A Study of Mechanisms of Nanobubble-Enhanced Flotation of Graphite
Abstract
:1. Introduction
2. Experimental
2.1. Sample
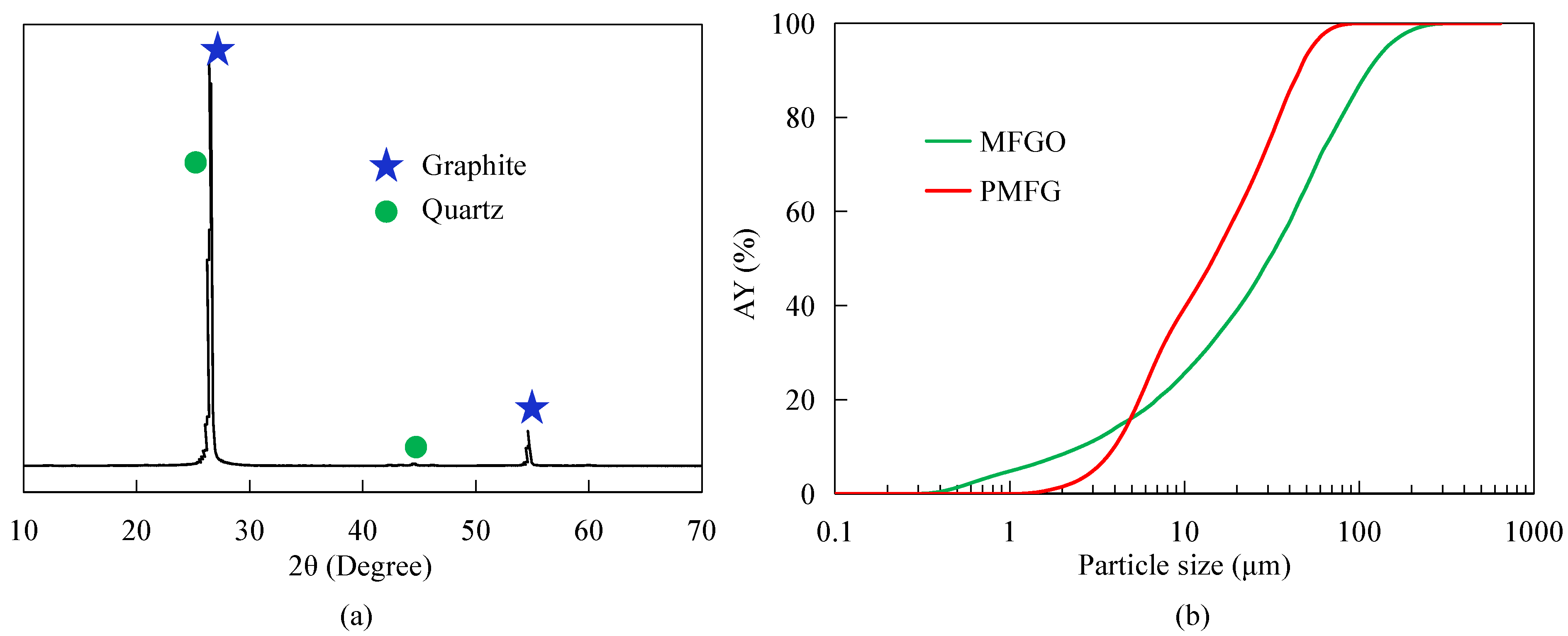
2.2. Sample Characterization
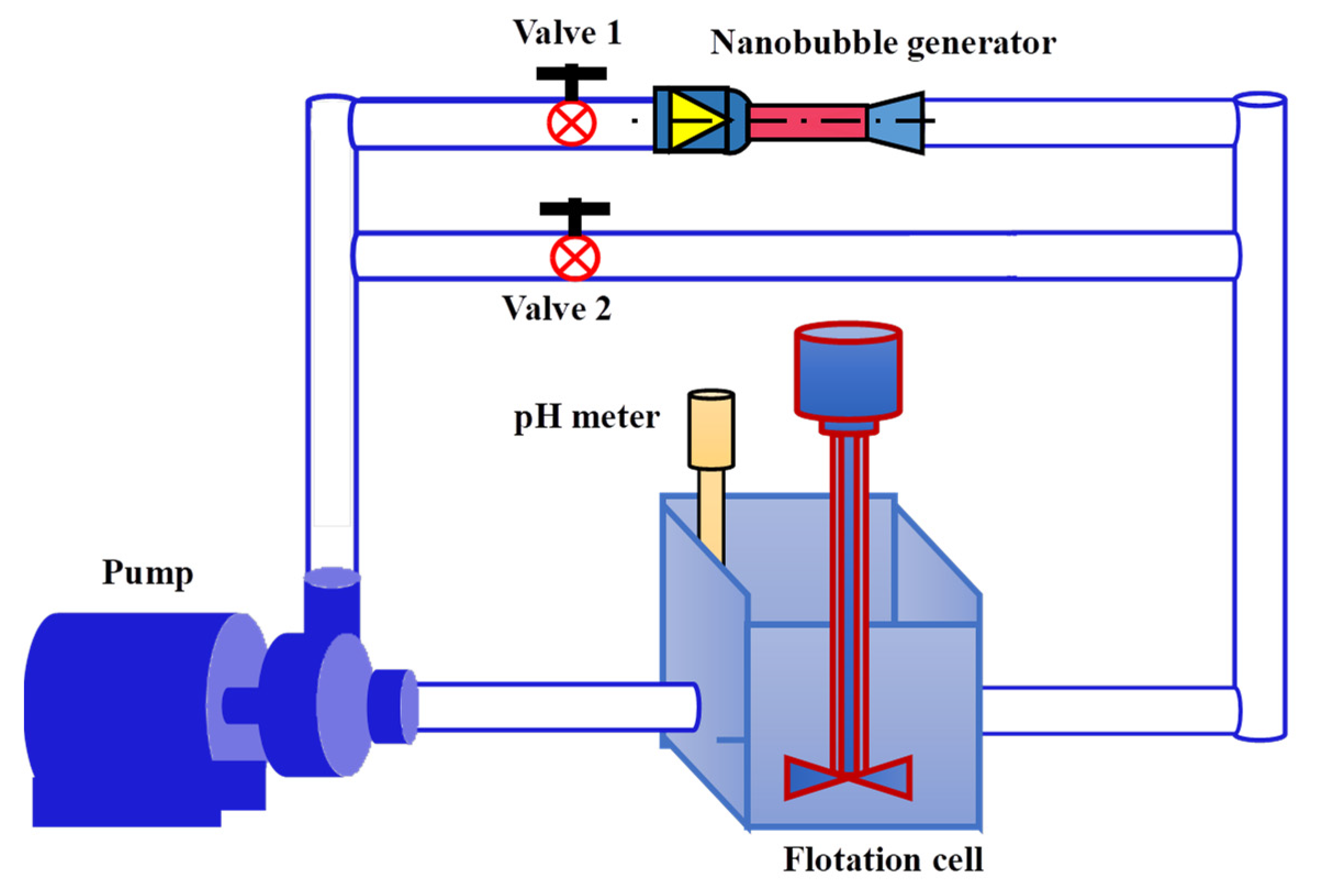
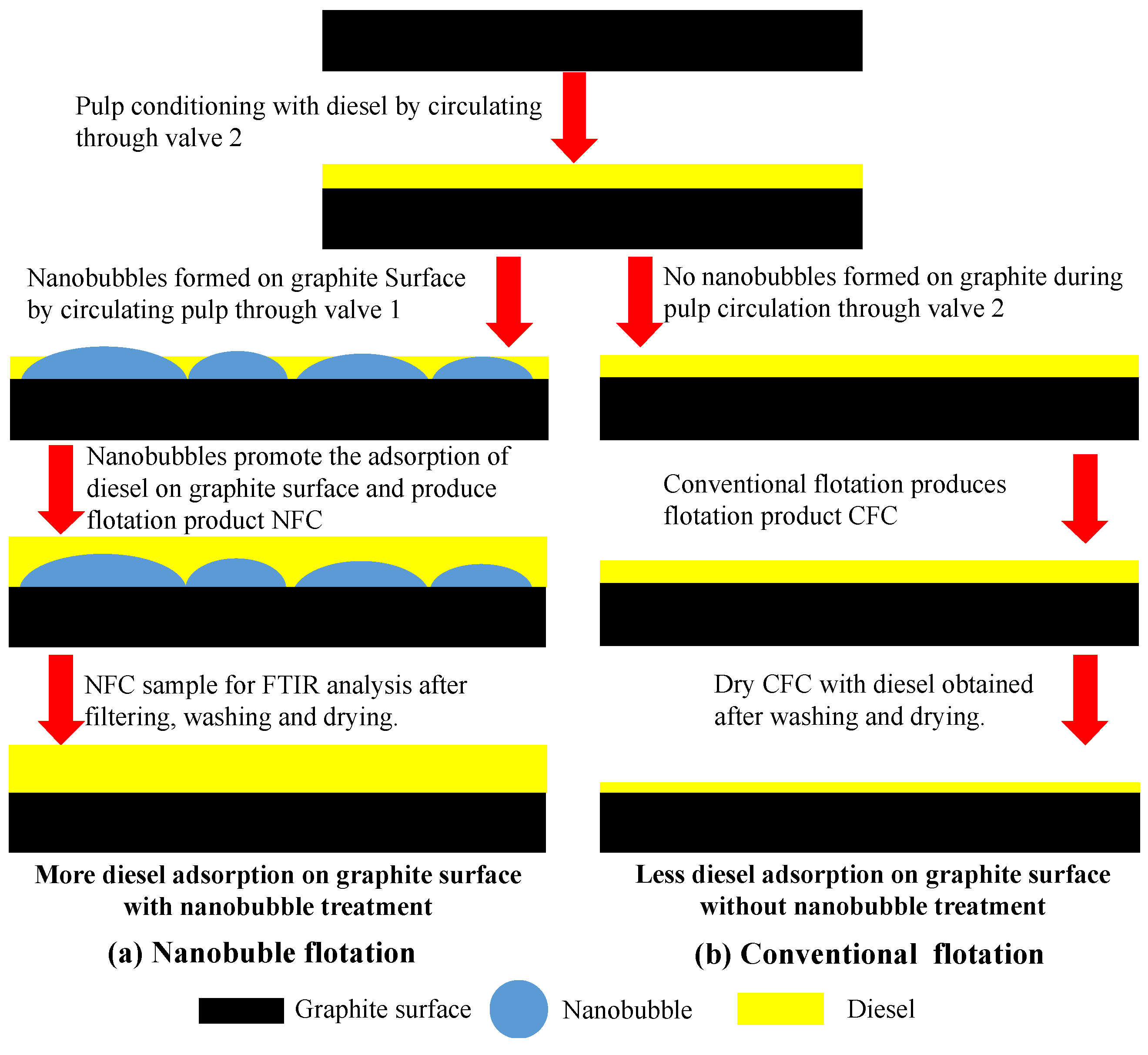
2.3. Flotation System
2.4. Bubble Size Measurement
2.5. Kinetic Flotation Tests
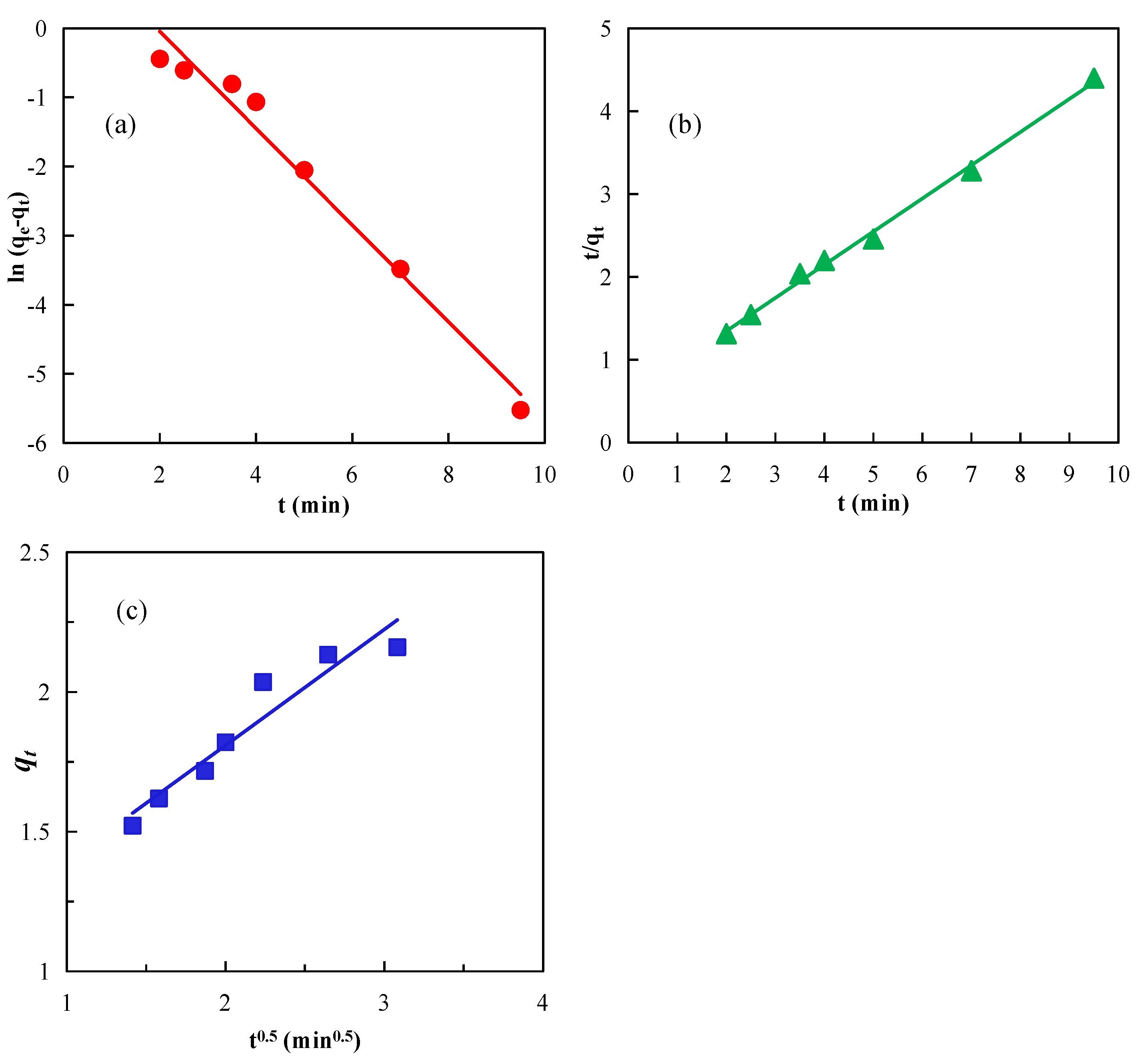
2.6. Adsorption Kinetics of Diesel on Graphite Surface
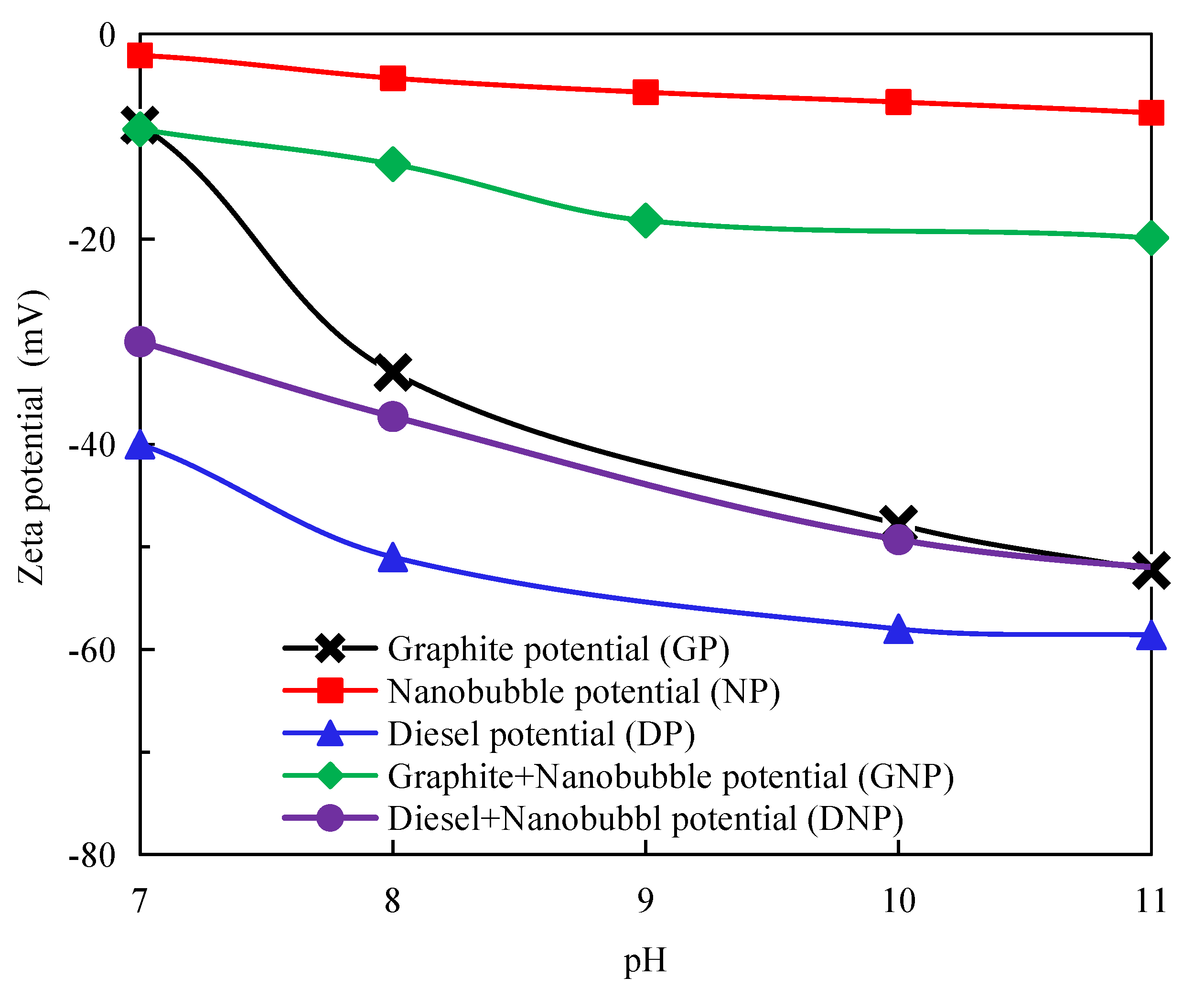
2.7. Zeta Potential Measurement
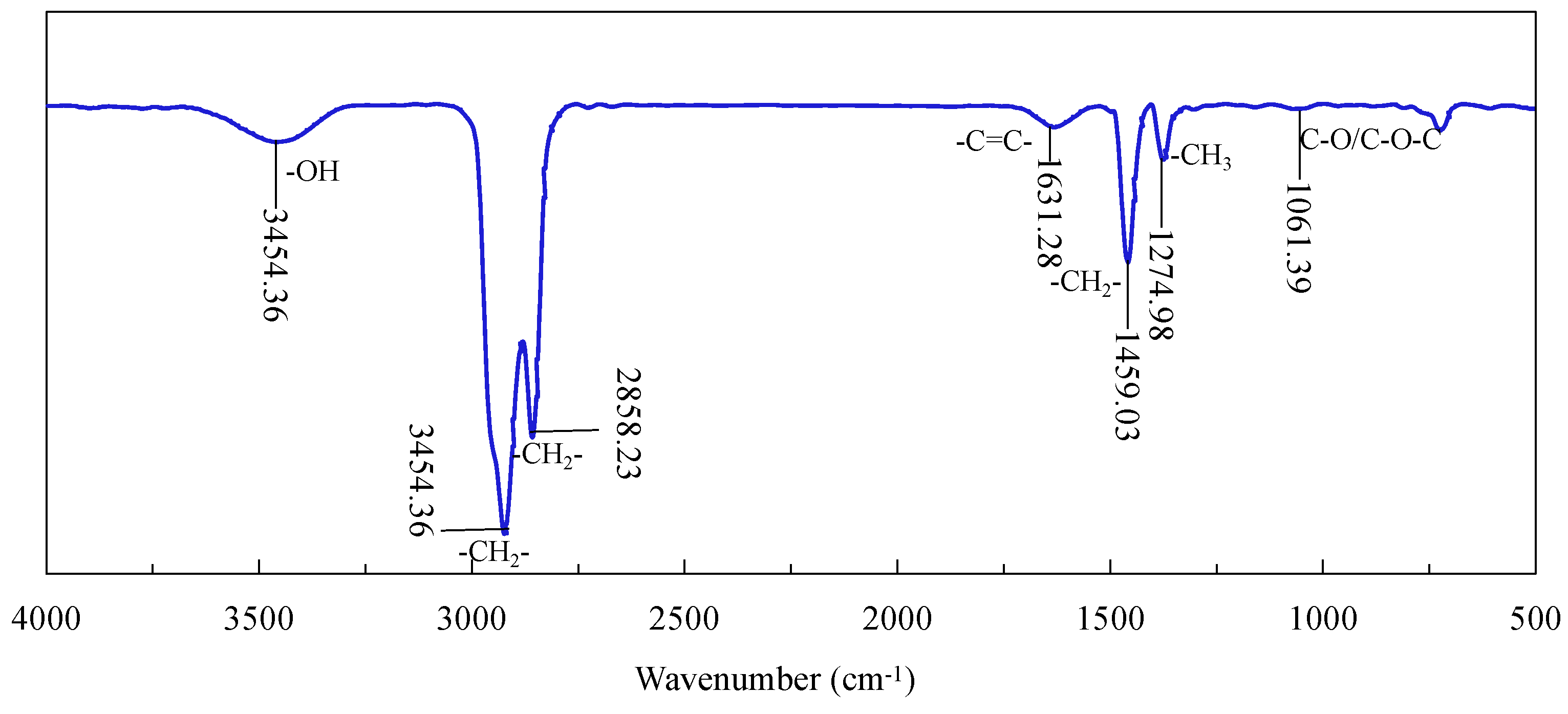
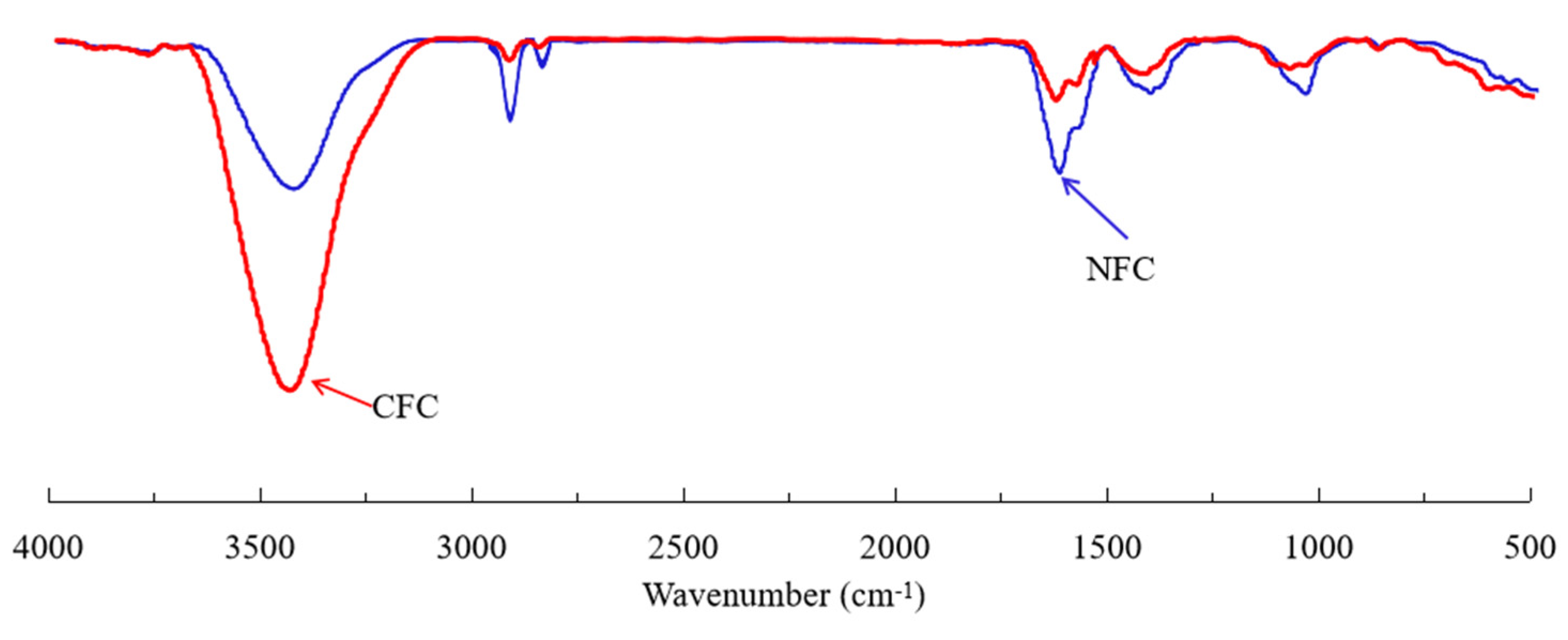
2.8. Contact Angle and FTIR Measurement
3. Results and Discussion
3.1. Nanobubble Size Distribution
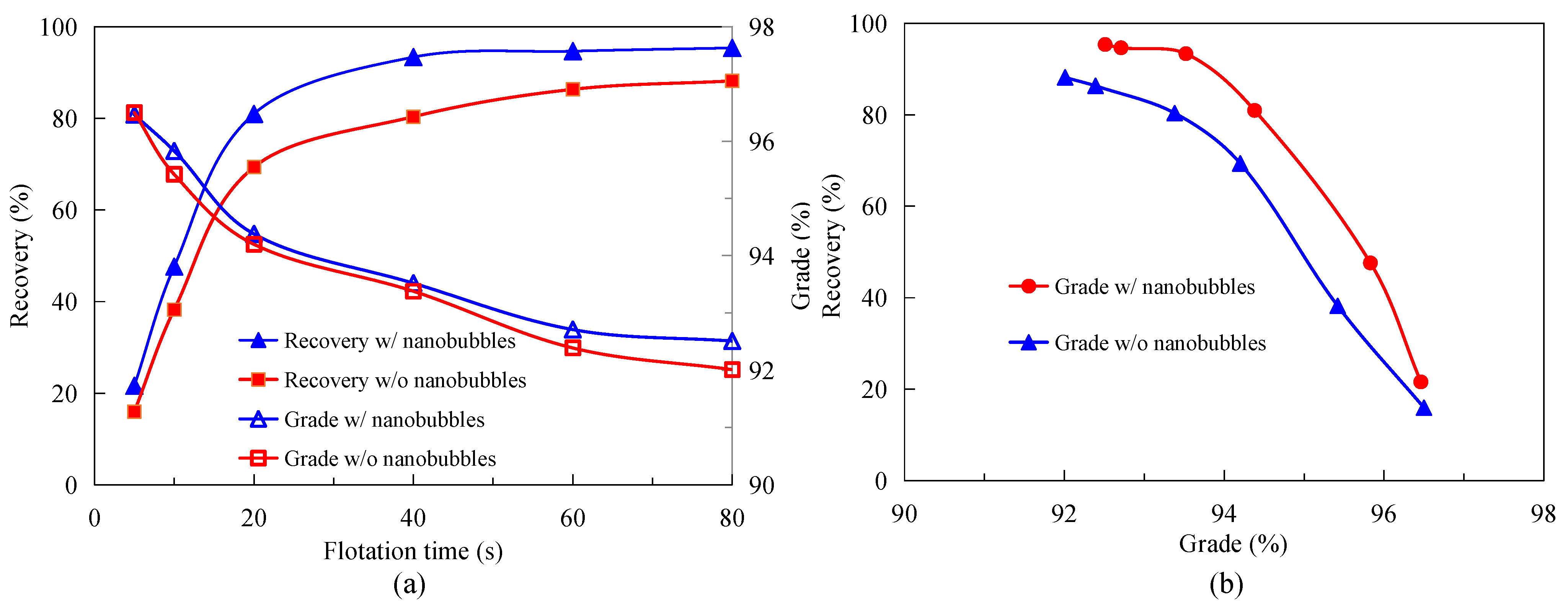
3.2. Effect of Nanobubbles on MFGO Flotation Kinetics
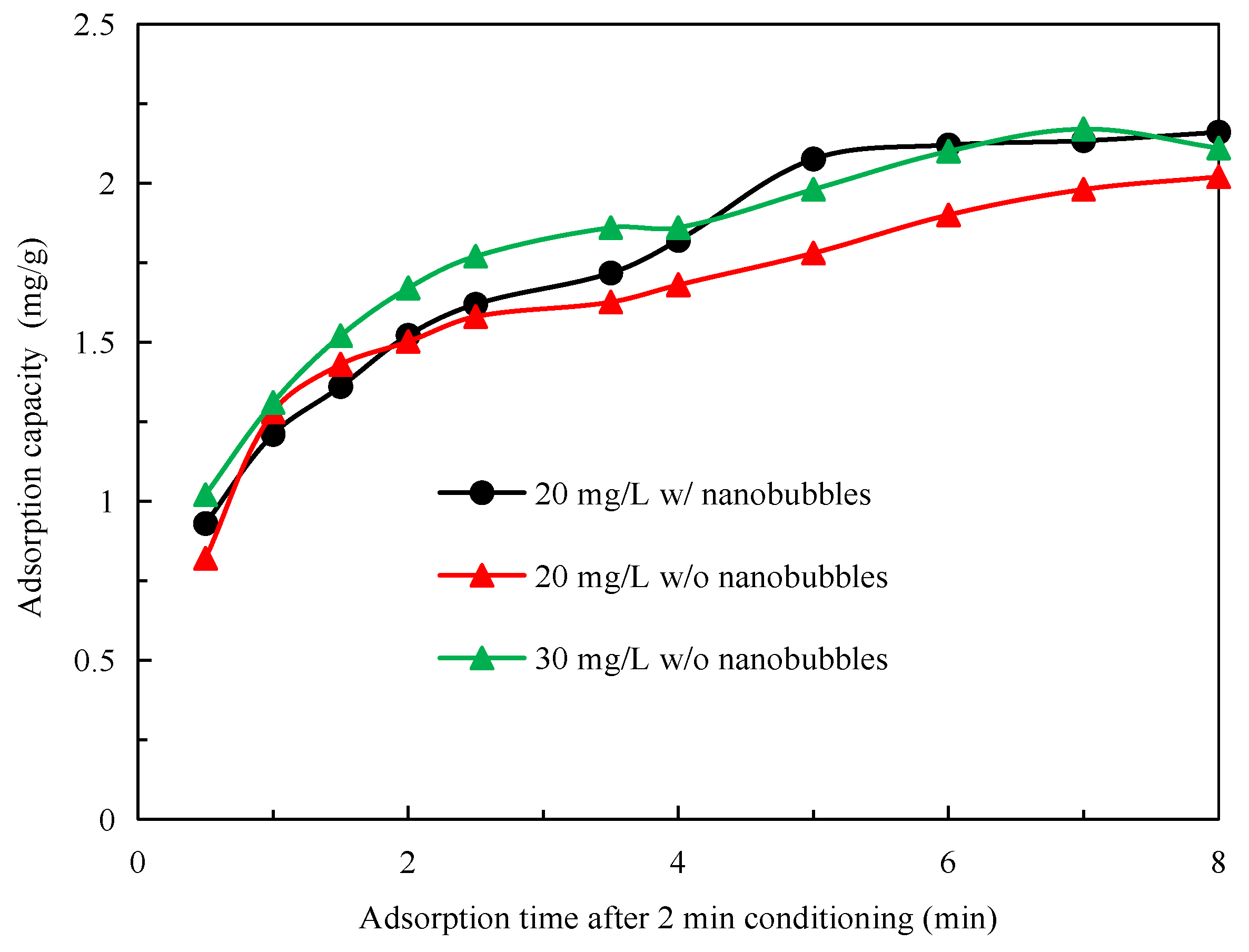
3.3. Effects of Nanobubbles on Diesel Adsorption Kinetics on Graphite
3.4. Measurement of Surface Potential of Graphite, Diesel Droplets, and Nanobubbles
3.5. FTIR Characterization of Diesel Adsorption on Graphite Surface
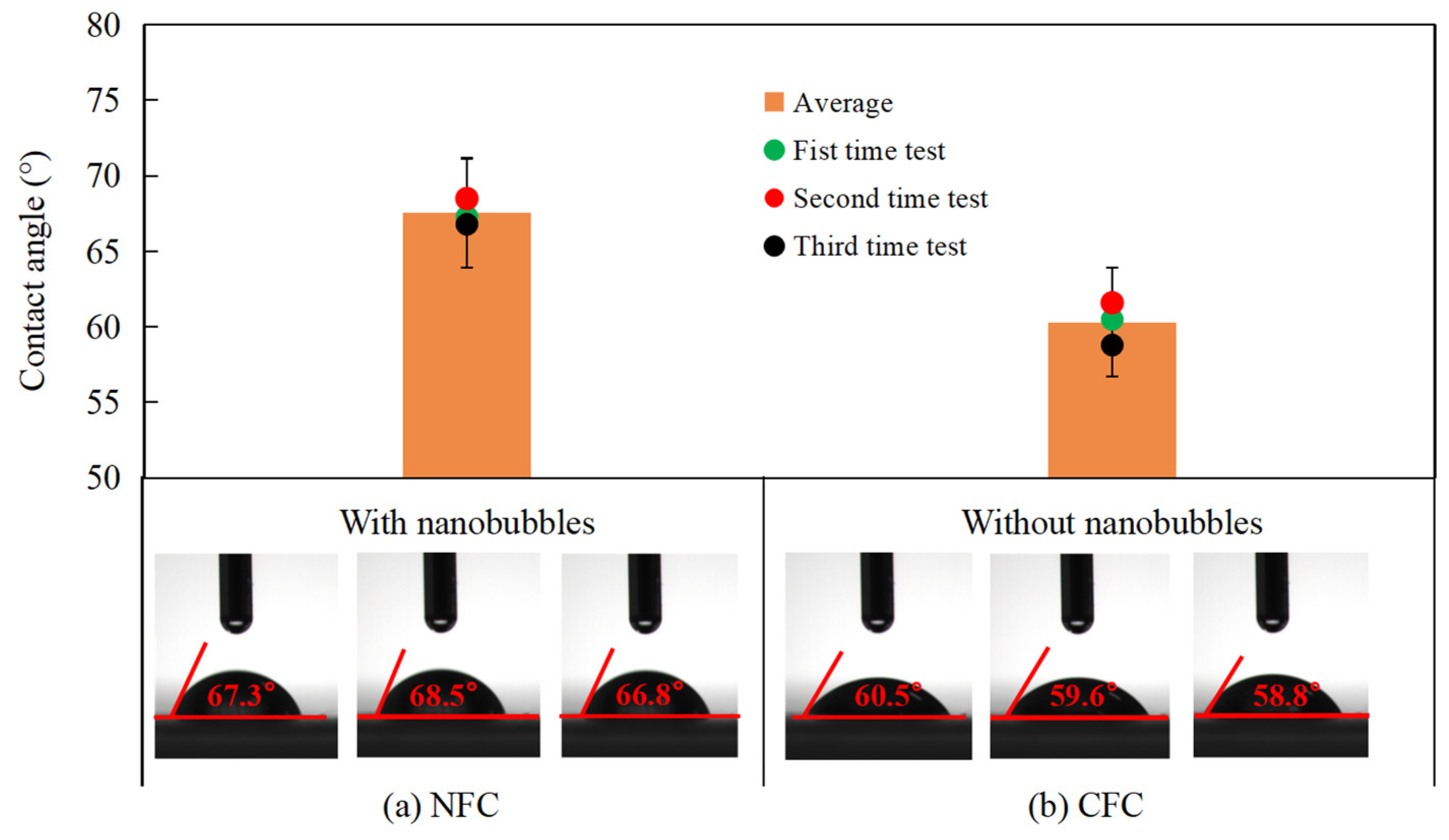
3.6. Contact Angle Measurements
4. Conclusions
- (1)
- The graphite flotation results showed that the flotation kinetics and the rate of recovery and the grade of the concentrate were significantly enhanced by the presence of nanobubbles;
- (2)
- The presence of the surface nanobubbles increased the adsorption rate and capacity of diesel on the graphite surface, significantly improving its hydrophobicity. The mineralization efficiency of the flotation process was also significantly improved by the surface nanobubbles, which is partly responsible for the increased graphite flotation kinetics and selectivity;
- (3)
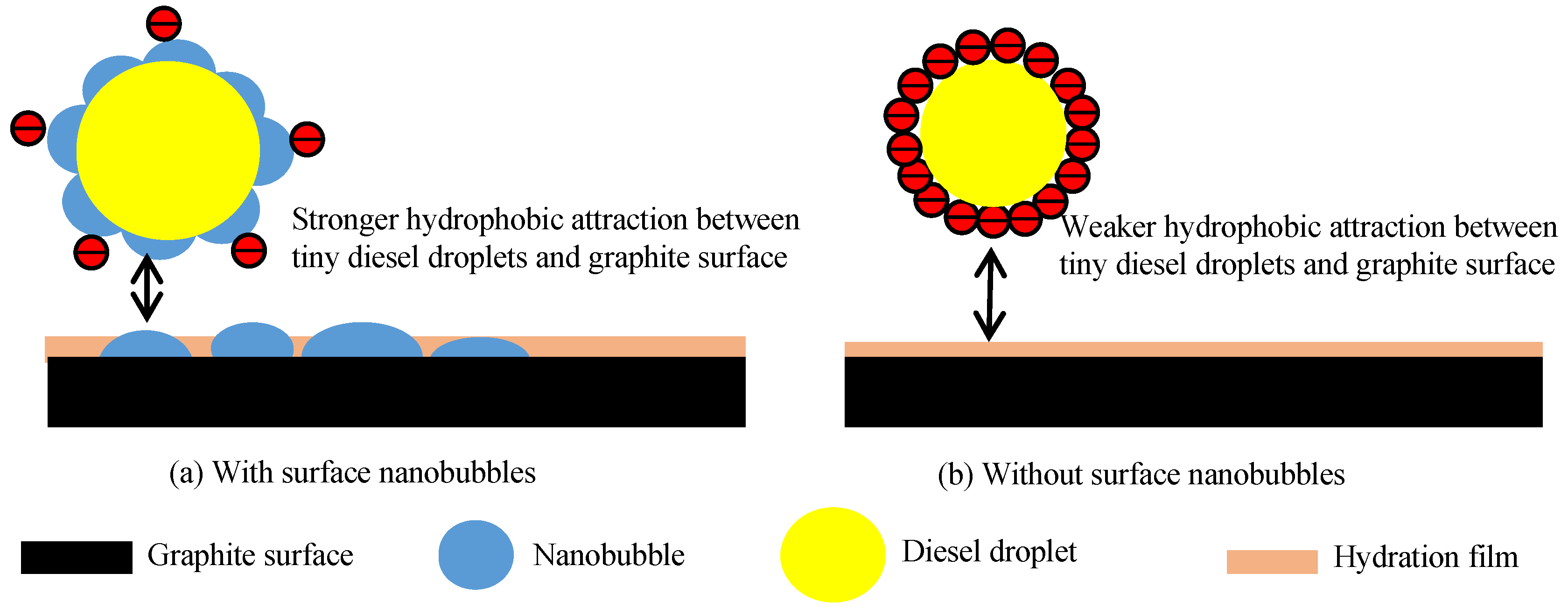
