Electro-Design of Bimetallic PdTe Electrocatalyst for Ethanol Oxidation: Combined Experimental Approach and Ab Initio Density Functional Theory (DFT)—Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Substrate Pretreatment
2.2. Electrochemical Characterization
2.3. Physical Characterization
3. Results and Discussion
3.1. Cyclic Voltammetry of Au Coated Glass Recorded in HClO4 Solution Te 4+ and Cu2+ Ions
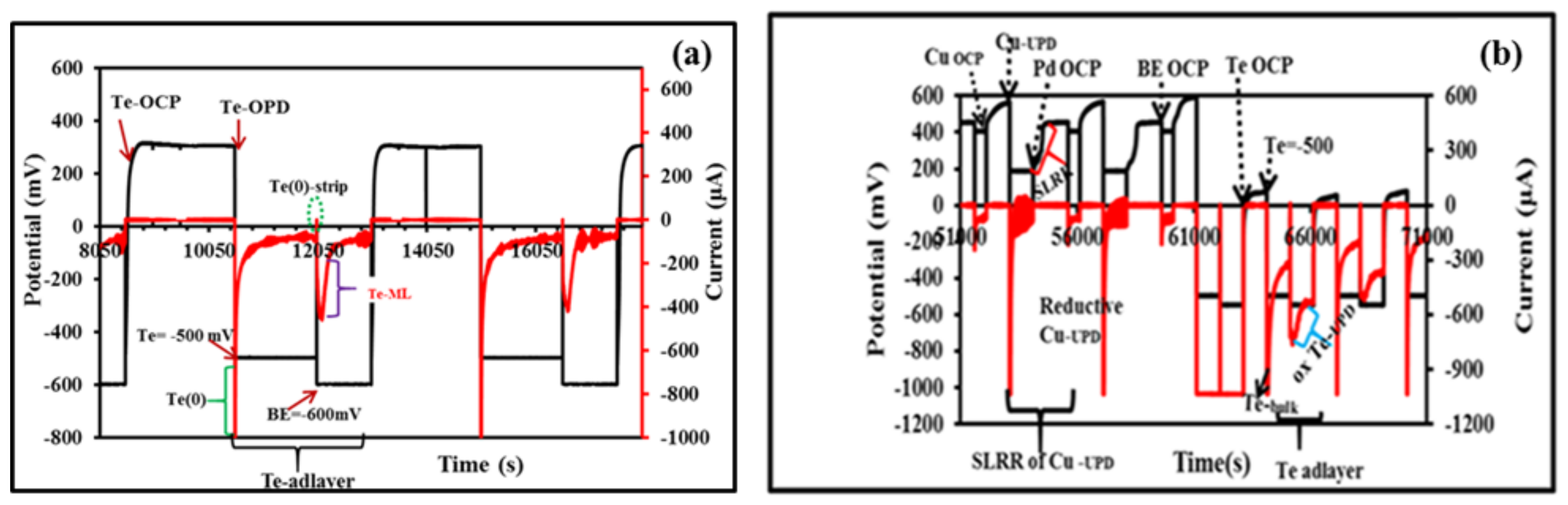
3.2. Electrodeposition of Monometallic (Te, Pd) and Bimetallic (PdTe, in Various Te Ratios) Nanofilms
3.3. Morphological Characterization
SEM and AFM Analysis
3.4. Electrochemical Performance of Pd-Based Electrode Catalysts and Their Kinetics during EOR
3.5. Electrochemical Impedance Spectroscopy (Pd vs. TePd)
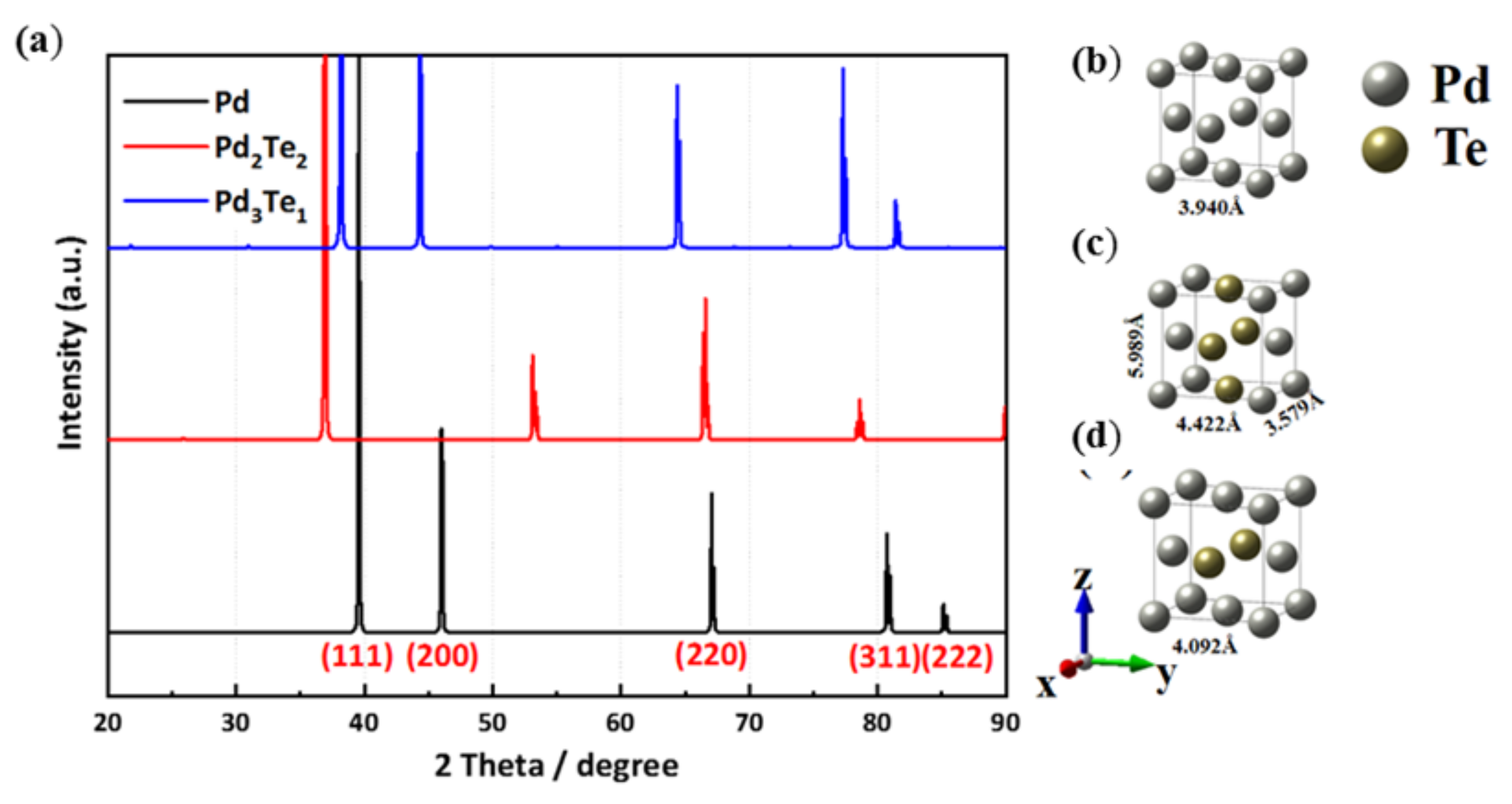
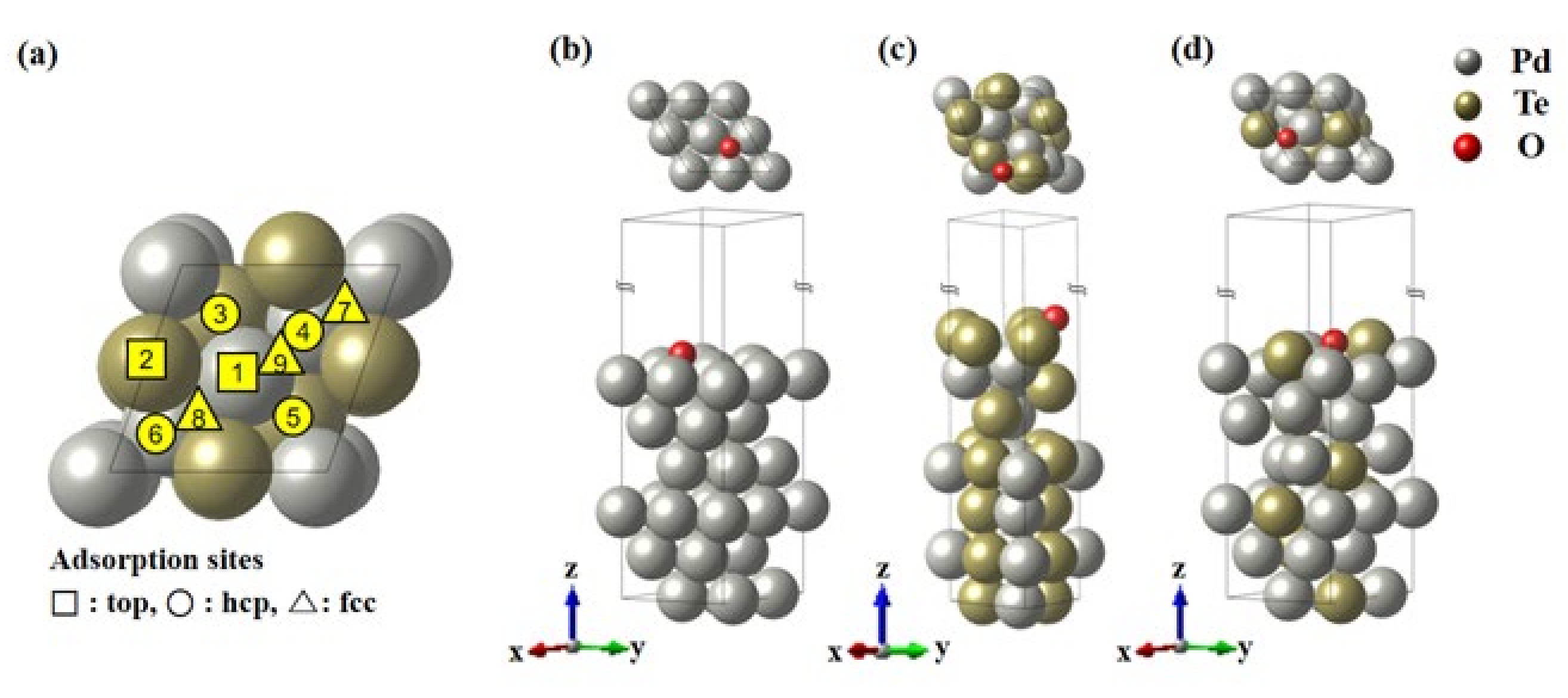
3.6. Computational Details
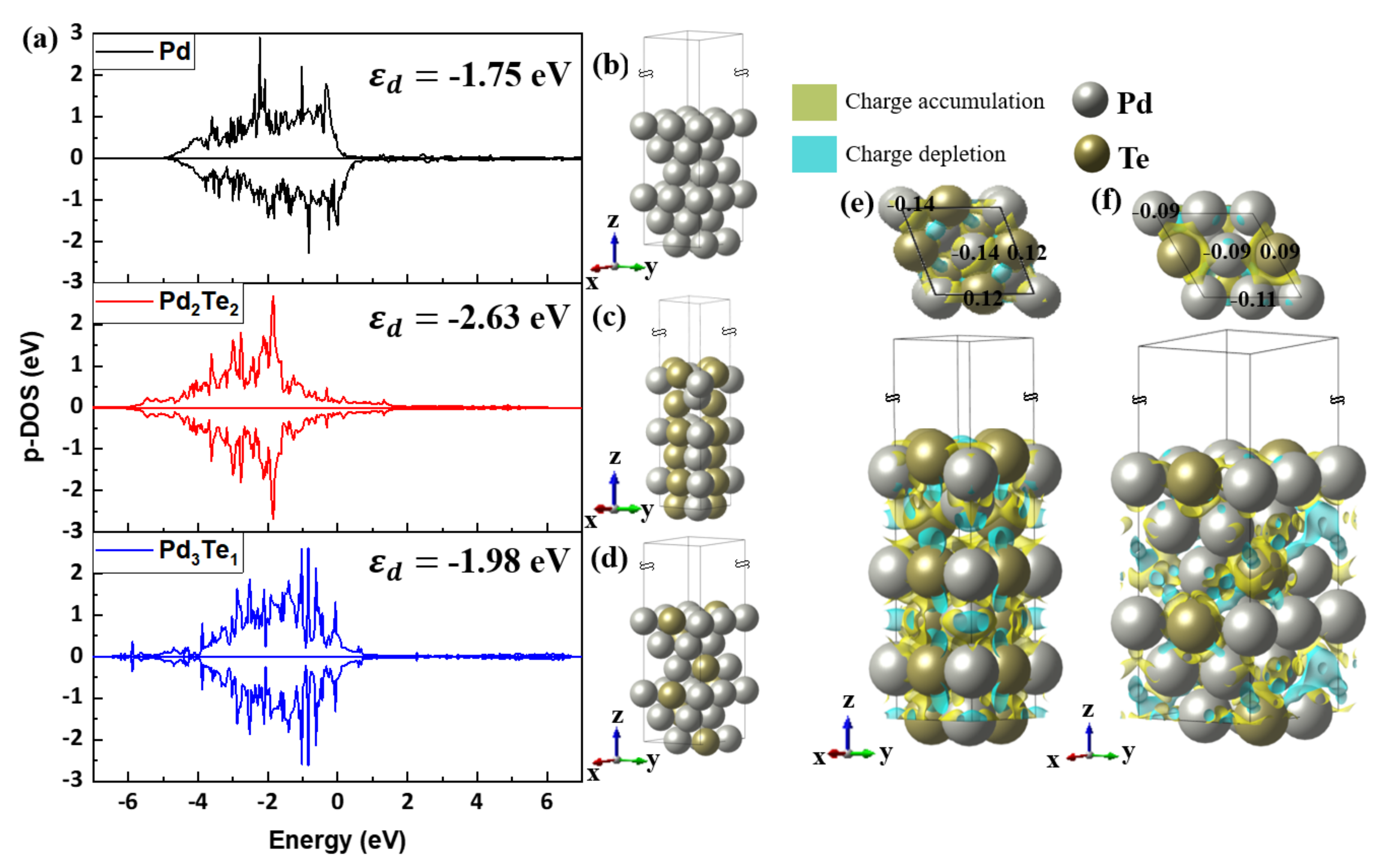
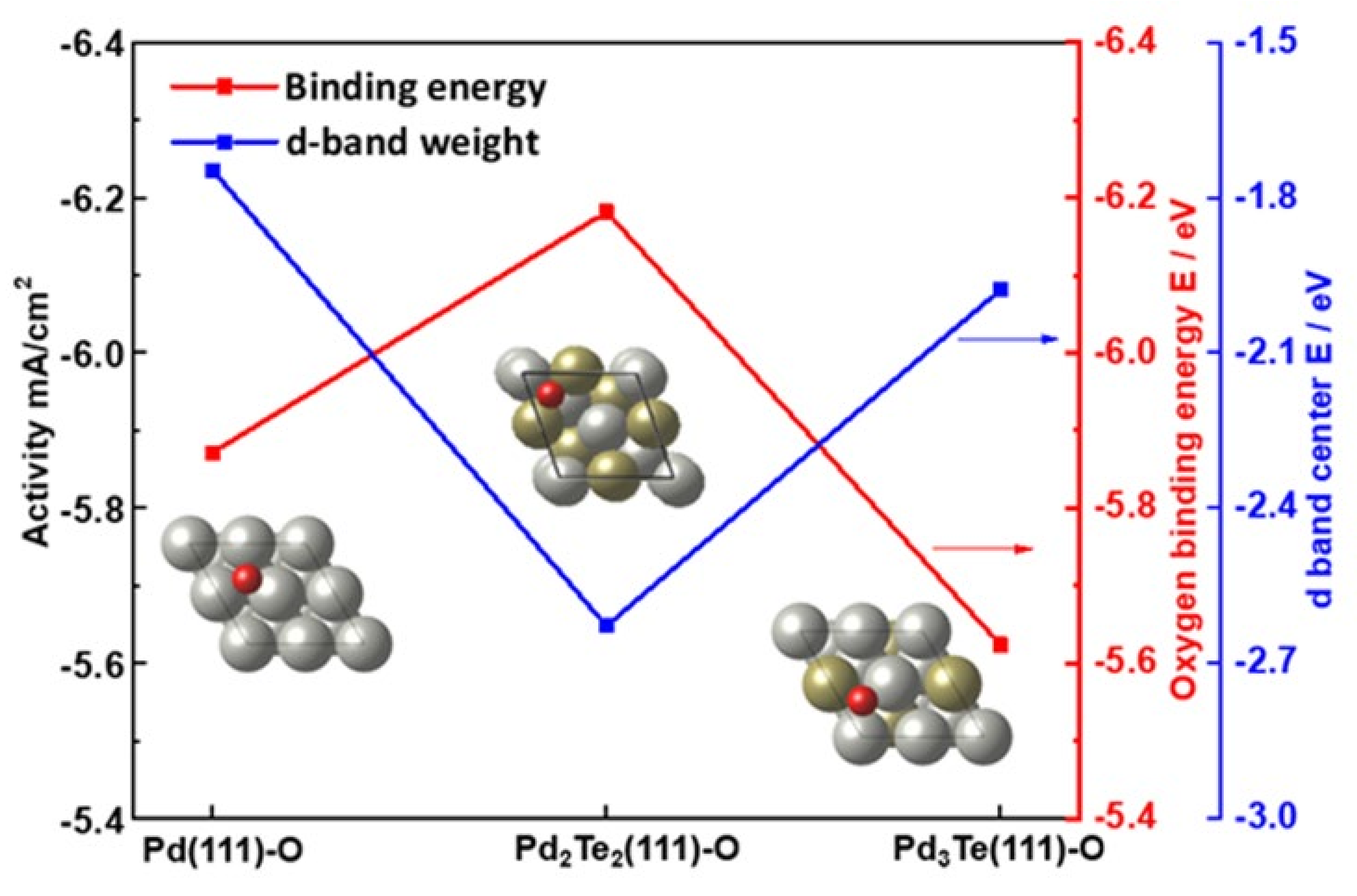
The First-Principle Studies on Pd and PdTe Surfaces Used for Ethanol Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Lodeiro, J.; Rodríguez-Gónzalez, B.; Novio, F.; Fernández-Lodeiro, A.; Ruiz-Molina, D.; Capelo, J.L.; Dos Santos, A.A.; Lodeiro, C. Synthesis and characterization of PtTe2 multi-crystallite nanoparticles using organotellurium nanocomposites. Sci. Rep. 2017, 7, 9889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Zhang, Y.; Ren, F.; Shiraishi, Y.; Du, Y. Universal surfactant-free strategy for self-standing 3D tremella-like Pd–M (M = Ag, Pb, and Au) nanosheets for superior alcohols electrocatalysis. Adv. Funct. Mater. 2020, 30, 2000255. [Google Scholar] [CrossRef]
