; Qin, L.Y.; Zhang, S.Y.; Xu, T.; Zhao, T.Y.; Yu, Z.Z. Ultraflexible reedlike carbon nanofiber membranes decorated with Ni-Co-S nanosheets and Fe2O3-C coreshell nanoneedle arrays as electrodes of flexible quasisolidstate asymmetric supercapacitors. ACS Appl. Energy Mater. 2021, 4, 1505–1516. [Google Scholar]
- Chen, Y.C.; Kang, C.X.; Ma, L.; Fu, L.K.; Li, G.H.; Hu, Q.; Liu, Q.M. MOF-derived Fe2O3 decorated with MnO2 nanosheet arrays as anode for high energy density hybrid supercapacitor. Chem. Eng. J. 2021, 417, 129243. [Google Scholar] [CrossRef]
- Hong, X.Y.; Li, J.H.; Zhu, G.S.; Xu, H.R.; Zhang, X.Y.; Zhao, Y.Y.; Zhang, J.; Yan, D.L.; Yu, A.B. Cobalt-nickel sulfide nanosheets modified by nitrogen-doped porous reduced graphene oxide as high-conductivity cathode materials for supercapacitor. Electrochim. Acta 2020, 362, 37156. [Google Scholar] [CrossRef]
- Ke, Q.; Guan, C.; Zhang, X.; Zheng, M.; Zhang, Y.; Cai, W.; Zhang, H.; Wang, J. Surface charge-mediated formation of H-TiO2@Ni(OH)2 heterostructures for high-performance supercapacitors. Adv. Mater. 2017, 29, 1604164. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.C.; Zhang, W.; Pu, J.; Wang, Z.H. Homogeneous core–shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- Hu, P.F.; Zhao, D.P.; Liu, H.Q.; Chen, K.F.; Wu, X. Engineering PPy decorated MnCo2O4 urchins for quasi-solid-state hybrid capacitors. CrystEngComm 2019, 21, 1600–1606. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.Z.; Dubbink, D.; Elshof, J.E.T. Inkjet Printing of δ-MnO2 Nanosheets for Flexible Solid-state Microsupercapacitor. Nano Energy 2018, 49, 481–488. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Z.X.; Xiao, J.F.; Cen, W.L.; Yuan, S.J. Polypyrrole-encapsulated Fe2O3 nanotube arrays on a carbon cloth support: Achieving synergistic effect for enhanced supercapacitor performance. Electrochim. Acta 2021, 386, 138486. [Google Scholar] [CrossRef]
