One-Dimensional CoMoP Nanostructures as Bifunctional Electrodes for Overall Water Splitting
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
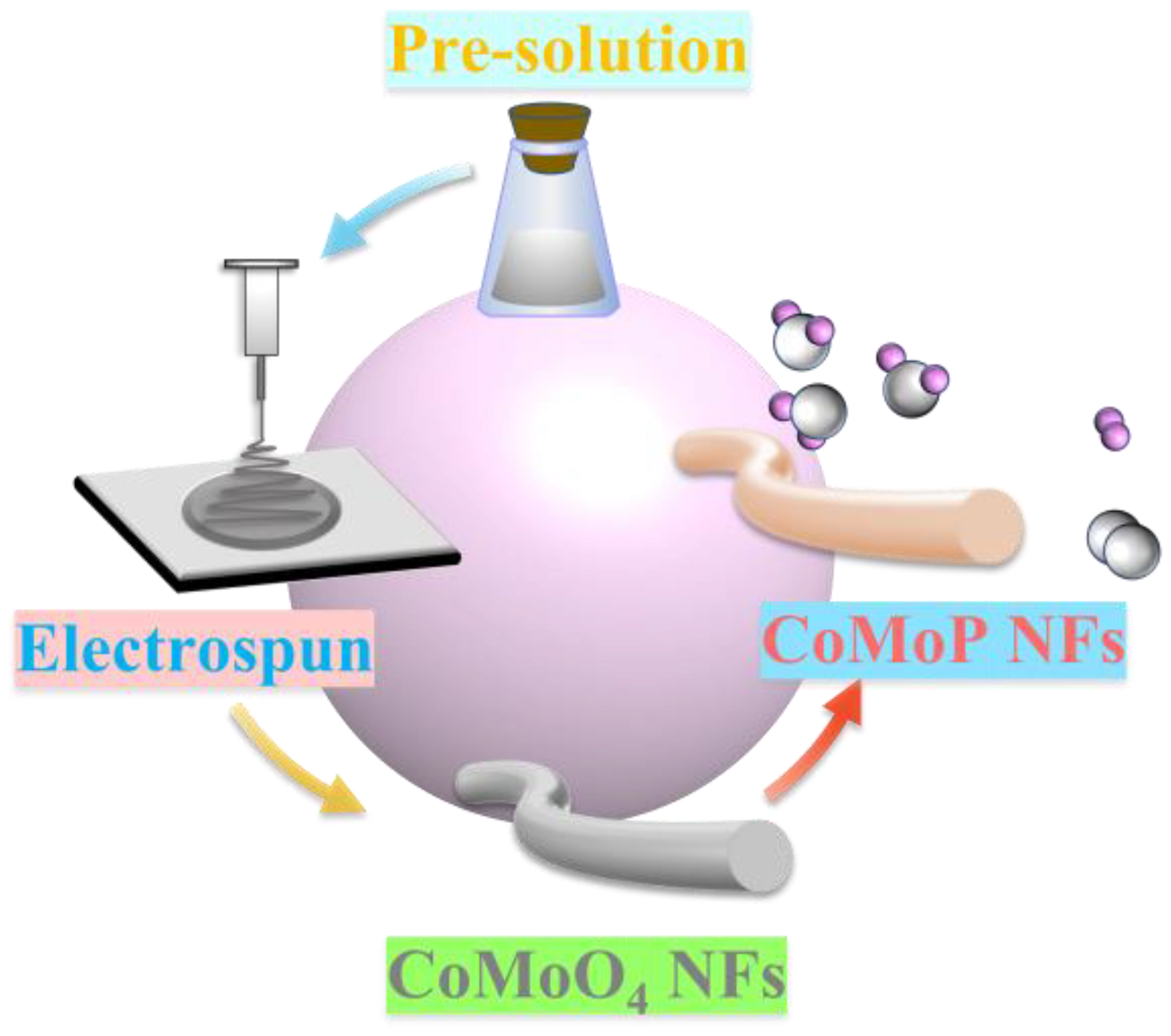
2.2. Synthesis of CoMoO4 Nanofibers
2.3. Synthesis of CoMoP Nanofibers
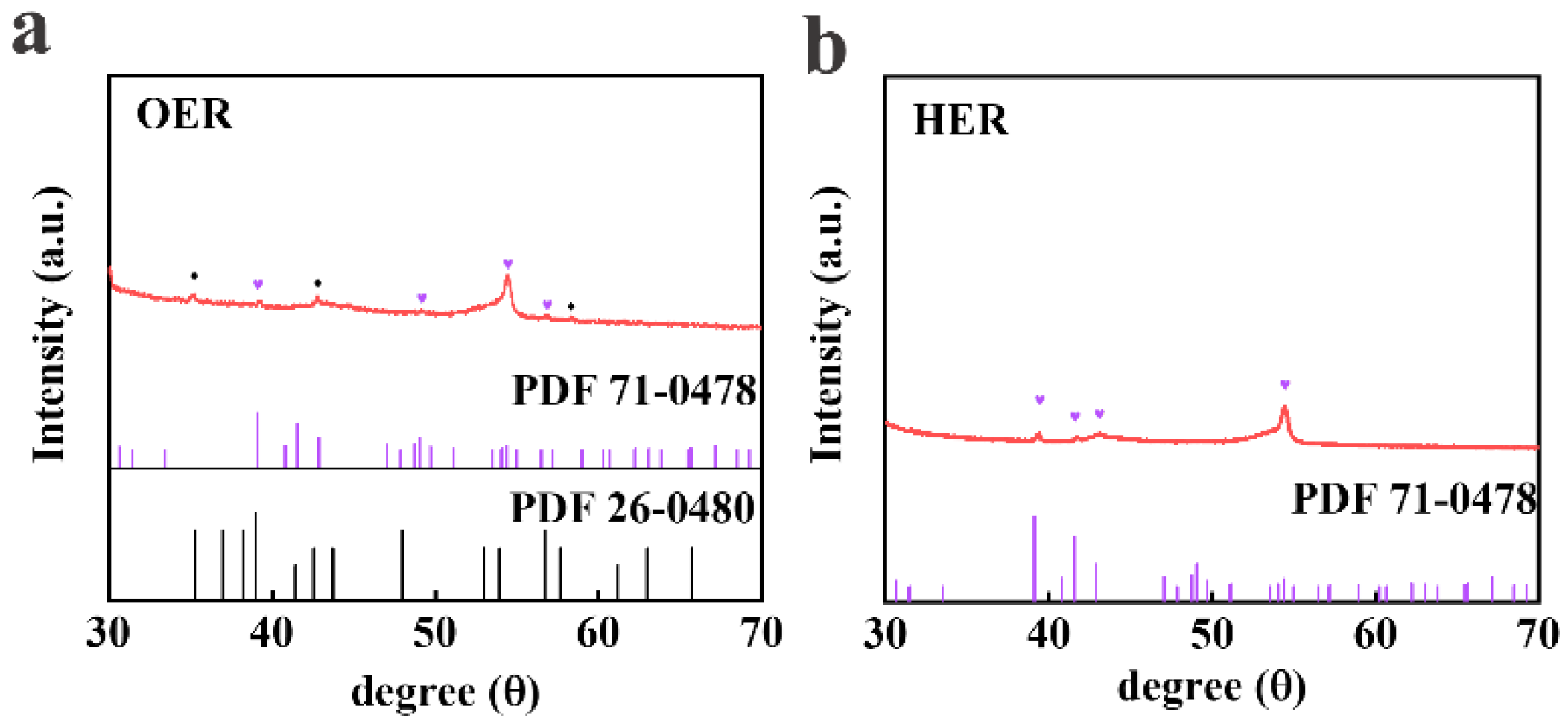
2.4. Characterizations
2.5. Electrochemical Measurements
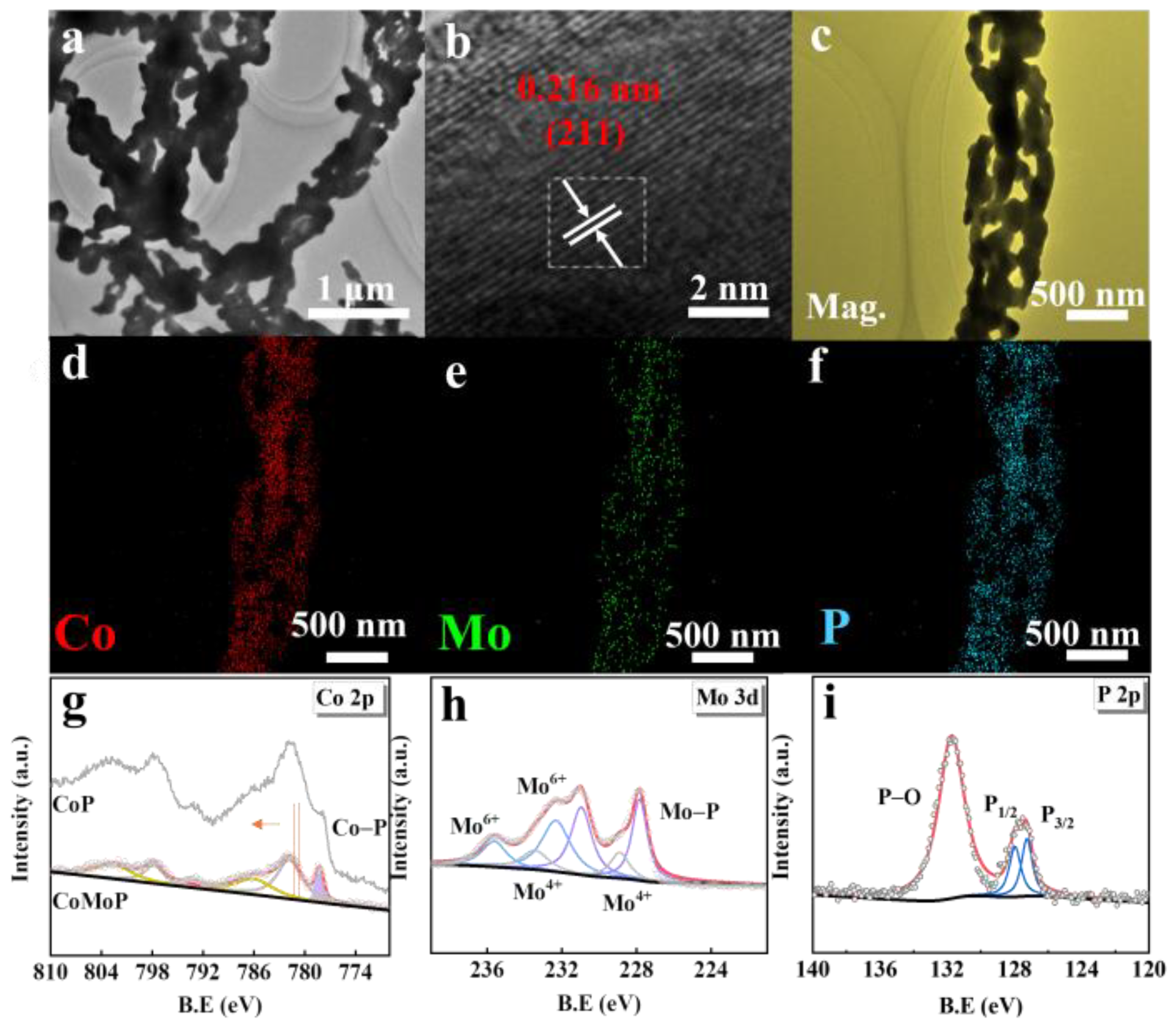
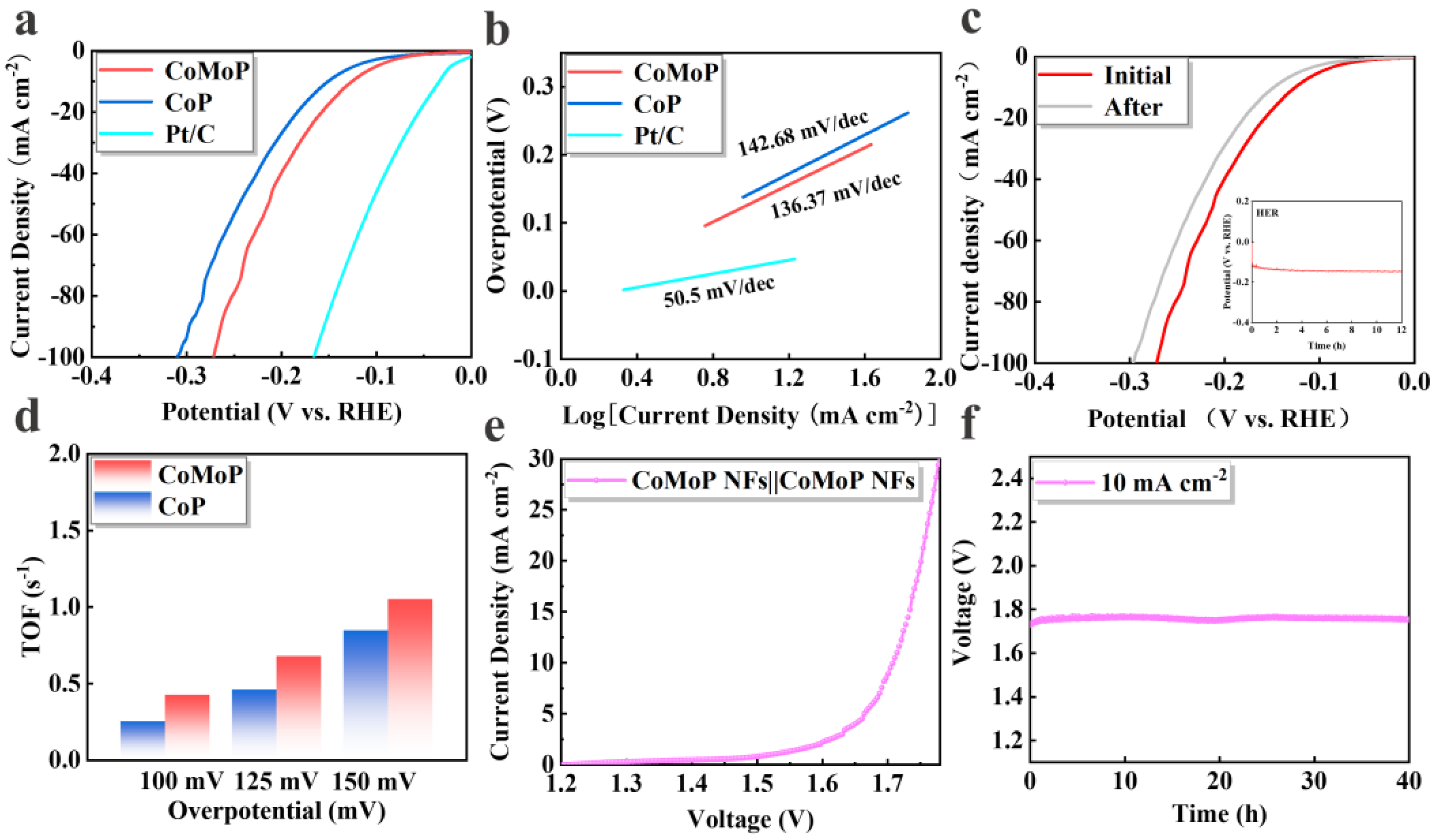
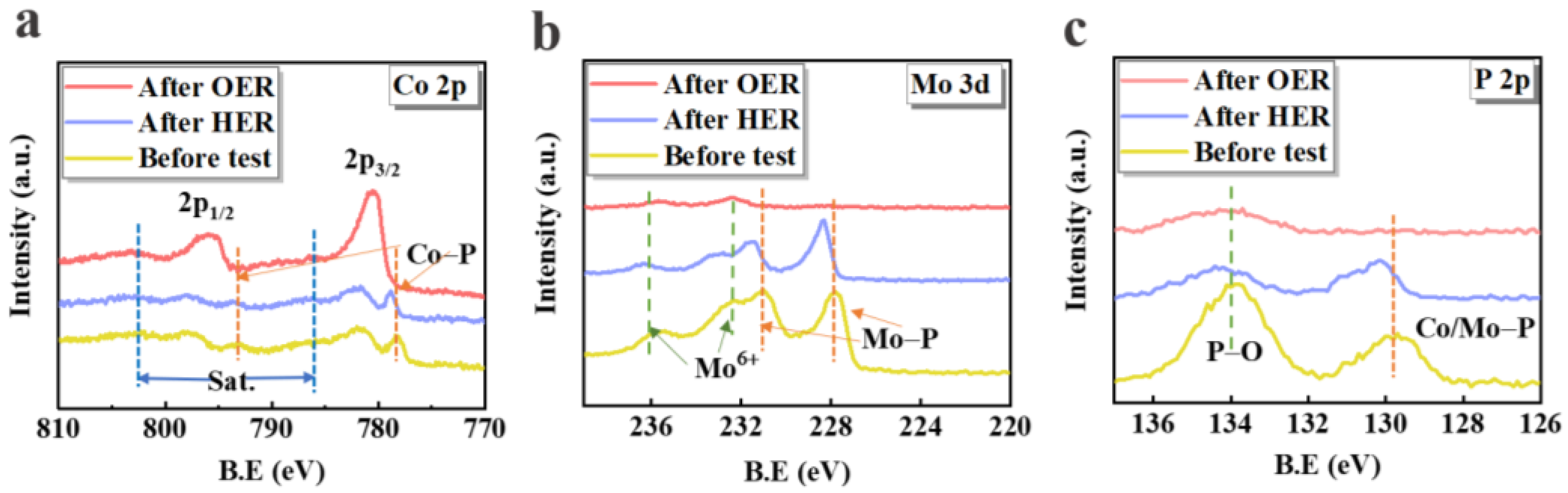
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouyang, T.; Wang, X.-T.; Mai, X.-Q.; Chen, A.-N.; Tang, Z.-Y.; Liu, Z.Q. Coupling Magnetic Single-Crystal Co2Mo3O8 with Ultrathin Nitrogen-Rich Carbon Layer for Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 11948–11957. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, L. N-enriched porous carbon encapsulated bimetallic phosphides with hierarchical structure derived from controlled electrodepositing multilayer ZIFs for electrochemical overall water splitting. Appl. Catal. B Environ. 2019, 259, 118053. [Google Scholar] [CrossRef]
- Anjum, M.; Okyay, M.-S.; Kim, M.; Lee, M.H.; Park, N.; Lee, J.S. Bifunctional sulfur-doped cobalt phosphide electrocatalyst outperforms all-noble-metal electrocatalysts in alkaline electrolyzer for overall water splitting. Nano Energy 2018, 53, 286–295. [Google Scholar] [CrossRef]
