Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy
Abstract
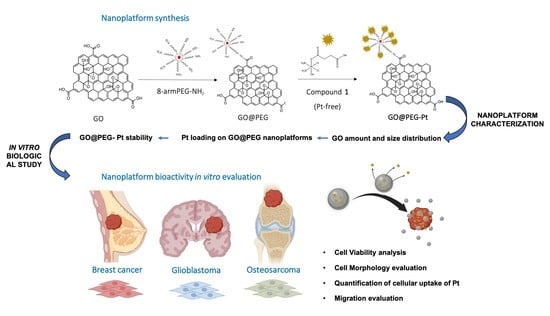
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Pt-Based Drug 1 (Pt-Free)
2.2. GO Flake Size Optimization
2.3. PEGylation of GO
2.4. Pt Loading on GO@PEG Nanoplaftorms
2.5. Characterization of GO-Based Nanoplatforms
2.5.1. Determination of GO Amount and Size Distribution
2.5.2. Pt Loading on GO-PEG Nanoplatforms
2.5.3. GO-PEG Pt Stability Testing
2.6. In Vitro Biological Study
2.6.1. Cell Culture
2.6.2. Cell Viability
2.6.3. Cell Morphology
2.6.4. Quantification of Cellular Uptake of Pt
2.6.5. Migration Assay
2.6.6. Statistical Analyses
3. Results and Discussion
3.1. Characterization of GO-Based Nanoplatforms
3.2. Synthesis of the Platinum-Based Prodrug
3.3. In Vitro Evaluation of GO@PEG-Pt Bioactivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization—Cancer. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 February 2022).
- Golemis, E.A.; Scheet, P.; Beck, T.N.; Scolnick, E.M.; Hunter, D.J.; Hawk, E.; Hopkins, N. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018, 32, 868–902. [Google Scholar] [CrossRef] [Green Version]
- Hulvat, M.C. Cancer Incidence and Trends. Surg. Clin. North. Am. 2020, 100, 469–481. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Gmeiner, W.H.; Ghosh, S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2014, 3, 111–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A microfluidic 3D invitro model for specificity of breast cancer metastasis to bone. Biomaterials 2014, 35, 2454–2461. [Google Scholar] [CrossRef]
- Brook, N.; Brook, E.; Dharmarajan, A.; Dass, C.R.; Chan, A. Breast cancer bone metastases: Pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol. 2018, 96, 63–78. [Google Scholar] [CrossRef]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Ottaviani, G.J.N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, C.; Yang, J.; Lv, M.; Liu, R.; He, D.; Fan, C.; Huang, Q. Uniform ultrasmall graphene oxide nanosheets with low cytotoxicity and high cellular uptake. ACS Appl. Mater. Interfaces 2013, 5, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Vila, M.; Portolés, M.T.; Vallet-Regi, M.; Gracio, J.; Marques, P.A.A.P. Nano-graphene oxide: A potential multifunctional platform for cancer therapy. Adv. Healthc. Mater. 2013, 2, 1072–1090. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Sima, L.E.; Chiritoiu, G.; Negut, I.; Grumezescu, V.; Orobeti, S.; Munteanu, C.V.A.; Sima, F.; Axente, E. Functionalized Graphene Oxide Thin Films for Anti-tumor Drug Delivery to Melanoma Cells. Front. Chem. 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Wang, X.; Tang, Q.; Chen, H.; Zhang, Q.; Jiang, H.; Wang, Z. Functionalized graphene oxide against U251 glioma cells and its molecular mechanism. Mater. Sci. Eng. C 2020, 116, 111187. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug. Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Orecchioni, M.; Cabizza, R.; Bianco, A.; Delogu, L.G. Graphene as cancer theranostic tool: Progress and future challenges. Theranostics 2015, 5, 710–723. [Google Scholar] [CrossRef]
- Zhao, X.; Si, J.; Huang, D.; Li, K.; Xin, Y.; Sui, M. Application of star poly(ethylene glycol) derivatives in drug delivery and controlled release. J. Control. Release 2020, 323, 565–577. [Google Scholar] [CrossRef]
- Rajaratnam, V.; Islam, M.M.; Yang, M.; Slaby, R.; Ramirez, H.M.; Mirza, S.P. Glioblastoma: Pathogenesis and current status of chemotherapy and other novel treatments. Cancers 2020, 12, 937. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Daniel, W.L.; Giljohann, D.A.; Mirkin, C.A.; Lippard, S.J. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J. Am. Chem. Soc. 2009, 131, 14652–14653. [Google Scholar] [CrossRef]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Zhao, C.; Qin, G.; Xi, G.; Li, T.; Wang, X.; Chen, T. One-step reduction and PEGylation of graphene oxide for photothermally controlled drug delivery. Biomaterials 2014, 35, 4986–4995. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’Homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [Green Version]