- The nanobubbles formed on the surface of the graphite compound effectively reduced the electrostatic repulsion between the graphite particles, promoting the agglomeration of fine graphite particles and increasing the stability of the graphite agglomerates. The surface nanobubbles also reduced the electrostatic repulsion between the diesel droplets and graphite particles and increased the adsorption capacity of diesel on the graphite surface, which improved the degree of hydrophobicity of the graphite surface and the selectivity of flotation;
- (4)
- The FTIR results and contact angle measurements confirmed that the surface nanobubbles improved the hydrophobicity of the graphite surface, increased the hydrophobic attraction between the graphite particles and diesel droplets and the adsorption capacity of diesel on the graphite surface, further improving the degree of the hydrophobicity of the graphite surface;
- (5)
- Future studies are needed to investigate how nanobubbles function to mask the hydrophilic sites on graphite surfaces. The interactions of nanobubbles with oil droplets and the consequent effects on oil adsorption on graphite should also be studied to achieve a better understanding of the fundamentals of nanobubble-enhanced flotation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 20, 671–689. [Google Scholar] [CrossRef]
- Chehreh, C.S.; Rudolph, M.; Kratzsch, R.; Sandmann, D.; Gutzmer, J. A Review of Graphite Beneficiation Techniques. Miner. Process. Extr. Metall. Rev. 2016, 37, 58–68. [Google Scholar] [CrossRef]
- Billot, N.; Beyer, M.; Koch, N.; Ihle, C.; Reinhart, G. Development of an adhesion model for graphite-based lithium-ion battery anodes. J. Manuf. Syst. 2021, 58, 131–142. [Google Scholar] [CrossRef]
- Yin, Q.; Chen, L.; Chen, Y.; Zhan, F. A high-performance flexible aqueous silver–zinc rechargeable battery based on AgNP/CNT-graphite paper and ZnNF-graphite paper. Compos. Commun. 2021, 26, 100728. [Google Scholar] [CrossRef]