- Du, C.; Shang, M.; Mao, J.; Song, W. Hierarchical MoP/Ni2P heterostructures on nickel foam for efficient water splitting. J. Mater. Chem. A 2017, 5, 15940–15949. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, Z.; Fu, H.; Zhang, X.; Tian, G.; Fu, H. NiSe-Ni0.85Se Heterostructure Nanoflake Arrays on Carbon Paper as Efficient Electrocatalysts for Overall Water Splitting. Small 2018, 14, 1800763. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Sivanantham, A.; Shanmugam, S.; Kalai Selvan, R. Electrospun Carbon Nanofibers Encapsulated with NiCoP: A Multifunctional Electrode for Supercapattery and Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. Adv. Energy Mater. 2018, 8, 1800555. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, R.; Dong, S.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries. Energy Environ. Sci. 2019, 12, 2422–2432. [Google Scholar] [CrossRef]
- Louie, M.-W.; Bell, A.-T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yan, L.; Luo, H.; Mustain, W.; Xu, H. Recent progress and perspectives of bifunctional oxygen reduction/evolution catalyst development for regenerative anion exchange membrane fuel cells. Nano Energy 2018, 47, 172–198. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Paudel, D.R.; Dhakal, P.P.; Kim, N.H.; Lee, J.H. Hybridized bimetallic phosphides of Ni–Mo, Co–Mo, and Co–Ni in a single ultrathin-3D-nanosheets for efficient HER and OER in alkaline media. Compos. B Eng. 2022, 239, 109992. [Google Scholar] [CrossRef]