- Cai, J.; Huang, Y.; Guo, Y. PdTex/C nanocatalysts with high catalytic activity for ethanol electro-oxidation in alkaline medium. Appl. Catal. B 2014, 150, 230–237. [Google Scholar] [CrossRef]
- Mkhohlakali, A.; Fuku, X.; Modibedi, M.; Khotseng, L.; Mathe, M. Electrodeposition of ternary BiTePd nanofilm electrocatalyst using surface-limited reaction for direct ethanol fuel cell. J. Electrochem. Soc. 2022, 169, 046520. [Google Scholar] [CrossRef]
- Meihua, H.; Lirong, L.; Yonglang, G. Microwave heated polyol synthesis of Pt3Te/C catalysts. Electrochim. Acta 2009, 54, 3303–3308. [Google Scholar]
- Elsheikh, A.; McGregor, J. Synthesis and Characterization of PdAgNi/C Trimetallic Nanoparticles for Ethanol Electrooxidation. Nanomaterials 2021, 11, 2244. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Wu, Z.; You, H.; Du, Y. Universal strategies to multi-dimensional noble-metal-based catalysts for electrocatalysis. Coord. Chem. Rev. 2021, 436, 213825. [Google Scholar] [CrossRef]
- Zhang, R.L.; Duan, J.J.; Han, Z.; Feng, J.J.; Huang, H.; Zhang, Q.L.; Wang, A.J. One-step aqueous synthesis of hierarchically multi-branched PdRuCu nanoassemblies with highly boosted catalytic activity for ethanol and ethylene glycol oxidation reactions. Appl. Surf. Sci. 2020, 506, 144791. [Google Scholar] [CrossRef]
- Duan, J.J.; Feng, J.J.; Zhang, L.; Yuan, J.; Zhang, Q.L.; Wang, A.J. Facile one-pot aqueous fabrication of interconnected ultrathin PtPbPd nanowires as advanced electrocatalysts for ethanol oxidation and oxygen reduction reactions. Int. J. Hydrog. Energy 2019, 44, 27455–27464. [Google Scholar] [CrossRef]
- Feng, Y.G.; Niu, H.J.; Mei, L.P.; Feng, J.J.; Fang, K.M.; Wang, A.J. Engineering 3D hierarchical thorn-like PtPdNiCu alloyed nanotripods with enhanced performances for methanol and ethanol electrooxidation. J. Colloid Interface Sci. 2020, 575, 425–432. [Google Scholar] [CrossRef]