- Yu, J.; Lin, M.; Tan, Q.; Li, J. High-value utilization of graphite electrodes in spent lithium-ion batteries: From 3D waste graphite to 2D graphene oxide. J. Hazard. Mater. 2020, 401, 123715. [Google Scholar] [CrossRef]
- Huang, H.; Ming, X.; Wang, Y.; Guo, F.; Liu, Y.; Xu, Z.; Li, P.; Gao, C. Polyacrylonitrile-derived thermally conductive graphite film via graphene template effect. Carbon 2021, 180, 197–203. [Google Scholar] [CrossRef]
- Morstein, C.E.; Dienwiebel, M. Graphite lubrication mechanisms under high mechanical load. Wear 2021, 477, 203794. [Google Scholar] [CrossRef]
- Sarath, C.K.; Sarkar, D. Structural properties of Al2O3–MgO–C refractory composites improved with YAG nanoparticle hybridized expandable graphite. Mat. Sci. Eng. A-Struct. 2021, 803, 140502. [Google Scholar] [CrossRef]
- Al-Qasir, I.I.; Cheng, Y.; Lin, J.Y.Y.; Campbell, A.A.; Sala, G.; Ramic, K.; Islam, F.F.; Qteish, A.; Marsden, B.; Abernathy, D.L.; et al. Neutron thermalization in nuclear graphite: A modern story of a classic moderator. Ann. Nucl. Energy 2021, 161, 108437. [Google Scholar] [CrossRef]
- Song, Y.; Zhai, G.; Song, J.; Li, G.; Shi, J.; Guo, Q.; Liu, L. Seal and wear properties of graphite from MCMBs/pitch-based carbon/phenolic-based carbon composites. Carbon 2006, 44, 2793–2796. [Google Scholar] [CrossRef]
- Gross, A.J.; Hammond, J.L.; Holzinger, M.; Cosnier, S. Flotation Assembly of Large-Area Ultrathin MWCNT Nanofilms for Construction of Bioelectrodes. Nanomaterials 2017, 7, 342. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, C.; Zheng, X.; Liu, Y.; Goh, W.; Lim, R.; Tan, S.; Yang, L. Three-dimensional highway-like graphite flakes/carbon fiber hybrid electrode for electrochemical biosensor. Mater. Today Adv. 2022, 14, 100238. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Feng, A.; Guo, Z.; Zhang, Y. Application of stirred mill to upgrading of graphite concentrate by flotation. Can. Met. Q. 2017, 57, 245–251. [Google Scholar] [CrossRef]