- Ullah, H.; Loh, A.; Trudgeon, D.P.; Li, X. Density Functional Theory Study of NiFeCo Trinary Oxy-Hydroxides for an Efficient and Stable Oxygen Evolution Reaction Catalyst. ACS Omega 2020, 32, 20517–20524. [Google Scholar] [CrossRef] [PubMed]
- Sookhakian, M.; Ullah, H.; Mat Teridi, M.A.; Tong, G.B.; Basirun, W.J.; Alias, Y. Boron-doped graphene-supported manganese oxide nanotubes as an efficient non-metal catalyst for the oxygen reduction reaction. Sustain. Energy Fuels 2020, 4, 737–749. [Google Scholar] [CrossRef]
- Wang, H.; Tong, Y.; Li, K.; Chen, P. Heterostructure engineering of iridium species on nickel/molybdenum nitride for highly-efficient anion exchange membrane water electrolyzer. J. Colloid Interface Sci. 2022, 628, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lv, Y.; Su, J.; Wang, Y.; Yang, Q.; Zhang, Y.; Zhou, J.; Xu, L.; Sun, D.; Tang, Y. Anchoring CoFe2O4 Nanoparticles on N-Doped Carbon Nanofibers for High-Performance Oxygen Evolution Reaction. Adv. Sci. 2017, 4, 1700226. [Google Scholar]
- Riyajuddin, S.; Azmi, K.; Pahuja, M.; Kumar, S.; Maruyama, T.; Bera, C.; Ghosh, K. Super-Hydrophilic Hierarchical Ni-Foam-Graphene-Carbon Nanotubes-Ni2P-CuP2 Nano-Architecture as Efficient Electrocatalyst for Overall Water Splitting. ACS Nano 2021, 15, 5586–5599. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Z.; Zhang, X.; Zhong, A.; Qin, W.; Liu, W.; Liu, Y. In-situ phosphatizing of cobalt-molybdenum nanosheet arrays on self-supporting rGO/CNTs film as efficient electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2021, 422, 130355. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.; He, L.; Jiang, X.; Asiri, A.-M.; Sun, X. Fe-Doped CoP Nanoarray: A Monolithic Multifunctional Catalyst for Highly Efficient Hydrogen Generation. Adv. Mater. 2017, 29, 1602441. [Google Scholar]
- He, K.; Tadesse Tsega, T.; Liu, X.; Zai, J.; Li, X.-H.; Liu, X.; Li, W.; Ali, N.; Qian, X. Utilizing the Space-Charge Region of the FeNi-LDH/CoP p-n Junction to Promote Performance in Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2019, 58, 11903–11909. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Russo, P.A.; Amsalem, P.; Koch, N.; Pinna, N. The Importance of Ligand Selection on the Formation of Metal Phosphonate-Derived CoMoP and CoMoP2 Nanoparticles for Catalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2020, 3, 4147–4156. [Google Scholar]
- Zhang, G.; Wang, G.; Liu, Y.; Liu, H.; Qu, J.; Li, J. Highly Active and Stable Catalysts of Phytic Acid-Derivative Transition Metal Phosphides for Full Water Splitting. J. Am. Chem. Soc. 2016, 138, 14686–14693. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, L. Efficient gel route to embed phosphorus into MOF-derived porous FePx as anodes for high performance lithium-ion batteries. Energy Storage Mater. 2018, 14, 367–375. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, B.; Zhao, D.; Wang, H.; Selomulya, C. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Z.; Chai, L.; Xu, L.; Zhu, Z.; Hu, Y.; Qian, J.; Huang, S. MOF derived N-doped carbon coated CoP particle/carbon nanotube composite for efficient oxygen evolution reaction. Carbon 2019, 141, 643–651. [Google Scholar] [CrossRef]
- Lv, X.; Li, X.; Yang, C.; Ding, X.; Zhang, Y.; Zheng, Y.-Z.; Li, S.; Sun, X.; Tao, X. Large-Size, Porous, Ultrathin NiCoP Nanosheets for Efficient Electro/Photocatalytic Water Splitting. Adv. Funct. Mater. 2020, 30, 1910830. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Yang, Z.-J.; Yang, D.-H.; Zhao, L.; Shi, X.-R.; Yang, G.; Han, B.-H. FeCoP2 Nanoparticles Embedded in N and P Co-doped Hierarchically Porous Carbon for Efficient Electrocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 8832–8843. [Google Scholar] [CrossRef]
- Li, D.; Baydoun, H.; Verani, C.N.; Brock, S.L. Efficient Water Oxidation Using CoMnP Nanoparticles. J. Am. Chem. Soc. 2016, 138, 4006–4009. [Google Scholar] [CrossRef]
- Zhao, T.; Shen, X.; Wang, Y.; Hocking, R.-K.; Li, Y.; Rong, C.; Dastafkan, K.; Su, Z.; Zhao, C. In Situ Reconstruction of V-Doped Ni2P Pre-Catalysts with Tunable Electronic Structures for Water Oxidation. Adv. Funct. Mater. 2021, 31, 2106614. [Google Scholar]
- Zhang, H.; Zhou, W.; Dong, J.; Lu, X.-F.; Lou, X.-W. Intramolecular electronic coupling in porous iron cobalt (oxy)phosphide nanoboxes enhances the electrocatalytic activity for oxygen evolution. Energy Environ. Sci. 2019, 12, 3348–3355. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Cao, J.; Guo, S.; Zheng, X.; Feng, J.; Qi, J. Activating and optimizing the activity of NiCoP nanosheets for electrocatalytic alkaline water splitting through the V doping effect enhanced by P vacancies. J. Mater. Chem. A 2019, 7, 24486–24492. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Lin, Y.; Cui, Y.; Sun, Y.; Liu, Y.; Liu, Y. Cobalt nickel phosphide nanoparticles decorated carbon nanotubes as advanced hybrid catalysts for hydrogen evolution. J. Mater. Chem. A 2016, 4, 14675–14686. [Google Scholar] [CrossRef]
- Cao, Y.; Xue, Y.; Niu, Y.; Qin, Y.; Sawangphruk, M.; Zhang, X.; Liu, X. Facile Electrodeposition of Ni-Cu-P Dendrite Nanotube Films with Enhanced Hydrogen Evolution Reaction Activity and Durability. ACS Appl. Mater. Interfaces 2018, 10, 35224–35233. [Google Scholar] [CrossRef]
- Bai, J.; Xi, B.; Mao, H.; Lin, Y.; Ma, X.; Feng, J.; Xiong, S. One-Step Construction of N, P-codoped Porous Carbon Sheets/CoP Hybrids with Enhanced Lithium and Potassium Storage. Adv. Mater. 2018, 30, 1802310. [Google Scholar]
- Duan, J.; Chen, S.; Zhao, C. Strained Nickel Phosphide Nanosheet Array. ACS Appl. Mater. Interfaces 2018, 10, 30029–30034. [Google Scholar] [CrossRef]
- Huang, X.; Xu, X.; Luan, X.; Cheng, D. CoP nanowires coupled with CoMoP nanosheets as a highly efficient cooperative catalyst for hydrogen evolution reaction. Nano Energy 2020, 68, 104332. [Google Scholar] [CrossRef]