- Sheridan, L.B.; Kim, Y.G.; Perdue, B.R.; Jagannathan, K.; Stickney, J.L.; Robinson, D.B. Hydrogen adsorption, absorption, and desorption at palladium nanofilms formed on Au (111) by electrochemical atomic layer deposition (E-ALD): Studies using voltammetry and in situ scanning tunneling microscopy. J. Phys. Chem. C 2013, 117, 15728–15740. [Google Scholar] [CrossRef]
- Mkwizu, T.S.; Mathe, M.K.; Cukrowski, I. Electrodeposition of multilayered bimetallic nanoclusters of ruthenium and platinum via surface-limited redox−replacement reactions for electrocatalytic applications. Langmuir 2010, 26, 570–580. [Google Scholar] [CrossRef]
- Xaba, N.; Modibedi, R.M.; Mathe, M.K.; Khotseng, L.E. Pd, PdSn, PdBi, and PdBiSn nanostructured thin films for the electro-oxidation of ethanol in alkaline media. Electrocatalysis 2019, 10, 332–341. [Google Scholar] [CrossRef]
- Brankovic, S.R.; Wang, J.X.; Adžić, R.R. Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf. Sci. 2001, 474, L173–L179. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, J.Y.; Zhou, D.X.; Bao, S.Q.; Fan, X.A.; Duan, X.K. Electrochemical characterization of the underpotential deposition of tellurium on Au electrode. Electrochim. Acta 2007, 52, 3660–3666. [Google Scholar] [CrossRef]
- Forni, F.; Innocenti, M.; Pezzatini, G.; Foresti, M.L. Electrochemical aspects of CdTe growth on the face (111) of silver by ECALE. Electrochim. Acta 2000, 45, 3225–3231. [Google Scholar] [CrossRef]