- You, D.; He, A.; Li, G. Research Progress of Graphite Flotation Technology. Mod. Min. 2020, 36, 130–134. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=7102769434 (accessed on 31 August 2022). (In Chinese).
- Ma, F.; Tao, D.; Tao, Y.; Liu, S. An innovative flake graphite upgrading process based on HPGR, stirred grinding mill, and nanobubble column flotation. Int. J. Min. Sci. Technol. 2021, 31, 1063–1074. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, T.; Chen, Y.; Peng, Y.; Xie, G.; Wu, E. Comparison of mechanical flotation cell and cyclonic microbubble flotation column in terms of separation performance for fine graphite. Physicochem. Probl. Miner. Process. 2018, 54, 732–740. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, T.; Peng, Y.; Xie, G.; Wu, E. Multi-Stage Flotation for the Removal of Ash from Fine Graphite Using Mechanical and Centrifugal Forces. Minerals 2018, 8, 15. [Google Scholar] [CrossRef]
- Kang, W.; Li, H. Enhancement of flaky graphite cleaning by ultrasonic treatment. R. Soc. Open Sci. 2019, 6, 191160. [Google Scholar] [CrossRef]
- Shi, Q.; Liang, X.; Feng, Q.; Chen, Y.; Wu, B. The relationship between the stability of emulsified diesel and flotation of graphite. Miner. Eng. 2015, 78, 89–92. [Google Scholar] [CrossRef]
- Tao, D. Recent advances in fundamentals and applications of nanobubble enhanced froth flotation: A review. Miner. Eng. 2022, 183, 107554. [Google Scholar] [CrossRef]
- Song, B.; Walczyk, W.; Schonherr, H. Contact angles of surface nanobubbles on mixed self-assembled monolayers with systematically varied macroscopic wettability by atomic force microscopy. Langmuir 2011, 27, 8223–8232. [Google Scholar] [CrossRef]