- Huang, H.; Cho, A.; Kim, S.; Jun, H.; Lee, A.; Han, J.-W.; Lee, J. Structural Design of Amorphous CoMoPx with Abundant Active Sites and Synergistic Catalysis Effect for Effective Water Splitting. Adv. Funct. Mater. 2020, 30, 2003889. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, X.; Pan, Q.; Sun, D.; Men, L.; Sun, B.; Xu, C.; Su, Z. Ammonium polyphosphate induced bimetallic phosphides nanoparticles coated with porous N-doped carbon for efficiently electrochemical hydrogen evolution. Chem. Eng. J. 2022, 431, 133696. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Yang, R.; Li, D.; Meng, S.; Chen, M. CoP3/CoMoP Heterogeneous Nanosheet Arrays as Robust Electrocatalyst for pH-Universal Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 9309–9317. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, J.; Wang, W.; Tsiakaras, P.; Li, Y. Oxygen Vacancy and Core-Shell Heterojunction Engineering of Anemone-Like CoP@CoOOH Bifunctional Electrocatalyst for Efficient Overall Water Splitting. Small 2022, 12, 2106012. [Google Scholar] [CrossRef]
- Li, X.; Hu, Q.; Wang, H.; Chen, M.; Hao, X.; Ma, Y.; Liu, J.; Tang, K.; Abudula, A.; Guan, G. Charge induced crystal distortion and morphology remodeling: Formation of Mn-CoP nanowire @ Mn-CoOOH nanosheet electrocatalyst with rich edge dislocation defects. Appl. Catal. B Environ. 2021, 292, 120172. [Google Scholar] [CrossRef]
