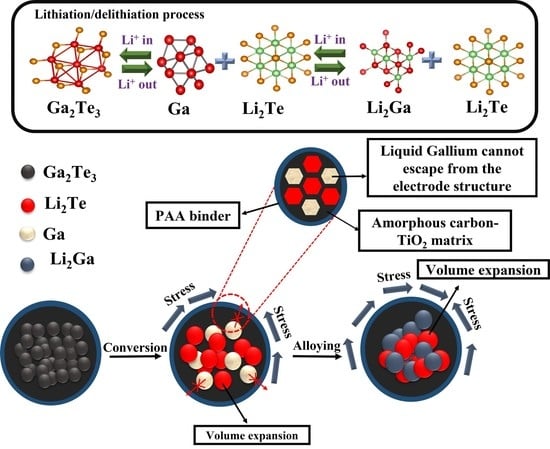
Gallium-Telluride-Based Composite as Promising Lithium Storage Material
Abstract
:1. Introduction
2. Experimental Materials and Methods
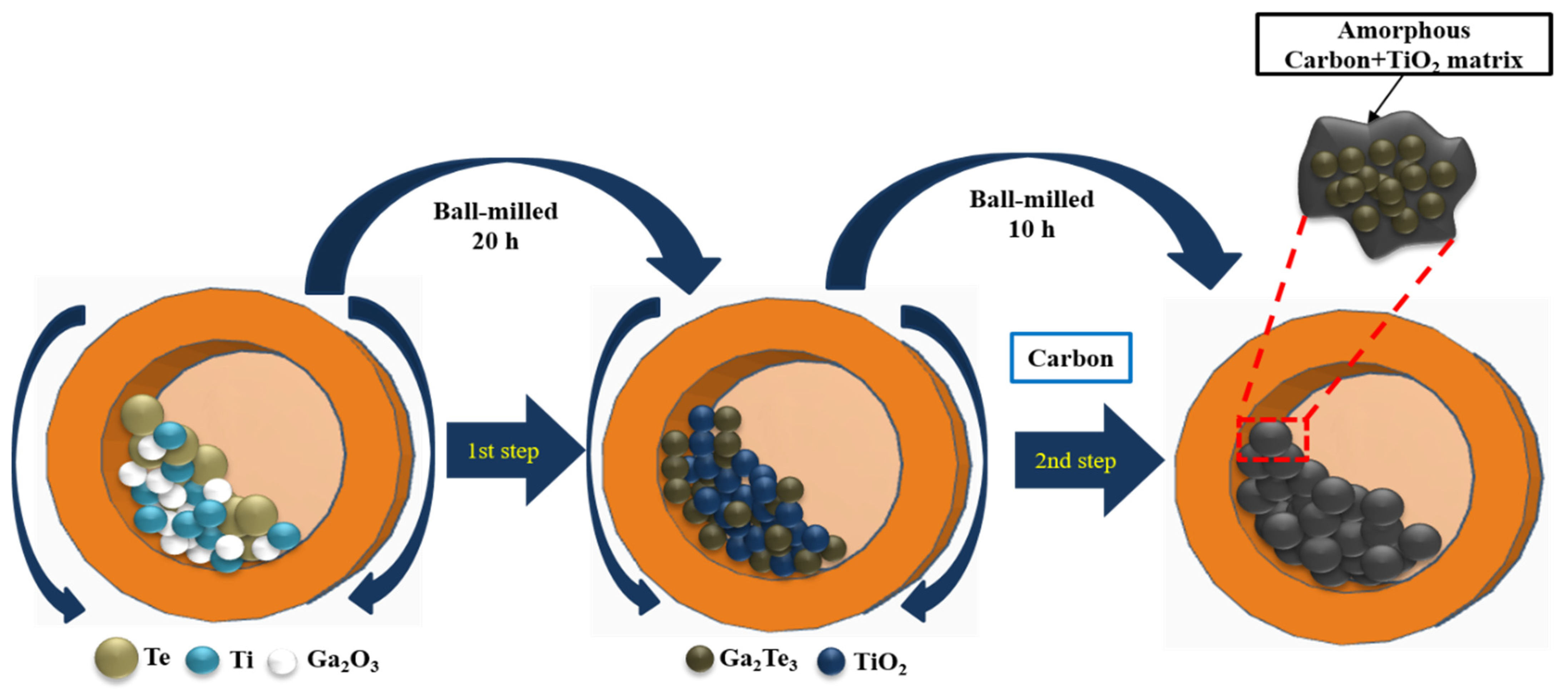
2.1. Material Synthesis
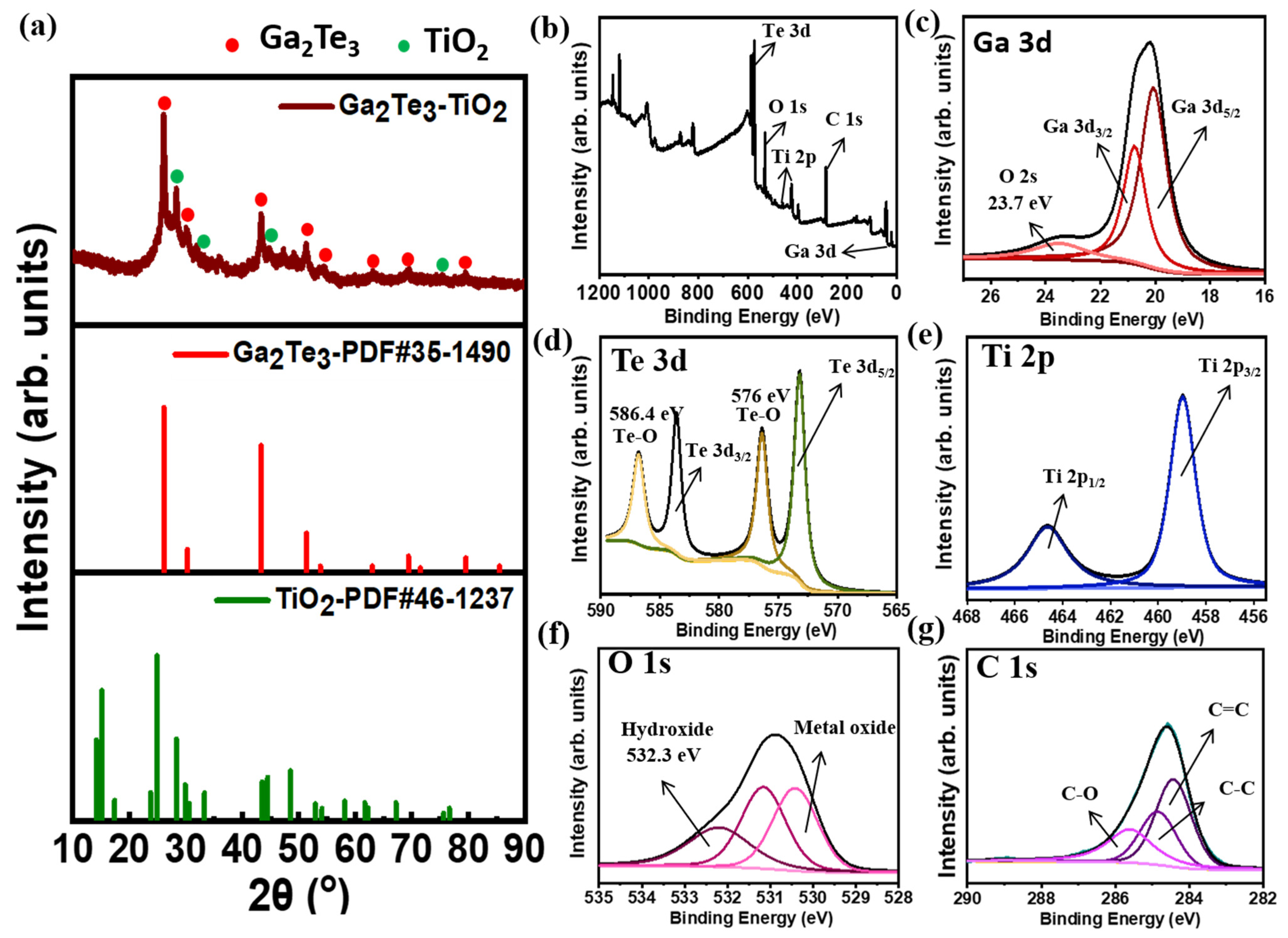
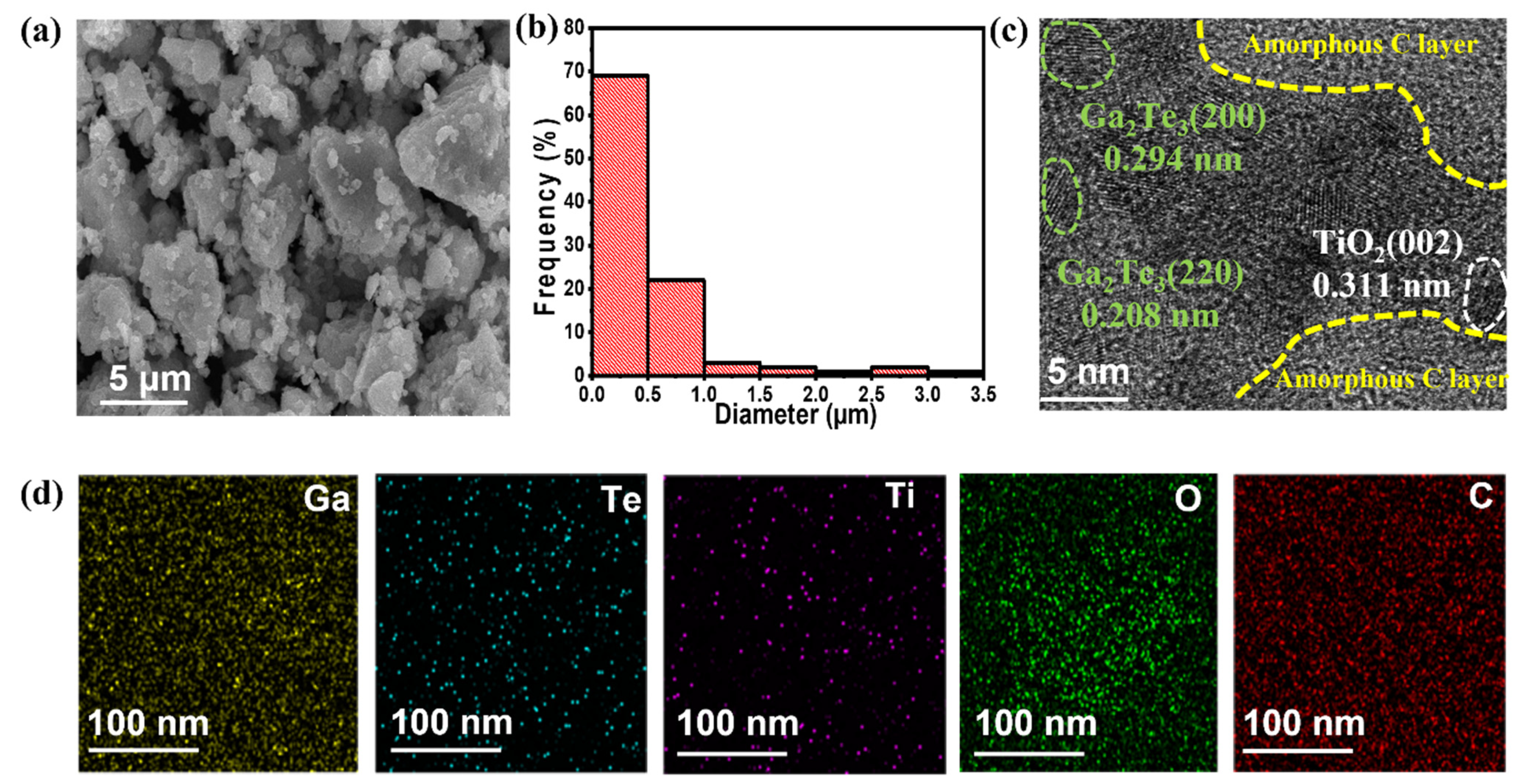
2.2. Material Characterization
2.3. Electrochemical Measurements
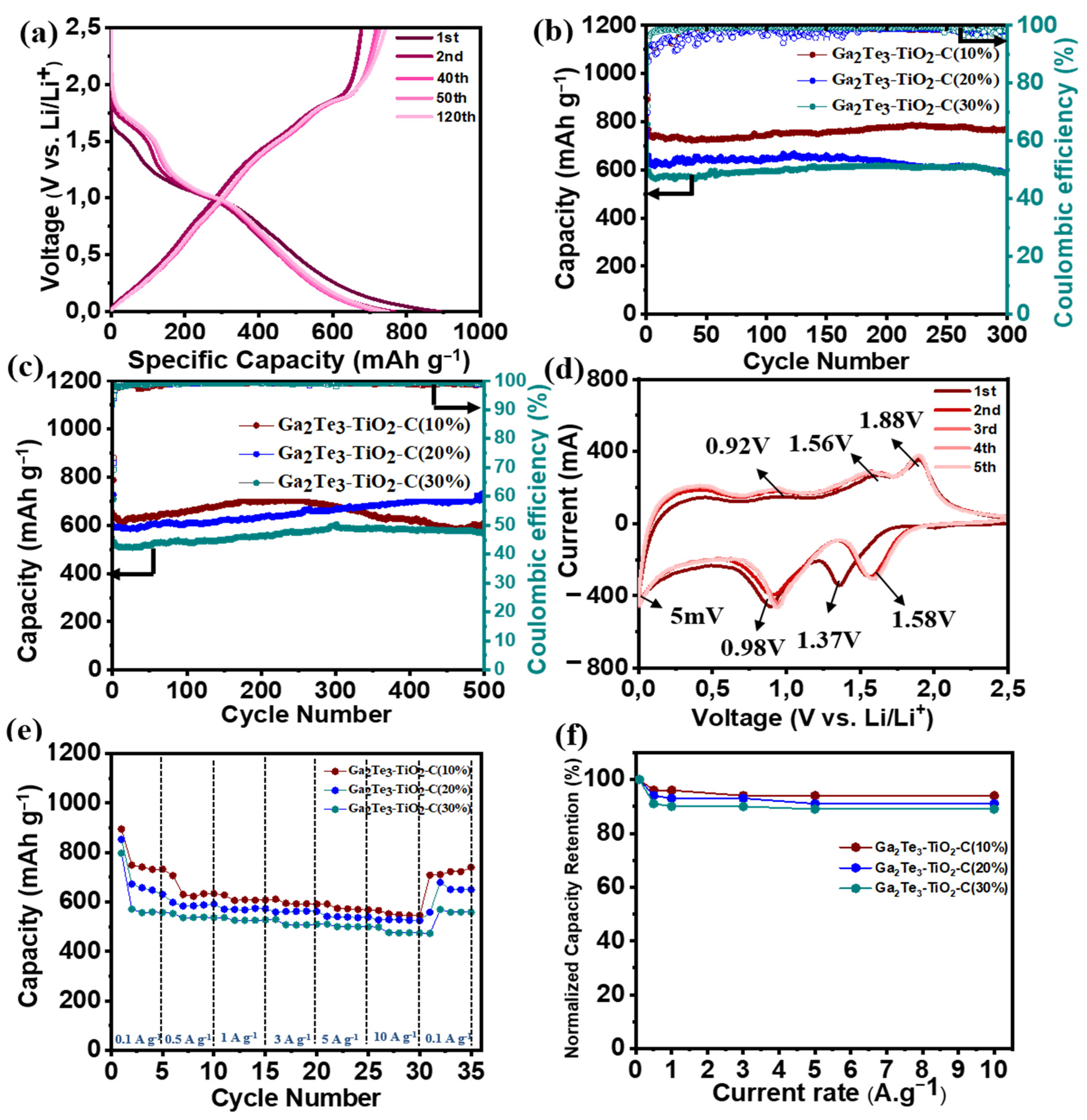
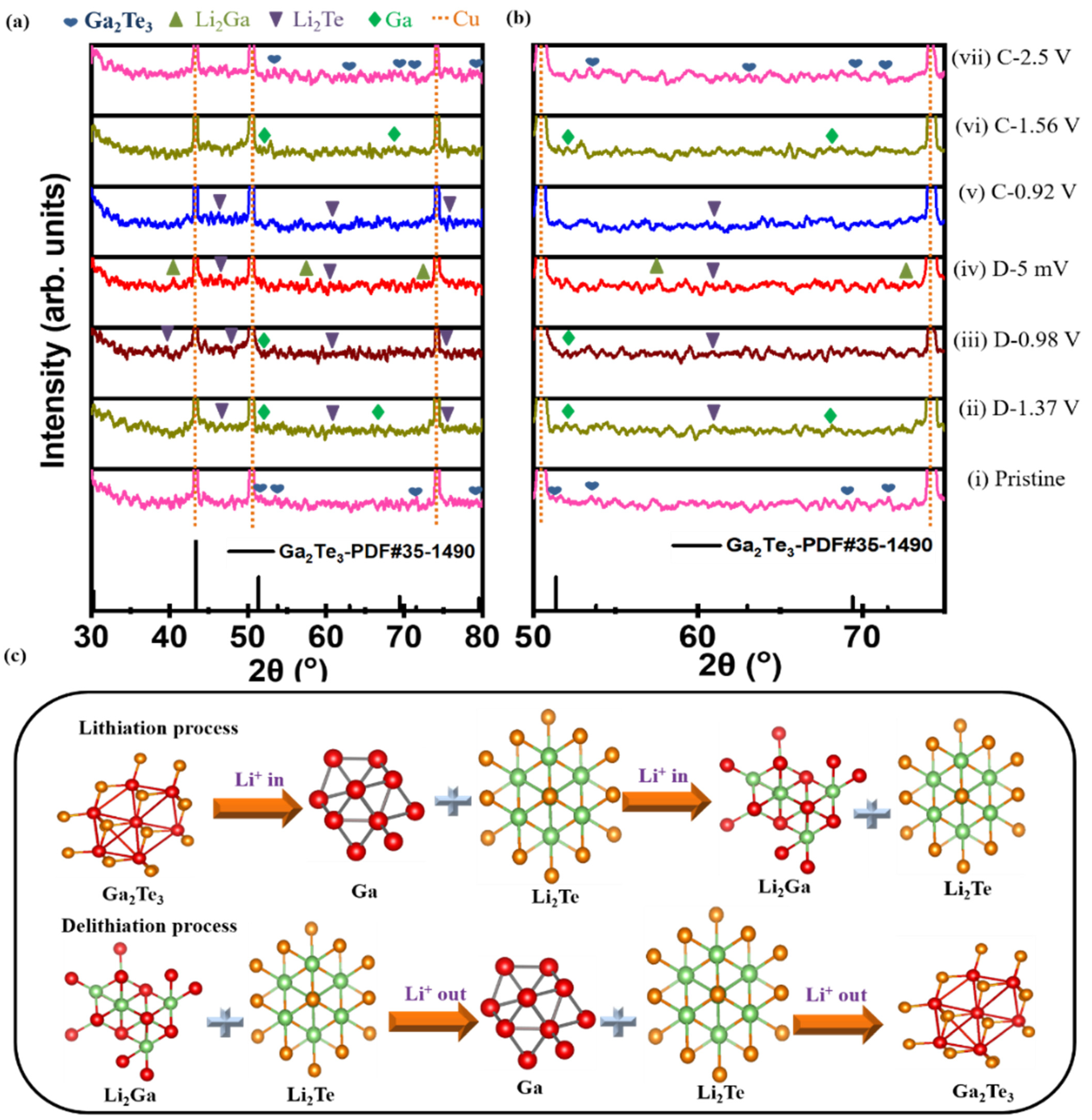
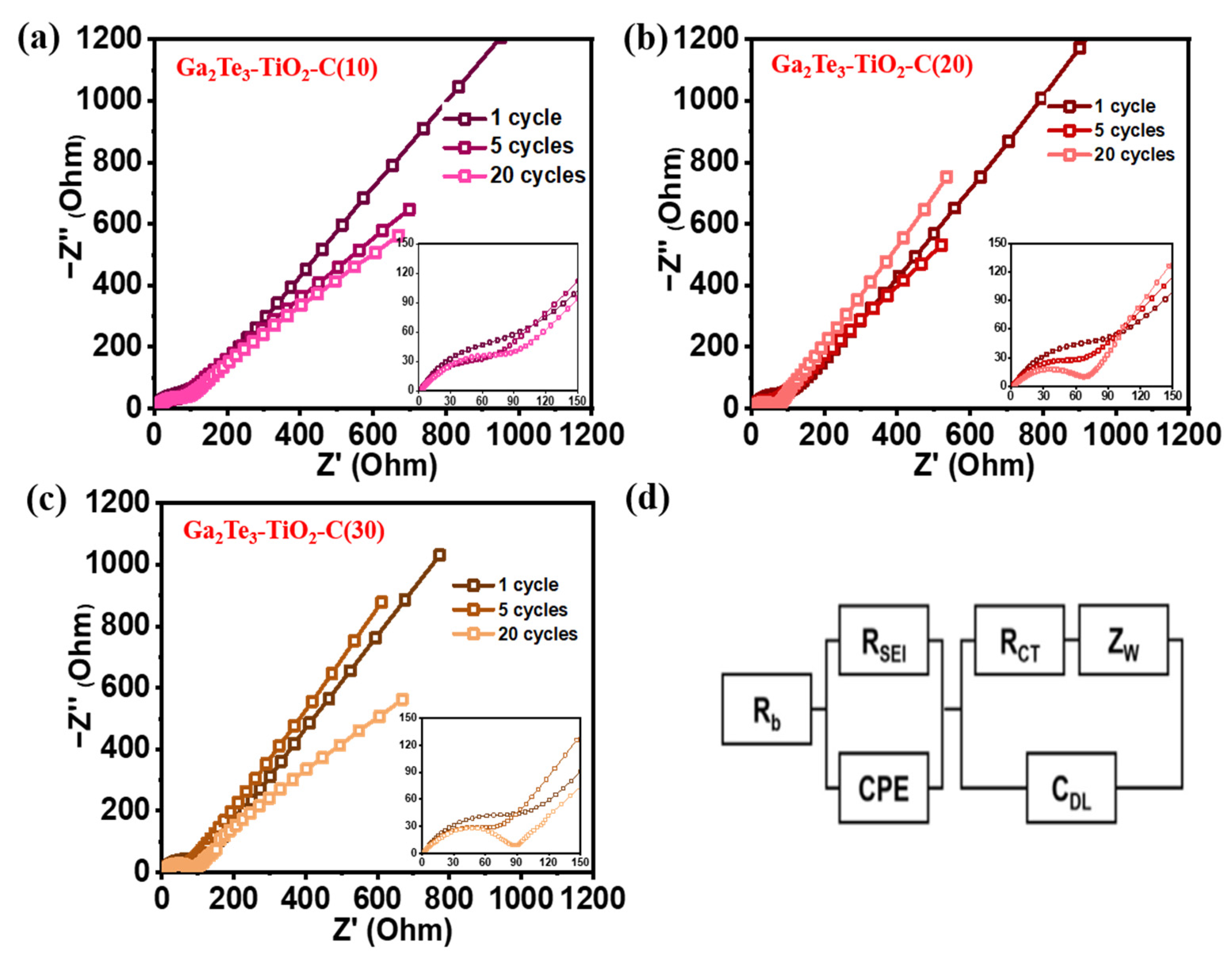
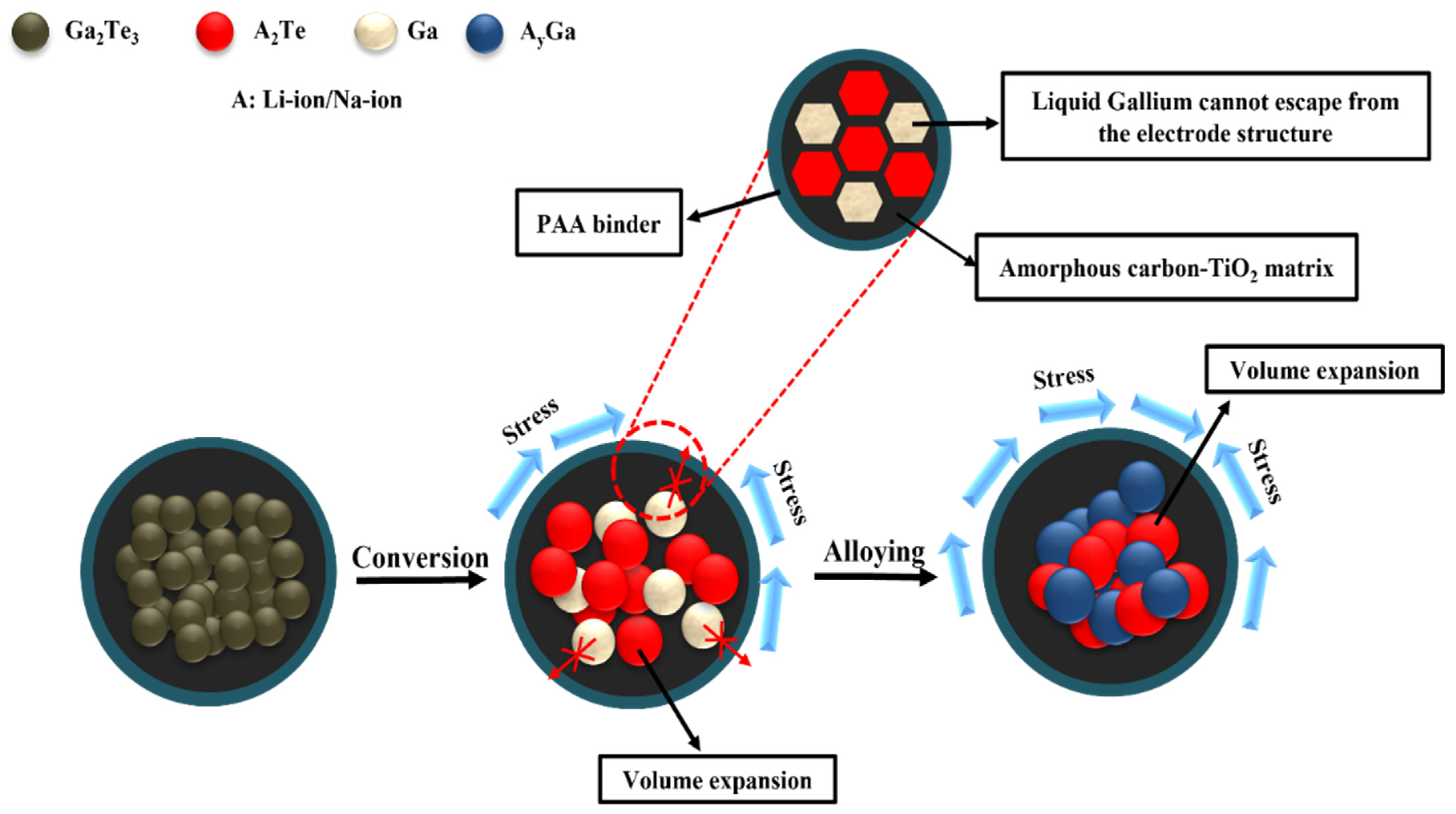
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Huggins, R.A. Advanced Batteries: Materials Science Aspects; Springer Science & Business Media: Berlin, Germany, 2009; pp. 1–474. [Google Scholar]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Weng, W.; Li, J.; Du, Y.; Ge, X.; Zhou, X.; Bao, J. Template-free synthesis of metal oxide hollow micro-/nanospheres via Ostwald ripening for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 10168–10175. [Google Scholar] [CrossRef]
- Zou, R.; Xu, M.; He, S.-A.; Han, X.; Lin, R.; Cui, Z.; He, G.; Brett, D.J.L.; Guo, Z.X.; Hu, J.; et al. Cobalt nickel nitride coated by a thin carbon layer anchoring on nitrogen-doped carbon nanotube anodes for high-performance lithium-ion batteries. J. Mater. Chem. A 2018, 6, 19853–19862. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Li, J.; Luo, H.; Mei, L.; Wang, T.; Zhang, Y. A germanium and zinc chalcogenide as an anode for a high-capacity and long cycle life lithium battery. RSC Adv. 2019, 9, 35045–35049. [Google Scholar] [CrossRef] [PubMed]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef]
- Mou, H.; Xiao, W.; Miao, C.; Li, R.; Yu, L. Tin and Tin Compound Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review. Front. Chem. 2020, 8, 141. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Ag Nanoparticle-Decorated MoS2 Nanosheets for Enhancing Electrochemical Performance in Lithium Storage. Nanomaterials 2021, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Kim, I.T. Self-Assembled Few-Layered MoS2 on SnO2 Anode for Enhancing Lithium-Ion Storage. Nanomaterials 2020, 10, 2558. [Google Scholar] [CrossRef] [PubMed]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.-K. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials 2020, 10, 1336. [Google Scholar] [CrossRef]
- Kim, W.S.; Vo, T.N.; Kim, I.T. GeTe-TiC-C Composite Anodes for Li-Ion Storage. Materials 2020, 13, 4222. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Kim, D.S.; Mun, Y.S.; Lee, H.J.; Ahn, S.-K.; Kim, I.T. Fast charging sodium-ion batteries based on Te-P-C composites and insights to low-frequency limits of four common equivalent impedance circuits. Chem. Eng. J. 2020, 398, 125703. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; Kim, I.T.; Hur, J. The Effects of the Binder and Buffering Matrix on InSb-Based Anodes for High-Performance Rechargeable Li-Ion Batteries. Nanomaterials 2021, 11, 3420. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Phung, V.D.; Kidanu, W.G.; Ahn, Y.N.; Nguyen, T.L.; Kim, I.T. Carbon-free Cu/SbxOy/Sb nanocomposites with yolk-shell and hollow structures as high-performance anodes for lithium-ion storage. J. Alloys Compd. 2021, 878, 160447. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Kim, I.T. In Situ Growth of W2C/WS2 with Carbon-Nanotube Networks for Lithium-Ion Storage. Nanomaterials 2022, 12, 1003. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Boron Oxide Enhancing Stability of MoS2 Anode Materials for Lithium-Ion Batteries. Materials 2022, 15, 2034. [Google Scholar] [CrossRef]
- Phan Nguyen, T.; Giang, T.T.; Kim, I.T. Restructuring NiO to LiNiO2: Ultrastable and reversible anodes for lithium-ion batteries. Chem. Eng. J. 2022, 437, 135292. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.-G. A PEO-assisted electrospun silicon–graphene composite as an anode material for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 9019–9023. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Seo, J.-U.; Park, C.-M. Nanostructured SnSb/MOx (M = Al or Mg)/C composites: Hybrid mechanochemical synthesis and excellent Li storage performances. J. Mater. Chem. A 2013, 1, 15316–15322. [Google Scholar] [CrossRef]
- Li, H.; Yamaguchi, T.; Matsumoto, S.; Hoshikawa, H.; Kumagai, T.; Okamoto, N.L.; Ichitsubo, T. Circumventing huge volume strain in alloy anodes of lithium batteries. Nat. Commun. 2020, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Moskalyk, R. Gallium: The Backbone of the Electronics Industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Lee, K.T.; Jung, Y.S.; Kim, T.; Kim, C.H.; Kim, J.H.; Kwon, J.Y.; Oh, S.M. Liquid Gallium Electrode Confined in Porous Carbon Matrix as Anode for Lithium Secondary Batteries. Electrochem. Solid-State Lett. 2008, 11, A21. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, X.; Tan, H.; Yang, D.; Feng, Y.; Rui, X.; Yu, Y. Gallium-based anodes for alkali metal ion batteries. J. Energy Chem. 2021, 55, 557–571. [Google Scholar] [CrossRef]
- Deshpande, R.D.; Li, J.; Cheng, Y.-T.; Verbrugge, M.W. Liquid Metal Alloys as Self-Healing Negative Electrodes for Lithium Ion Batteries. J. Electrochem. Soc. 2011, 158, A845. [Google Scholar] [CrossRef]
- Yarema, M.; Wörle, M.; Rossell, M.D.; Erni, R.; Caputo, R.; Protesescu, L.; Kravchyk, K.V.; Dirin, D.N.; Lienau, K.; von Rohr, F.; et al. Monodisperse Colloidal Gallium Nanoparticles: Synthesis, Low Temperature Crystallization, Surface Plasmon Resonance and Li-Ion Storage. J. Am. Chem. Soc. 2014, 136, 12422–12430. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Hong, L.; Yang, H.; Fan, F.; Liu, Y.; Li, H.; Li, J.; Huang, J.Y.; Chen, L.-Q.; Zhu, T.; et al. Nanovoid Formation and Annihilation in Gallium Nanodroplets under Lithiation–Delithiation Cycling. Nano Lett. 2013, 13, 5212–5217. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Ding, Y.; Goodenough, J.B.; Yu, G. Room-temperature liquid metal and alloy systems for energy storage applications. Energy Environ. Sci. 2019, 12, 2605–2619. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Y.; Huang, X.; Huang, L.; Cao, M.; Song, G.; Guo, X.; Sui, X.; Ren, R.; Chen, J. Self-healing liquid metal nanoparticles encapsulated in hollow carbon fibers as a free-standing anode for lithium-ion batteries. Nano Energy 2019, 62, 883–889. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.; Guo, Y.; Wan, L.-J. Effect of cations in ionic liquids on the electrochemical performance of lithium-sulfur batteries. Sci. China Chem. 2014, 57, 1564–1569. [Google Scholar] [CrossRef]
- Xin, S.; Gu, L.; Zhao, N.-H.; Yin, Y.-X.; Zhou, L.-J.; Guo, Y.-G.; Wan, L.-J. Smaller Sulfur Molecules Promise Better Lithium–Sulfur Batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef]
- Yang, C.-P.; Xin, S.; Yin, Y.-X.; Ye, H.; Zhang, J.; Guo, Y.-G. An Advanced Selenium–Carbon Cathode for Rechargeable Lithium–Selenium Batteries. Angew. Chem. Int. Ed. 2013, 52, 8363–8367. [Google Scholar] [CrossRef]
- Xin, S.; Yin, Y.-X.; Guo, Y.-G.; Wan, L.-J. A High-Energy Room-Temperature Sodium-Sulfur Battery. Adv. Mater. 2014, 26, 1261–1265. [Google Scholar] [CrossRef]
- Zeng, L.; Wei, X.; Wang, J.; Jiang, Y.; Li, W.; Yu, Y. Flexible one-dimensional carbon–selenium composite nanofibers with superior electrochemical performance for Li–Se/Na–Se batteries. J. Power Sources 2015, 281, 461–469. [Google Scholar] [CrossRef]
- Yang, C.-P.; Yin, Y.-X.; Guo, Y.-G. Elemental Selenium for Electrochemical Energy Storage. J. Phys. Chem. Lett. 2015, 6, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, H.; Zhang, J.; Liu, Z.; Yang, Y. A study of novel anode material CoS2 for lithium ion battery. J. Power Sources 2005, 146, 264–269. [Google Scholar] [CrossRef]
- Yin, J.; Cao, H.; Zhou, Z.; Zhang, J.; Qu, M. SnS2@reduced graphene oxide nanocomposites as anode materials with high capacity for rechargeable lithium ion batteries. J. Mater. Chem. 2012, 22, 23963–23970. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Li, J. SnSe/carbon nanocomposite synthesized by high energy ball milling as an anode material for sodium-ion and lithium-ion batteries. Electrochim. Acta 2015, 176, 1296–1301. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Li, J. Robust nanocube framework CoS2-based composites as high-performance anodes for Li- and Na-ion batteries. Compos. Part B Eng. 2022, 231, 109592. [Google Scholar]
- Nam, K.-H.; Park, C.-M. 2D layered Sb2Se3-based amorphous composite for high-performance Li- and Na-ion battery anodes. J. Power Sources 2019, 433, 126639. [Google Scholar] [CrossRef]
- Park, A.-R.; Jeon, K.-J.; Park, C.-M. Electrochemical mechanism of Li insertion/extraction in ZnS and ZnS/C anodes for Li-ion batteries. Electrochim. Acta 2018, 265, 107–114. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.-X.; You, Y.; Yan, Y.; Guo, Y.-G. A High-Capacity Tellurium@Carbon Anode Material for Lithium-Ion Batteries. Energy Technol. 2014, 2, 757–762. [Google Scholar] [CrossRef]