- Ishida, N.; Inoue, T.; Miyahara, M.; Higashitani, K. Nanobubbles on a hydrophobic surface in water observed by tapping-mode atomic force microscopy. Langmuir 2000, 16, 6377–6380. [Google Scholar] [CrossRef]
- Borkent, B.M.; Beer, S.D.; Mugele, F.; Lohse, D. On the shape of surface nanobubbles. Langmuir 2010, 26, 260–268. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Nanobubbles and the nanobubble bridging capillary force. Adv. Colloid Interface 2010, 154, 30–55. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z. Nanobubble generation and its applications in froth flotation (part IV): Mechanical cells and specially designed column flotation of coal. Min. Sci. Technol. 2010, 20, 641–671. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. Nanobubble column flotation of fine coal particles and associated fundamentals. Int. J. Miner. Process. 2013, 124, 109–116. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, C.; Lv, H.; Zhao, B.; Liu, K.; Ou, L. Nanobubbles heterogeneous nucleation induced by temperature rise and its influence on minerals flotation. Appl. Surf. Sci. 2020, 508, 145282. [Google Scholar] [CrossRef]
- Hernandez, C.; Nieves, L.; Leon, A.C.; Advincula, R.; Exner, A.A. Role of Surface Tension in Gas Nanobubble Stability Under Ultrasound. ACS Appl. Mater. Interface 2018, 10, 9949–9956. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, X.; Wang, W. Contact line pinning and the relationship between nanobubbles and substrates. J. Chem. Phys. 2014, 140, 054705. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, D.; Wang, D.; Maeda, N. Stability of Interfacial Nanobubbles. Langmuir 2013, 29, 1017–1023. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Wu, Z.; Tao, D. An experimental study on size distribution and zeta potential of bulk cavitation nanobubbles. Int. J. Min. Met. Mater. 2020, 27, 152–161. [Google Scholar] [CrossRef]