- Yang, P.H.; Xiao, X.; Li, Y.Z.; Ding, Y.; Qiang, P.F.; Tan, X.H.; Mai, W.J.; Lin, Z.Y.; Wu, W.Z.; Li, T.Q.; et al. Hydrogenated ZnO Core-Shell Nanocables for Flexible Supercapacitors and Self-Powered Systems. ACS Nano 2013, 7, 2617–2626. [Google Scholar] [CrossRef]
- Wu, X.H.; Chen, Y.H.; Liang, K.J.; Yu, X.A.; Zhuang, Q.Q.; Yang, Q.; Liu, S.F.; Liao, S.; Lia, N.; Zhang, H.Y. Fe2O3 nanowire arrays on Ni-coated yarns as excellent electrodes for high performance wearable yarn-supercapacitor. J. Alloys Compd. 2021, 886, 158156. [Google Scholar] [CrossRef]

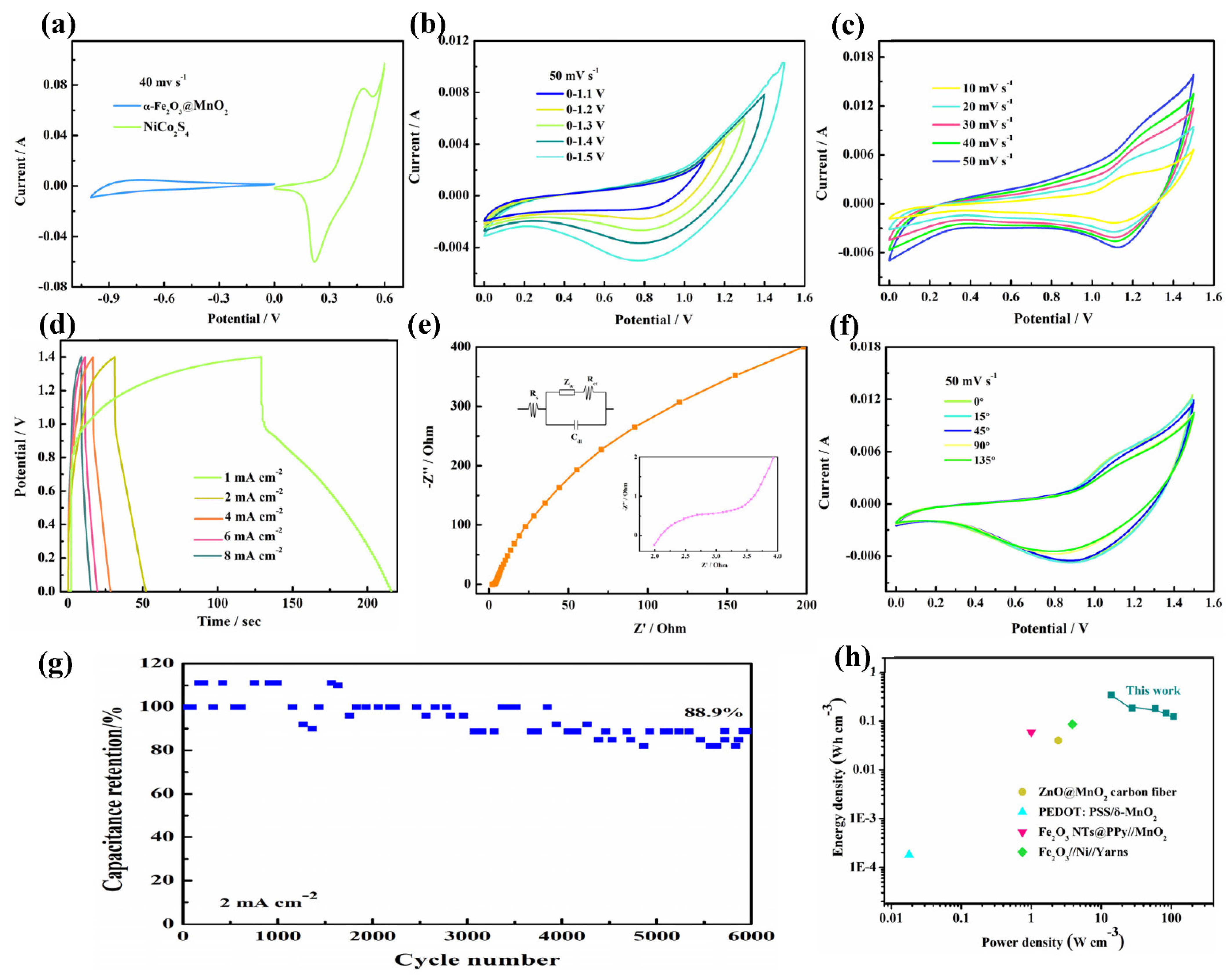
| Supercapacitor | Capacitance | Energy Density (mWh cm−3) | Power Density (W cm−2) | Capacitance Retention | Ref. |
|---|---|---|---|---|---|
| PEDOT: PSS/δ-MnO2 | 2.4 F cm−3 | 0.018 | 0.018 | 88% | [38] |
| Fe2O3NTs@PPy//MnO2 | - | 0.0594 | 1 | 92% | [39] |
| ZnO@MnO2 | 26 mF cm−2 | 0.04 | 2.44 | 87.5% | [40] |
| Fe2O3//Ni/Yarns | 0.67 F cm−3 | 0.086 | 3.87 | 87.1% | [41] |
| α-Fe2O3@MnO2//NiCo2S4 | 37.8 mF cm−2 | 0.102 | 4.2 | 88.9% | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Shang, D.; Li, Z. Micro/Nano Energy Storage Devices Based on Composite Electrode Materials. Nanomaterials 2022, 12, 2202. https://doi.org/10.3390/nano12132202
Niu Y, Shang D, Li Z. Micro/Nano Energy Storage Devices Based on Composite Electrode Materials. Nanomaterials. 2022; 12(13):2202. https://doi.org/10.3390/nano12132202
Chicago/Turabian StyleNiu, Yanqi, Deyong Shang, and Zhanping Li. 2022. "Micro/Nano Energy Storage Devices Based on Composite Electrode Materials" Nanomaterials 12, no. 13: 2202. https://doi.org/10.3390/nano12132202