- Nagulapati, V.M.; Kim, D.S.; Oh, J.; Lee, J.H.; Hur, J.; Kim, I.T.; Lee, S.G. Enhancing the Electrochemical Performance of SbTe Bimetallic Anodes for High-Performance Sodium-Ion Batteries: Roles of the Binder and Carbon Support Matrix. Nanomaterials 2019, 9, 1134. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.-X.; Guo, Y.-G. High-Capacity Te Anode Confined in Microporous Carbon for Long-Life Na-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 27838–27844. [Google Scholar] [CrossRef]
- Seo, J.-U.; Seong, G.-K.; Park, C.-M. Te/C nanocomposites for Li-Te Secondary Batteries. Sci. Rep. 2015, 5, 7969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, M.-Q.; Chen, Y.; Hu, L.; Liu, T.; Bao, S.; Xu, M. Muscle-like electrode design for Li-Te batteries. Energy Storage Mater. 2018, 10, 10–15. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Lv, W.; Wen, K.; Wang, Z.; Zhang, W.; Li, Y.; Qin, W.; He, W. Three-Dimensional Hierarchical Reduced Graphene Oxide/Tellurium Nanowires: A High-Performance Freestanding Cathode for Li–Te Batteries. ACS Nano 2016, 10, 8837–8842. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Chen, S.-F.; Geng, D.-S.; Chien, S.-W.; An, T.; Hor, T.S.A.; Liu, Z.-L.; Yu, S.-H.; Zong, Y. Tellurium@Ordered Macroporous Carbon Composite and Free-Standing Tellurium Nanowire Mat as Cathode Materials for Rechargeable Lithium–Tellurium Batteries. Adv. Energy Mater. 2015, 5, 1401999. [Google Scholar] [CrossRef]
- Park, A.-R.; Park, C.-M. Cubic Crystal-Structured SnTe for Superior Li- and Na-Ion Battery Anodes. ACS Nano 2017, 11, 6074–6084. [Google Scholar] [CrossRef]
- Nam, K.-H.; Park, C.-M. Layered Sb2Te3 and its nanocomposite: A new and outstanding electrode material for superior rechargeable Li-ion batteries. J. Mater. Chem. A 2016, 4, 8562–8565. [Google Scholar] [CrossRef]
- Sun, D.; Liu, S.; Zhang, G.; Zhou, J. NiTe2/N-doped graphitic carbon nanosheets derived from Ni-hexamine coordination frameworks for Na-ion storage. Chem. Eng. J. 2019, 359, 1659–1667. [Google Scholar] [CrossRef]
- Nam, K.H.; Sung, G.K.; Choi, J.H.; Youn, J.S.; Jeon, K.J.; Park, C.M. New high-energy-density GeTe-based anodes for Li-ion batteries. J. Mater. Chem. A 2019, 7, 3278–3288. [Google Scholar] [CrossRef]
- Seo, J.-U.; Park, C.-M. ZnTe and ZnTe/C nanocomposite: A new electrode material for high-performance rechargeable Li-ion batteries. J. Mater. Chem. A 2014, 2, 20075–20082. [Google Scholar] [CrossRef]
- Ganesan, V.; Nam, K.-H.; Park, C.-M. Robust Polyhedral CoTe2–C Nanocomposites as High-Performance Li- and Na-Ion Battery Anodes. ACS Appl. Energy Mater. 2020, 3, 4877–4887. [Google Scholar] [CrossRef]