- Ma, F.; Tao, D.; Tao, Y. Effects of nanobubbles in column flotation of Chinese sub-bituminous coal. Int. J. Coal Prep. Util. 2022, 42, 1126–1142. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, P.; Tao, D. Surface nanobubble characterization and its enhancement mechanisms for fine-particle flotation: A review. Int. J. Min. Met. Mater. 2022, 29, 727–738. [Google Scholar] [CrossRef]
- Ma, F. Study on High-Efficiency Nanobubble Flotation of Graphite Ore and Its Mechanisms. Ph.D. Thesis, China University of Mining and Technology, Xuzhou, China, 2021. [Google Scholar]
- Li, Y.; Hu, Z.; Xia, W.; Shao, H.; Zheng, K.; Liang, L.; Peng, Y.; Xie, G. Application of compound reagent H511 in the flotation removal of unburned carbon from fly ash. Colloid Surface A 2020, 595, 124699. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; He, H.; Yang, Z.; Chen, L. Exploration on the mechanism of oily-bubble flotation of long-flame coal. Fuel 2018, 216, 427–435. [Google Scholar] [CrossRef]
- Oliveira, H.; Azevedo, A.; Rubio, J. Nanobubbles generation in a high-rate hydrodynamic cavitation tube. Miner. Eng. 2018, 116, 32–34. [Google Scholar] [CrossRef]
- Finch, J.A.; Nesset, J.E.; Acuña, C. Role of frother on bubble production and behaviour in flotation. Miner. Eng. 2008, 21, 949–957. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, H.; Ou, L.; Shi, Q. Aggregation of ultra-fine scheelite particles induced by hydrodynamic cavitation. Int. J. Miner. Process. 2016, 157, 236–240. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation using a multiphase pump, properties and features in flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Ahmadi, R.; Khodadadi, D.A.; Abdollahy, M.; Fan, M. Nano-microbubble flotation of fine and ultrafine chalcopyrite particles. Int. J. Min. Sci. Technol. 2014, 24, 559–566. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. High-Efficiency Nanobubble Coal Flotation. Int. J. Coal Prep. Util. 2013, 33, 242–256. [Google Scholar] [CrossRef]