- Seo, M.H.; Choi, S.M.; Seo, J.K.; Noh, S.H.; Kim, W.B.; Han, B. The graphene-supported palladium and palladium–yttrium nanoparticles for the oxygen reduction and ethanol oxidation reactions: Experimental measurement and computational validation. Appl. Catal. B 2013, 129, 163–171. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, H.; Jin, S.; Choi, S.M.; Kim, H.J.; Seo, M.H.; Kim, W.B. A review on recent progress in the aspect of stability of oxygen reduction electrocatalysts for proton-exchange membrane fuel cell: Quantum mechanics and experimental approaches. Energy Technol. 2019, 7, 1900312. [Google Scholar] [CrossRef]
- Molina Martín, L.M.; Benito, A.; Alonso Martín, J.A. Ab initio studies of ethanol dehydrogenation at binary AuPd nanocatalysts. Mol. Catal. 2018, 449, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Gregory, B.W.; Norton, M.L.; Stickney, J.L. CdTe acidic bath-UPD. J. Electroanal. Chem. Interfacial Electrochem. 1990, 293, 85–101. [Google Scholar] [CrossRef]
- Dursun, Z.; Aoun, S.B.; Taniguchi, I. Electrocatalytic oxidation of D-glucose using a Cd ad-atom-modified Au (111) electrode in alkaline solution. Turk. J. Chem. 2008, 32, 423–430. [Google Scholar]
- Kolb, D.M.; Przasnyski, M.; Gerischer, H. Underpotential deposition of metals and work function differences. J. Electroanal. Chem. Interfacial Electrochem. 1974, 54, 25–38. [Google Scholar] [CrossRef]
- Holzle, M.H.; Zwing, V.; Kolb, D.M. The influence of steps on the deposition of Cu onto Au (111). Electrochim. Acta 1995, 40, 1237–1247. [Google Scholar] [CrossRef]
- Kuzume, A.; Herrero, E.; Feliu, J.M.; Nichols, R.J.; Schiffrin, D.J. Copper underpotential deposition at high index single crystal surfaces of Au. J. Electroanal. Chem. 2004, 570, 157–161. [Google Scholar] [CrossRef]