- Abed Al- Abbas, S.S.; Muhsin, M.K.; Jappor, H.R. Tunable optical and electronic properties of gallium telluride monolayer for photovoltaic absorbers and ultraviolet detectors. Chem. Phys. Lett. 2018, 713, 46–51. [Google Scholar] [CrossRef]
- Bose, D.N.; De Purkayastha, S. Dielectric and photoconducting properties of Ga2Te3 and In2Te3 crystals. Mater. Res. Bull. 1981, 16, 635–642. [Google Scholar] [CrossRef]
- Aydoğan, Ş.; Karacalı, T.; Yoğurtçu, Y.K. Investigation of the switching phenomena in Ga2Te3 single crystals. J. Cryst. Growth 2005, 279, 110–113. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Abdul-Jabbar, N.M.; Wirth, B.D. Theoretical study of Ga2Se3,Ga2Te3 and Ga2(Se1−xTex)3: Band-gap engineering. Acta Mater. 2014, 71, 349–369. [Google Scholar] [CrossRef]
- Antonopoulos, J.G.; Karakostas, T.; Bleris, G.L.; Economou, N.A. On the phase diagram of the Ga-Te system in the composition range 55 at % Te. J. Mater. Sci. 1981, 16, 733–738. [Google Scholar] [CrossRef]
- David, R. (Ed.) CRC Handbook of Chemistry and Physics, 87th ed.; Lide (National Institute of Standards and Technology); CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2006; Volume 2608, ISBN 0-8493-0487-3. [Google Scholar]
- Gnanamuthu, R.M.; Lee, C.W. Electrochemical properties of Super P carbon black as an anode active material for lithium-ion batteries. Mater. Chem. Phys. 2011, 130, 831–834. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Ma, J.; Kim, N.; Lee, H.-W.; Cui, Y.; Cho, J. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat. Energy 2016, 1, 16113. [Google Scholar] [CrossRef]
- Ng, S.; Wang, J.; Guo, Z.; Chen, J.; Wang, G.; Liu, H. Single wall carbon nanotube paper as anode for lithium-ion battery. Electrochim. Acta 2005, 51, 23–28. [Google Scholar] [CrossRef]
- Qin, S.; Liu, D.; Lei, W.; Chen, Y. Synthesis of an indium oxide nanoparticle embedded graphene three-dimensional architecture for enhanced lithium-ion storage. J. Mater. Chem. A 2015, 3, 18238–18243. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676–681. [Google Scholar] [CrossRef]
- Ni, S.; Li, T.; Lv, X.; Yang, X.; Zhang, L. Designed constitution of NiO/Ni nanostructured electrode for high performance lithium ion battery. Electrochim. Acta 2013, 91, 267–274. [Google Scholar] [CrossRef]
- Taberna, P.-L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.-M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Huang, G.; Liang, F.; Yin, D.; Wang, L. A Core—Shell Fe/Fe2O3 Nanowire as a High-Performance Anode Material for Lithium-Ion Batteries. Chem. A Eur. J. 2016, 22, 12081–12087. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Liu, F.; Zhu, J.; Hou, Y. Hybrid of Co3Sn2@Co Nanoparticles and Nitrogen-Doped Graphene as a Lithium Ion Battery Anode. ACS Nano 2013, 7, 10307–10318. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Jung, C.-H.; Kim, W.-S.; Hong, S.-H. V4P7@C nanocomposite as a high performance anode material for lithium-ion batteries. J. Power Sources 2018, 400, 204–211. [Google Scholar] [CrossRef]