- Ma, F.; Dai, S.; Tao, D.; Tao, Y.; Ma, Z. Study on the adsorption mechanisms of gold chloride on the surface of fine quartz. Energy Source Part A 2019, 43, 1151–1161. [Google Scholar] [CrossRef]
- Chen, D. Study on Modification of Fe-Mn Binary Oxide Foradsorption of Pb2+ and Cu2+ in Aqueous Solution Andits Mechanism. Ph.D. Thesis, Guangdong University of Technology, Guanghzou, China, 2020; pp. 23–25. Available online: https://d.wanfangdata.com.cn/thesis/D02080620 (accessed on 31 August 2022). (In Chinese).
- Zhang, J. Study on the Pollution Status and Adsoiption Behavior of Microplastics and Heavy Metals in the Soil of Coal Mining Subsidence Area. Ph.D. Thesis, Anhui University of Science and Technology, Chuzhou, China, 2020; pp. 33–35. Available online: https://d.wanfangdata.com.cn/thesis/Y3748507 (accessed on 31 August 2022). (In Chinese).
- Stöckelhuber, K.W.; Radoev, B.; Wenger, A.; Schulze, H.J. Rupture of Wetting Films Caused by Nanobubbles. Langmuir 2004, 20, 164–168. [Google Scholar] [CrossRef]
- Tong, X.; Bai, Z.; Wu, J. Study of the Microstructure and Morphology Features of Cryptocrystalline Graphite. China Non-Met. Min. Ind. 2019, 4, 10–12+37. Available online: https://www.doc88.com/p18247387244244.html?r=1 (accessed on 31 August 2022). (In Chinese).
- Qiu, Y.; Yu, Y.; Zhang, L.; Peng, W.; Qian, Y. Dispersion and agglomeration mechanism of flaky graphite particles in aqueous solution. J. Disper. Sci. Technol. 2016, 38, 796–800. [Google Scholar] [CrossRef]
- Yoon, R.H.; Yordan, J.L. Zeta-potential measurements on microbubbles generated using various surfactants. J. Colloid Interface Sci. 1986, 113, 430–438. [Google Scholar] [CrossRef]