- Sahin, O.; Kivrak, H.; Karaman, M.; Atbas, D. The effect of iridium addition to platinum on the alcohol electrooxidation activity. Am. J. Mater. Sci. Eng. 2015, 3, 15–20. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Banga, D.O.; Vaidyanathan, R.; Xuehai, L.; Stickney, J.L.; Cox, S.; Happeck, U. Formation of PbTe nanofilms by electrochemical atomic layer deposition (ALD). Electrochim. Acta 2008, 53, 6988–6994. [Google Scholar] [CrossRef]
- Mkhohlakali, A.C.; Fuku, X.; Modibedi, R.M.; Khotseng, L.E.; Ray, S.C.; Mathe, M.K. Electrosynthesis and characterization of PdIr using electrochemical atomic layer deposition for ethanol oxidation in alkaline electrolyte. Appl. Surf. Sci. 2020, 502, 144158. [Google Scholar] [CrossRef]
- Sheridan, L.B.; Gebregziabiher, D.K.; Stickney, J.L.; Robinson, D.B. Formation of palladium nanofilms using electrochemical atomic layer deposition (E-ALD) with chloride complexation. Langmuir 2013, 29, 1592–1600. [Google Scholar] [CrossRef]
- Ma, Y.; Wijesekara, W.; Palmqvist, A.E. Electrochemical deposition and characterization of thermoelectric ternary (BixSb1-x)2Te3 and Bi2(Te1-ySey)3 thin films. J. Electron. Mater. 2012, 41, 1138–1146. [Google Scholar] [CrossRef]
- Wang, X.; Ni, Y. A facile electrochemical route for fast deposition of featherlike tellurium microstructures. RSC Adv. 2012, 2, 2340–2345. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, H.S. Formation mechanism of PbTe dendritic nanostructures grown by electrodeposition. Mater. Chem. Phys. 2017, 187, 82–87. [Google Scholar] [CrossRef]