- Lee, H.J.; Shim, H.W.; Kim, J.C.; Kim, D.W. Mo-MoO3-graphene nanocomposites as anode materials for lithium-ion batteries: Scalable, facile preparation and characterization. Electrochim. Acta 2017, 251, 81–90. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.; Kim, S.; Choi, J. SiO2/TiO2 Composite Film for High Capacity and Excellent Cycling Stability in Lithium-Ion Battery Anodes. Adv. Funct. Mater. 2017, 27, 1703538. [Google Scholar] [CrossRef]
- Allcorn, E.; Manthiram, A. FeSb2–Al2O3–C Nanocomposite Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 10886–10891. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huo, K.; Hu, L.; Liu, N.; Cha, J.J.; McDowell, M.T.; Chu, P.K.; Cui, Y. Highly Conductive, Mechanically Robust, and Electrochemically Inactive TiC/C Nanofiber Scaffold for High-Performance Silicon Anode Batteries. ACS Nano 2011, 5, 8346–8351. [Google Scholar] [CrossRef]
- Xu, B.; Shen, H.; Ge, J.; Tang, Q. Improved cycling performance of SiOx/MgO/Mg2SiO4/C composite anode materials for lithium-ion battery. Appl. Surf. Sci. 2021, 546, 148814. [Google Scholar] [CrossRef]
- Xiao, Z.; Lei, C.; Yu, C.; Chen, X.; Zhu, Z.; Jiang, H.; Wei, F. Si@Si3N4@C composite with egg-like structure as high-performance anode material for lithium ion batteries. Energy Storage Mater. 2020, 24, 565–573. [Google Scholar] [CrossRef]
- So, S.; Ko, J.; Ahn, Y.N.; Kim, I.T.; Hur, J. Unraveling improved electrochemical kinetics of In2Te3-based anodes embedded in hybrid matrix for Li-ion batteries. Chem. Eng. J. 2022, 429, 132395. [Google Scholar] [CrossRef]
- Huy, V.P.H.; So, S.; Kim, I.T.; Hur, J. Self-healing gallium phosphide embedded in a hybrid matrix for high-performance Li-ion batteries. Energy Storage Mater. 2021, 34, 669–681. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Zabinski, J.S. Load-adaptive crystalline–amorphous nanocomposites. J. Mater. Sci. 1998, 33, 319–327. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Shi, Q.; Xin, S.; Wu, J.; Zhang, C.; Qiu, L.; Zhang, C. Facile Synthesis of Carbon-Coated Porous Sb2Te3 Nanoplates with High Alkali Metal Ion Storage. ACS Appl. Mater. Interfaces 2019, 11, 29934–29940. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Ju, H.S.; Lee, J.-K.; Kang, Y.C. Carbon/two-dimensional MoTe2 core/shell-structured microspheres as an anode material for Na-ion batteries. Nanoscale 2017, 9, 1942–1950. [Google Scholar] [CrossRef]
- Bondino, F.; Duman, S.; Nappini, S.; D'Olimpio, G.; Ghica, C.; Menteş, T.O.; Mazzola, F.; Istrate, M.C.; Jugovac, M.; Vorokhta, M.; et al. Improving the Efficiency of Gallium Telluride for Photocatalysis, Electrocatalysis, and Chemical Sensing through Defects Engineering and Interfacing with its Native Oxide. Adv. Funct. Mater. 2022, 2205923. [Google Scholar] [CrossRef]
- Huang, C.; Mu, W.; Zhou, H.; Zhu, Y.; Xu, X.; Jia, Z.; Zheng, L.; Tao, X. Effect of OH− on chemical mechanical polishing of β-Ga2O3 (100) substrate using an alkaline slurry. RSC Adv. 2018, 8, 6544–6550. [Google Scholar] [CrossRef]