- Jung, I.; Pelton, M.; Piner, R.; Dikin, D.A.; Stankovich, S.; Watcharotone, S.; Hausner, A.M.; Ruoff, R.S. Simple approach for high-contrast optical imaging and characterization of graphene-based sheets. Nano Lett. 2007, 7, 3569–3575. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Sazonova, E.V.; Kopeina, G.S.; Imyanitov, E.N.; Zhivotovsky, B. Platinum drugs and taxanes: Can we overcome resistance? Cell Death Discov. 2021, 7, 155. [Google Scholar] [CrossRef]
- Sowder, M.E.; Johnson, R.W. Bone as a Preferential Site for Metastasis. JBMR Plus 2019, 3, e10126. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Q.; Ding, F.; Yu, Y.; Wu, F. CRGDyK-modified camretastain A4-loaded graphene oxide nanosheets for targeted anticancer drug delivery. RSC Adv. 2015, 5, 40258–40268. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.; Lu, Y.; Pan, Z. Bio-inspired cisplatin annocarriers for osteosarcoma treatment. Biomater. Sci. 2016, 4, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Son, K.D.; Kim, Y.J. Anticancer activity of drug-loaded calcium phosphate nanocomposites against human osteosarcoma. Biomater. Res. 2017, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Yu, H.Y.; Hai, S.; Liu, J.G. Cisplatin-loaded poly(L-glutamic acid)-g-methoxy poly(ethylene glycol) nanoparticles as a potential chemotherapeutic agent against osteosarcoma. Chin. J. Polym. Sci. 2015, 33, 763–771. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Salaheldin, T.A.; Ramadan, M.A.; Farroh, K.Y.; Abdallah, Z.F.; Eloahed, T.Y.A. Synthesis, characterization and cytotoxic evaluation of graphene oxide nanosheets: In vitro liver cancer model. Asian Pacific J. Cancer Prev. 2017, 18, 955–961. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Sun, T.; Li, L. Crystal phase transition and properties of titanium oxide nanotube arrays prepared by anodization. J. Alloys Compd. 2007, 434–435, 792–795. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Yang, J.; Ma, G.; Zhang, Z.; Zhang, X. Dual sensitive and temporally controlled camptothecin prodrug liposomes codelivery of siRNA for high efficiency tumor therapy. Biomaterials 2014, 35, 9731–9745. [Google Scholar] [CrossRef]
- Jang, D.J.; Moon, C.; Oh, E. Improved tumor targeting and antitumor activity of camptothecin loaded solid lipid nanoparticles by preinjection of blank solid lipid nanoparticles. Biomed. Pharmacother. 2016, 80, 162–172. [Google Scholar] [CrossRef]
- Charmi, J.; Nosrati, H.; Amjad, J.M.; Mohammadkhani, R.; Danafar, H. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5, e01466. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Singh, D.P.; Kumar, R.; Matsuda, A. Graphene oxide for drug delivery and cancer therapy. In Nanostructured Polymer Composites for Biomedical Applications; Nanostructured Polymer Composites for Biomedical Applications Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–488. [Google Scholar] [CrossRef]
- Li, W.; Zhan, P.; De Clercq, E.; Lou, H.; Liu, X. Current drug research on PEGylation with small molecular agents. Prog. Polym. Sci. 2013, 38, 421–444. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. Linkers: The key elements for the creation of efficient nanotherapeutics. J. Control. Release 2018, 270, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Du, L.; Wu, S.; Li, Y.; Zhao, X.; Ju, X.; Wang, Y. Cytotoxicity of PEGylated graphene oxide on lymphoma cells. Biomed. Mater. Eng. 2014, 24, 2135–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Tsuchiya, H. Hemotherapy for osteosarcoma Where does it come from What is it Where is it going. Expert Opin. Pharm. 2013, 14, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Zhao, N.; Wang, C.; Kamar, S.; Zhou, Y.; He, Z.; Yang, J.; Sun, B.; Shi, X.; et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett. 2018, 16, 6228–6237. [Google Scholar] [CrossRef] [Green Version]