- Xu, S.; Gao, X.; Deshmukh, A.; Zhou, J.; Chen, N.; Peng, W.; Gong, Y.; Yao, Z.; Finkelstein, K.-D.; Wan, B.; et al. Pressure-promoted irregular CoMoP2 nanoparticles activated by surface reconstruction for oxygen evolution reaction electrocatalysts. J. Mater. Chem. A 2020, 8, 2001–2007. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Kou, Z.; Yu, Y.; Liu, X.; Gao, X.; Zheng, L.; Zou, H.; Pang, Y.; Wang, Z.; Pan, Z.; He, J.; et al. Potential-Dependent Phase Transition and Mo-Enriched Surface Reconstruction of γ-CoOOH in a Heterostructured Co-Mo2C Precatalyst Enable Water Oxidation. ACS Catal. 2020, 10, 4411–4419. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.-J.; Chen, Z. CoP Nanoframes as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. ACS Catal. 2019, 10, 412–419. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Yan, J.; Ding, X.; Jia, Y.; Li, S.; Zhang, M. One-Dimensional CoMoP Nanostructures as Bifunctional Electrodes for Overall Water Splitting. Nanomaterials 2022, 12, 3886. https://doi.org/10.3390/nano12213886
Chang X, Yan J, Ding X, Jia Y, Li S, Zhang M. One-Dimensional CoMoP Nanostructures as Bifunctional Electrodes for Overall Water Splitting. Nanomaterials. 2022; 12(21):3886. https://doi.org/10.3390/nano12213886
Chicago/Turabian StyleChang, Xin, Jun Yan, Xinyao Ding, Yaozu Jia, Shijie Li, and Mingyi Zhang. 2022. "One-Dimensional CoMoP Nanostructures as Bifunctional Electrodes for Overall Water Splitting" Nanomaterials 12, no. 21: 3886. https://doi.org/10.3390/nano12213886