- Kang, W.; Tang, Y.; Li, W.; Yang, X.; Xue, H.; Yang, Q.; Lee, C.S. High interfacial storage capability of porous NiMn2O4/C hierarchical tremella-like nanostructures as the lithium ion battery anode. Nanoscale 2015, 7, 225–231. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, H.; Lv, P.; Zhang, Z.; Wang, J.; Xia, Q. Electrochemical properties of iron oxides/carbon nanotubes as anode material for lithium ion batteries. J. Power Sources 2015, 274, 1091–1099. [Google Scholar] [CrossRef]
- Zhu, X.J.; Guo, Z.P.; Zhang, P.; Du, G.D.; Zeng, R.; Chen, Z.X.; Li, S.; Liu, H.K. Highly porous reticular tin–cobalt oxide composite thin film anodes for lithium ion batteries. J. Mater. Chem. 2009, 19, 8360–8365. [Google Scholar] [CrossRef]
- Jung, J.-W.; Kim, C.; Cheong, J.Y.; Kim, I.-D. Gallium Nitride Nanoparticles Embedded in a Carbon Nanofiber Anode for Ultralong-Cycle-Life Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 44263–44269. [Google Scholar] [CrossRef]
- Ni, S.; Chen, Q.; Liu, J.; Yang, S.; Li, T.; Yang, X.; Zhao, J. New insights into the Li-storage mechanism in α-Ga2O3 anode and the optimized electrode design. J. Power Sources 2019, 433, 126681. [Google Scholar] [CrossRef]
- Wang, K.; Ye, W.; Yin, W.; Chai, W.; Rui, Y.; Tang, B. Several carbon-coated Ga2O3 anodes: Efficient coating of reduced graphene oxide enhanced the electrochemical performance of lithium ion batteries. Dalton Trans. 2021, 50, 3660–3670. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ye, W.; Yin, W.; Chai, W.; Tang, B.; Rui, Y. One-step synthesis of MOF-derived Ga/Ga2O3@C dodecahedra as an anode material for high-performance lithium-ion batteries. Dalton Trans. 2019, 48, 12386–12390. [Google Scholar] [CrossRef]
- Guo, X.; Ding, Y.; Xue, L.; Zhang, L.; Zhang, C.; Goodenough, J.B.; Yu, G. A Self-Healing Room-Temperature Liquid-Metal Anode for Alkali-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1804649. [Google Scholar] [CrossRef]
- Sun, C.; Yang, M.; Wang, T.; Shao, Y.; Wu, Y.; Hao, X. A self-healing CuGa2 anode for high-performance Li ion batteries. J. Power Sources 2019, 437, 226889. [Google Scholar]
- Sun, C.; Yang, M.; Wang, T.; Shao, Y.; Wu, Y.; Hao, X. Graphene-Oxide-Assisted Synthesis of GaN Nanosheets as a New Anode Material for Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 26631–26636. [Google Scholar] [CrossRef]
- Tang, X.; Huang, X.; Huang, Y.; Gou, Y.; Pastore, J.; Yang, Y.; Xiong, Y.; Qian, J.; Brock, J.D.; Lu, J.; et al. High-Performance Ga2O3 Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5519–5526. [Google Scholar] [CrossRef]
- Gómez-Cámer, J.L.; Novák, P. Polyacrylate bound TiSb2 electrodes for Li-ion batteries. J. Power Sources 2015, 273, 174–179. [Google Scholar] [CrossRef]