- He, X.; Nie, Y.; Lu, X.; Ji, J. Intensification of Liquid-liquid Heterogeneous Micromixing for Preparing Biodiesel by Hydrodynamic Cavitation. Adv. Fine Petrochem. 2011, 12, 47–51. Available online: http://www.doc88.com/p-9179578498179.html (accessed on 31 August 2022). (In Chinese).
- Zheng, K.; Xia, W.; Zhang, W. Reverse flotation of non-coking coal fines using non-ionic surfactant triton X-100 as depressant. Colloid Surface A 2021, 611, 125794. [Google Scholar] [CrossRef]
- Calgaroto, S.; Azevedo, A.; Rubio, J. Flotation of quartz particles assisted by nanobubbles. Int. J. Miner. Process. 2015, 137, 64–70. [Google Scholar] [CrossRef]



| Mineral | Graphite | Quartz | Muscovite | Pyrite | Garnet | Calcite | K-Feldspar |
|---|---|---|---|---|---|---|---|
| Content (%) | 84.06 | 3.47 | 4.78 | 2.69 | 1.12 | 1.07 | 0.98 |
| Model | Linear Fitting Equation | Parameter | Parameter Values |
|---|---|---|---|
| Quasi-first-order dynamics | y = −0.6996x + 1.3511 | qe | 2.1636 |
| qe1 | 3.8617 | ||
| k1 | 0.6996 | ||
| R2 | 0.9768 | ||
| Quasi-second-order dynamics | y = 0.4004x + 0.5452 | qe | 2.1636 |
| qe1 | 2.4975 | ||
| k2 | 0.2941 | ||
| R2 | 0.9962 | ||
| Intra-particle diffusion | y = 0.4141x + 0.9808 | k | 0.4141 |
| C | 0.9808 | ||
| R2 | 0.9151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Tao, D. A Study of Mechanisms of Nanobubble-Enhanced Flotation of Graphite. Nanomaterials 2022, 12, 3361. https://doi.org/10.3390/nano12193361
Ma F, Tao D. A Study of Mechanisms of Nanobubble-Enhanced Flotation of Graphite. Nanomaterials. 2022; 12(19):3361. https://doi.org/10.3390/nano12193361
Chicago/Turabian StyleMa, Fangyuan, and Dongping Tao. 2022. "A Study of Mechanisms of Nanobubble-Enhanced Flotation of Graphite" Nanomaterials 12, no. 19: 3361. https://doi.org/10.3390/nano12193361