- Geraldes, A.N.; Da Silva, D.F.; Pino, E.S.; Da Silva, J.C.; De Souza, R.F.; Hammer, P.; Spinace, E.V.; Oliveira, A.N.; Linardi, M.; dos Santos, M.C. Ethanol electro-oxidation in an alkaline medium using Pd/C., Au/C and PdAu/C electrocatalysts prepared by electron beam irradiation. Electrochim. Acta 2013, 111, 455–465. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Y.Y.; Zhao, Z.G. Surface voltammetric dealloying investigation on PdCu/C electrocatalysts toward ethanol oxidation in alkaline media. J. Nanopart. Res. 2018, 20, 314. [Google Scholar] [CrossRef]
- Ding, K.; Zhao, Y.; Liu, L.; Li, Y.; Liu, L.; Wang, Y.; Gu, H.; Wei, H.; Guo, Z. Multi-walled carbon nanotubes supported Pd composite nanoparticles hydrothermally produced from technical grade PdO precursor. Electrochim. Acta 2015, 176, 1256–1265. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, S.; Xu, H.; Zhang, K.; Yan, B.; Du, Y. Newly designed ternary metallic PtPdBi hollow catalyst with high performance for methanol and ethanol oxidation. Catalysts 2017, 7, 208. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; La Russa, D.J.; Miller, B. Effect of the iridium oxide thin film on the electrochemical activity of platinum nanoparticles. Langmuir 2004, 20, 9695–9702. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, K.; Xu, H.; Du, Y.; Goh, M.C. Anchoring gold nanoparticles on poly (3, 4-ethylenedioxythiophene)(PEDOT) nanonet as three-dimensional electrocatalysts toward ethanol and 2-propanol oxidation. J. Colloid. Interface Sci. 2019, 541, 258–268. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Hammer, B.H.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.S.; Park, D.; Jeon, S. Ultrasmall PdmMn1−mOx binary alloyed nanoparticles on graphene catalysts for ethanol oxidation in alkaline media. J. Power Sources 2016, 308, 180–188. [Google Scholar] [CrossRef]