- Senoh, H.; Kageyama, H.; Takeuchi, T.; Nakanishi, K.; Ohta, T.; Sakaebe, H.; Yao, M.; Sakai, T.; Yasuda, K. Gallium (III) sulfide as an active material in lithium secondary batteries. Lancet 2011, 196, 5631–5636. [Google Scholar] [CrossRef]
- Meng, X.; He, K.; Su, D.; Zhang, X.; Sun, C.; Ren, Y.; Wang, H.-H.; Weng, W.; Trahey, L.; Canlas, C.P.; et al. Gallium Sulfide–Single-Walled Carbon Nanotube Composites: High-Performance Anodes for Lithium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 5435–5442. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Jung, D.-W.; Oh, E.-S. Lithium storage characteristics of a new promising gallium selenide anodic material. J. Alloys Compd. 2014, 613, 42–45. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Hwa, Y.; Park, C.-M. Novel high-performance Ga2Te3 anodes for Li-ion batteries. J. Mater. Chem. A 2021, 9, 20553–20564. [Google Scholar] [CrossRef]
| Anode | Cycling Performance | Rate Capability | Synthesis Method | Ref. |
|---|---|---|---|---|
| GaN-CNFs | 405 mAh g−1 after 1200 cycles at 3 A g−1 | 310 mAh g−1 at 5 A g−1 | Electrospinning | [95] |
| α-Ga2O3@G | 350 mAh g−1 after 50 cycles at 0.15 A g−1 | 344 mAh g−1 at 0.5 A g−1 | Hydrothermal and sintering process | [96] |
| Ga2O3/rGO | 411 mAh g−1 after 600 cycles at 1 A g−1 | 222 mAh g−1 at 2 A g−1 | Sol–gel method | [97] |
| Ga2O3/C | 542 mAh g−1 after 200 cycles at 1 A g−1 | 192 mAh g−1 at 5 A g−1 | One-step hydrogen reduction | [98] |
| Ga-Ni | 420 mAh g−1 after 500 cycles at 3 C | 410 mAh g−1 at 5C | Heating process | [99] |
| CuGa2 | 510 mAh g−1 after 65 cycles at 2 A g−1 | 440 mAh g−1 at 4 A g−1 | Painting liquid Ga onto Cu foil | [100] |
| GaN/G | 600 mAh g−1 after 1000 cycles at 1 A g−1 | 200 mAh g−1 at 10 A g−1 | Wet chemical method | [101] |
| Ga2O3 NPs | 721 mAh g−1 after 200 cycles at 0.5 A g−1 | 280 mAh g−1 at 2 A g−1 | Hydrothermal carbonization method | [102] |
| Ga2S3 | 400 mAh g−1 after 20 cycles at 0.1 A g−1 | - | Commercial material | [103,104] |
| SWCNT-GaSx | 590 mAh g−1 after 100 cycles at 0.6 A g−1 | - | Atomic layer deposition | [105] |
| GaSe | 760 mAh g−1 after 50 cycles at 0.1 A g−1 | 450 mAh g−1 at 5 A g−1 | Chemical reduction method | [106] |
| Ball-milled Ga2Te3/C | 590 mAh g−1 after 500 cycles at 0.1 A g−1 | 495 mAh g−1 at 1C | Ball milling | [107] |
| Ga2Te3-TiO2-C | 769 mAh g−1 after 300 cycles at 0.1 A g−1 | 600 mAh g−1 at 10 Ag−1 | Ball milling | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Huy, V.P.; Kim, I.T.; Hur, J. Gallium-Telluride-Based Composite as Promising Lithium Storage Material. Nanomaterials 2022, 12, 3362. https://doi.org/10.3390/nano12193362
Hoang Huy VP, Kim IT, Hur J. Gallium-Telluride-Based Composite as Promising Lithium Storage Material. Nanomaterials. 2022; 12(19):3362. https://doi.org/10.3390/nano12193362
Chicago/Turabian StyleHoang Huy, Vo Pham, Il Tae Kim, and Jaehyun Hur. 2022. "Gallium-Telluride-Based Composite as Promising Lithium Storage Material" Nanomaterials 12, no. 19: 3362. https://doi.org/10.3390/nano12193362