- Robl, B.; Botter, S.M.; Pellegrini, G.; Neklyudova, O.; Fuchs, B. Evaluation of intraarterial and intravenous cisplatin chemotherapy in the treatment of metastatic osteosarcoma using an orthotopic xenograft mouse model. J. Exp. Clin. Cancer Res. 2016, 35, 113. [Google Scholar] [CrossRef] [Green Version]
- Winkler, K.; Bielack, S.; Delling, G.; Salzer-Kuntschik, M.; Kotz, R.; Greenshaw, C.; Jürgens, H.; Ritter, J.; Kusnierz-Glaz, C.; Erttmann, R. Effect of Intraarterial Versus Intravenous Cisplatin in Addition to Systemic Doxorubicin, High-Dose Methotrexate, and Ifosfamide on Histologic Tumor Response in Osteosarcoma (Study COSS-8s). Cancer 1990, 66, 1703–1710. [Google Scholar] [CrossRef]
- Mortezaee, K. Organ tropism in solid tumor metastasis: An updated review. Future Oncol. 2021, 17, 194. [Google Scholar] [CrossRef]
- Phromnoi, K.; Yodkeeree, S.; Anuchapreeda, S.; Limtrakul, P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol. Sin. 2009, 30, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J.E. Animal Models for Studying Prevention and Treatment of Breast Cancer; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chou, C.K.; Kim, M.; Vasisht, R.; Kuo, Y.-A.; Ang, P.; Liu, C.; Perillo, E.P.; Chen, Y.-I.; Blocher, K.; et al. Assessing metastatic potential of breast cancer cells based on EGFR dynamics. Sci. Rep. 2019, 9, 3395. [Google Scholar] [CrossRef] [PubMed]
- Kutwin, M.; Sawosz, E.; Jaworski, S.; Wierzbicki, M.; Strojny, B.; Grodzik, M.; Chwalibog, A. Assessment of the proliferation status of glioblastoma cell and tumour tissue after nanoplatinum treatment. PLoS ONE 2017, 12, e0178277. [Google Scholar] [CrossRef] [Green Version]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef]
- Du, G.Y.; He, S.W.; Zhang, L.; Sun, C.X.; Mi, L.D.; Sun, Z.G. Hesperidin exhibits in vitro and in vivo antitumor effects in human osteosarcoma MG-63 cells and xenograft mice models via inhibition of cell migration and invasion, cell cycle arrest and induction of mitochondrial-mediated apoptosis. Oncol Lett. 2018, 16, 6299–6306. [Google Scholar] [CrossRef] [Green Version]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer 2018, 65, 315–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Zhang, B.; Zheng, J.; Yu, M.; Zhou, T.; Zhao, K.; Jia, Y.; Gao, X.; Chen, C.; Wei, T. The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials 2014, 35, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug. Deliv. Rev. 2016, 105, 145–162. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giusto, E.; Žárská, L.; Beirne, D.F.; Rossi, A.; Bassi, G.; Ruffini, A.; Montesi, M.; Montagner, D.; Ranc, V.; Panseri, S. Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy. Nanomaterials 2022, 12, 2372. https://doi.org/10.3390/nano12142372
Giusto E, Žárská L, Beirne DF, Rossi A, Bassi G, Ruffini A, Montesi M, Montagner D, Ranc V, Panseri S. Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy. Nanomaterials. 2022; 12(14):2372. https://doi.org/10.3390/nano12142372
Chicago/Turabian StyleGiusto, Elena, Ludmila Žárská, Darren Fergal Beirne, Arianna Rossi, Giada Bassi, Andrea Ruffini, Monica Montesi, Diego Montagner, Vaclav Ranc, and Silvia Panseri. 2022. "Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy" Nanomaterials 12, no. 14: 2372. https://doi.org/10.3390/nano12142372