- Shao, M.; Huang, T.; Liu, P.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Adzic, R.R. Palladium monolayer and palladium alloy electrocatalysts for oxygen reduction. Langmuir 2006, 22, 10409–10415. [Google Scholar] [CrossRef]
- Lima, F.H.; Zhang, J.; Shao, M.H.; Sasaki, K.; Vukmirovic, M.B.; Ticianelli, A.E.; Adzic, R.R. Catalytic activity−d-band center correlation for the O2 reduction reaction on platinum in alkaline solutions. J. Phys. Chem. C 2007, 111, 404–410. [Google Scholar] [CrossRef]
- Bader, R.F. Atoms in Molecules: A Quantum Theory, 1st ed.; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Xu, C.; Shen, P.K.; Liu, Y. Ethanol electrooxidation on Pt/C and Pd/C catalysts promoted with oxide. J. Power Sources 2007, 164, 527–531. [Google Scholar] [CrossRef]
- Zheng, H.T.; Chen, S.; Shen, P.K. Spontaneous formation of platinum particles on electrodeposited palladium. Electrochem. Commun. 2007, 9, 1563–1566. [Google Scholar] [CrossRef]
- Zheng, H.T.; Li, Y.; Chen, S.; Shen, P.K. Effect of support on the activity of Pd electrocatalyst for ethanol oxidation. J. Power Sources 2006, 163, 371–375. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Xu, C.; Tong, Y. Kinetics of ethanol electrooxidation at Pd electrodeposited on Ti. Electrochem. Commun. 2007, 9, 2334–2339. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Sun, K.; Li, W. Pd–Ni electrocatalysts for efficient ethanol oxidation reaction in alkaline electrolyte. Int. J. Hydrogen Energy 2011, 36, 12686–12697. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.; Blizanac, B.B.; Arenz, M.; Stamenkovic, V.R.; Ross, P.N.; Markovic, N.M. The impact of geometric and surface electronic properties of Pt-catalysts on the particle size effect in electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef]
- Tal, A.A.; Posada-Borbón, A.; Grönbeck, H.J.; Abrikosov, I.A. Correlation between ethylene adsorption energies and core-level shifts for Pd nanoclusters. J. Phys. Chem. C 2018, 123, 2544–2548. [Google Scholar] [CrossRef]
- Hu, J.; Tanabe, M.; Sato, J.; Uosaki, K.; Ikeda, K. Effects of atomic geometry and electronic structure of platinum surfaces on molecular adsorbates studied by gap-mode SERS. J. Am. Chem. Soc. 2014, 136, 10299–10307. [Google Scholar] [CrossRef] [PubMed]


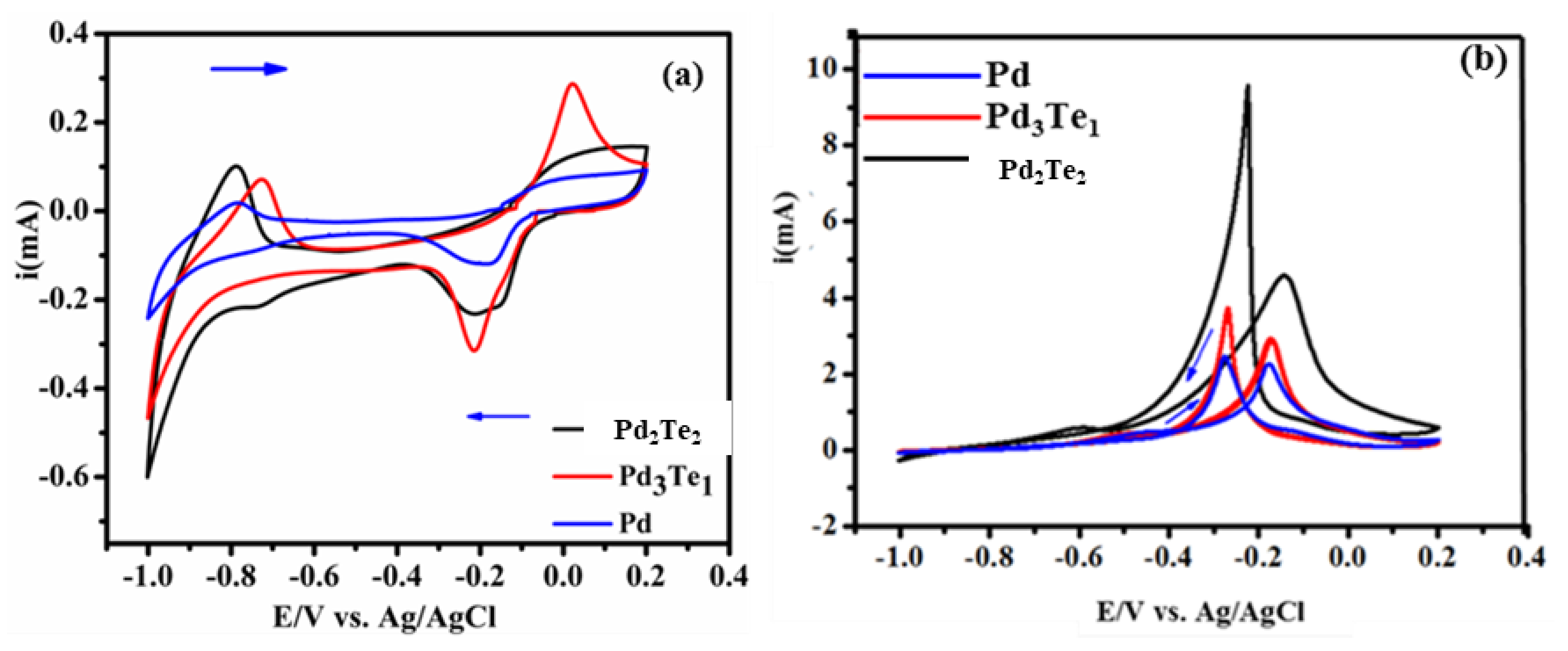


| Catalyst (mV) | if (mA) | ib (mA) | if/ib (mA) | Onset (E/V) |
|---|---|---|---|---|
| Pd | 2.0 | 2.4 | 0.9 | −4 |
| Pd3T1 | 3.2 | 3.75 | 0.8 | −0.42 |
| Pd2T2 | 5.0 | 9.5 | 0.52 | −0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkhohlakali, A.; Fuku, X.; Seo, M.H.; Modibedi, M.; Khotseng, L.; Mathe, M. Electro-Design of Bimetallic PdTe Electrocatalyst for Ethanol Oxidation: Combined Experimental Approach and Ab Initio Density Functional Theory (DFT)—Based Study. Nanomaterials 2022, 12, 3607. https://doi.org/10.3390/nano12203607
Mkhohlakali A, Fuku X, Seo MH, Modibedi M, Khotseng L, Mathe M. Electro-Design of Bimetallic PdTe Electrocatalyst for Ethanol Oxidation: Combined Experimental Approach and Ab Initio Density Functional Theory (DFT)—Based Study. Nanomaterials. 2022; 12(20):3607. https://doi.org/10.3390/nano12203607
Chicago/Turabian StyleMkhohlakali, Andile, Xolile Fuku, Min Ho Seo, Mmalewane Modibedi, Lindiwe Khotseng, and Mkhulu Mathe. 2022. "Electro-Design of Bimetallic PdTe Electrocatalyst for Ethanol Oxidation: Combined Experimental Approach and Ab Initio Density Functional Theory (DFT)—Based Study" Nanomaterials 12, no. 20: 3607. https://doi.org/10.3390/nano12203607





