Sustainable Synthesis of Sulfur-Single Walled Carbon Nanohorns Composite for Long Cycle Life Lithium-Sulfur Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrolyte Preparation
2.2. Active Materials and Electrode Preparation
2.3. Material Characterization
2.4. Electrochemical Characterization
3. Results
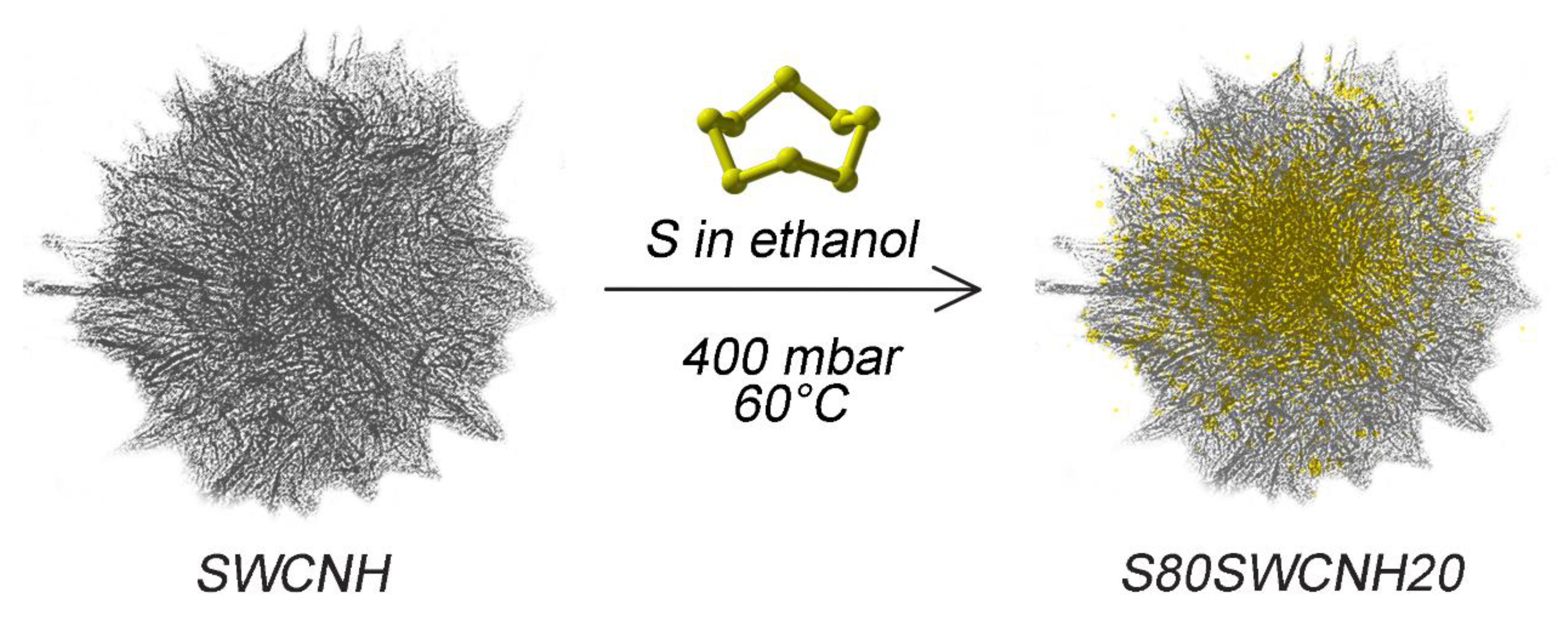
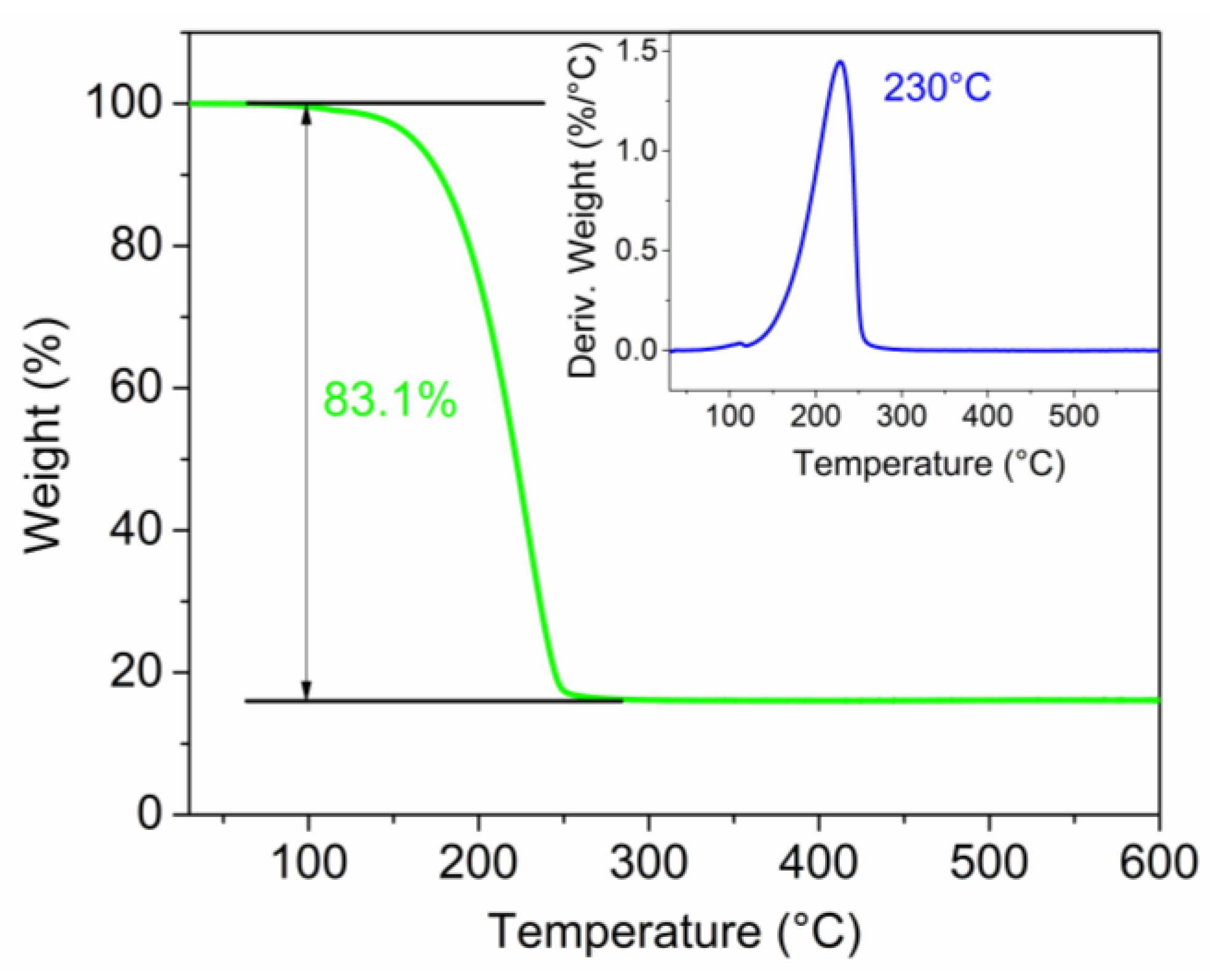
3.1. Active Material Characterization
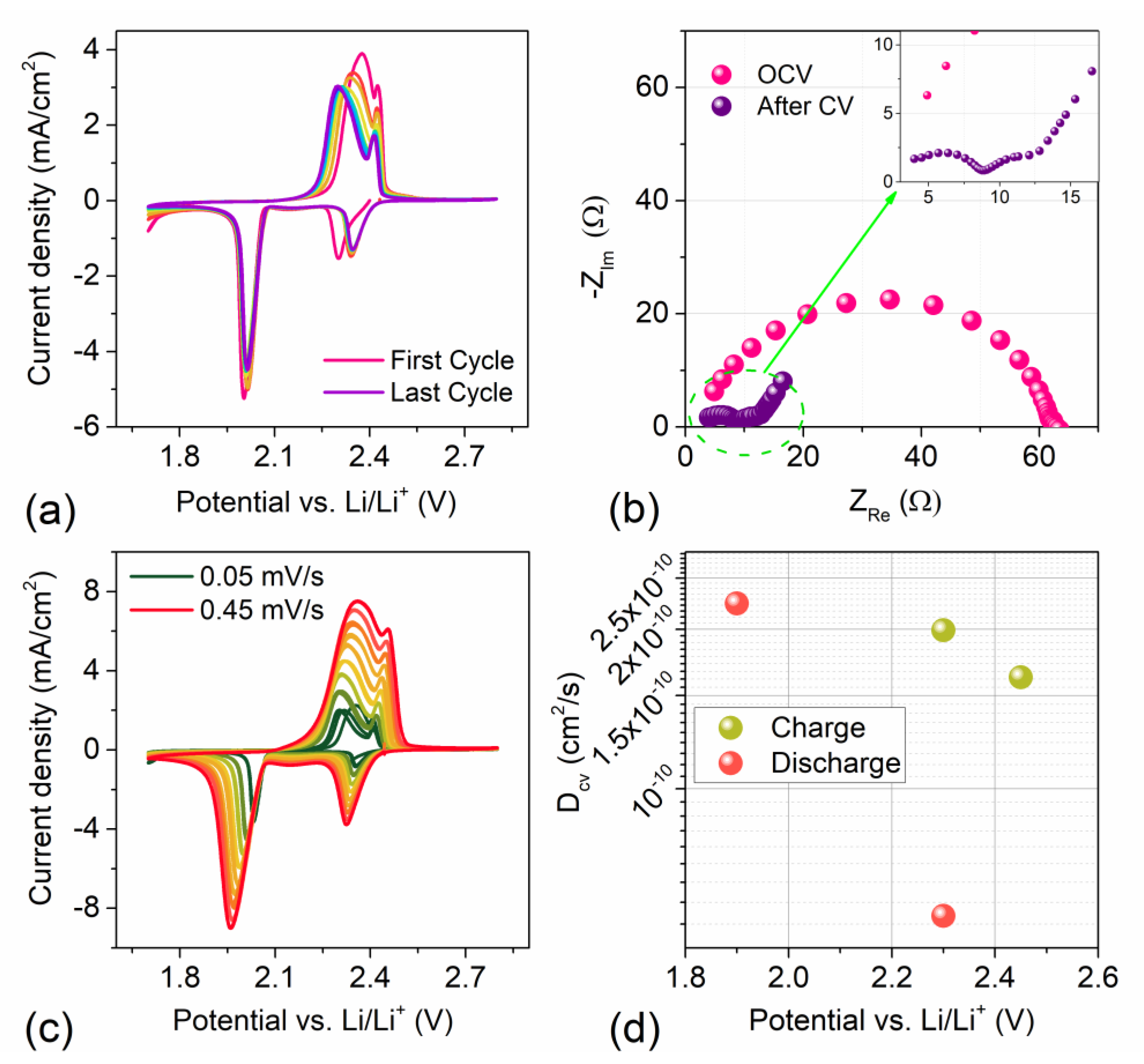
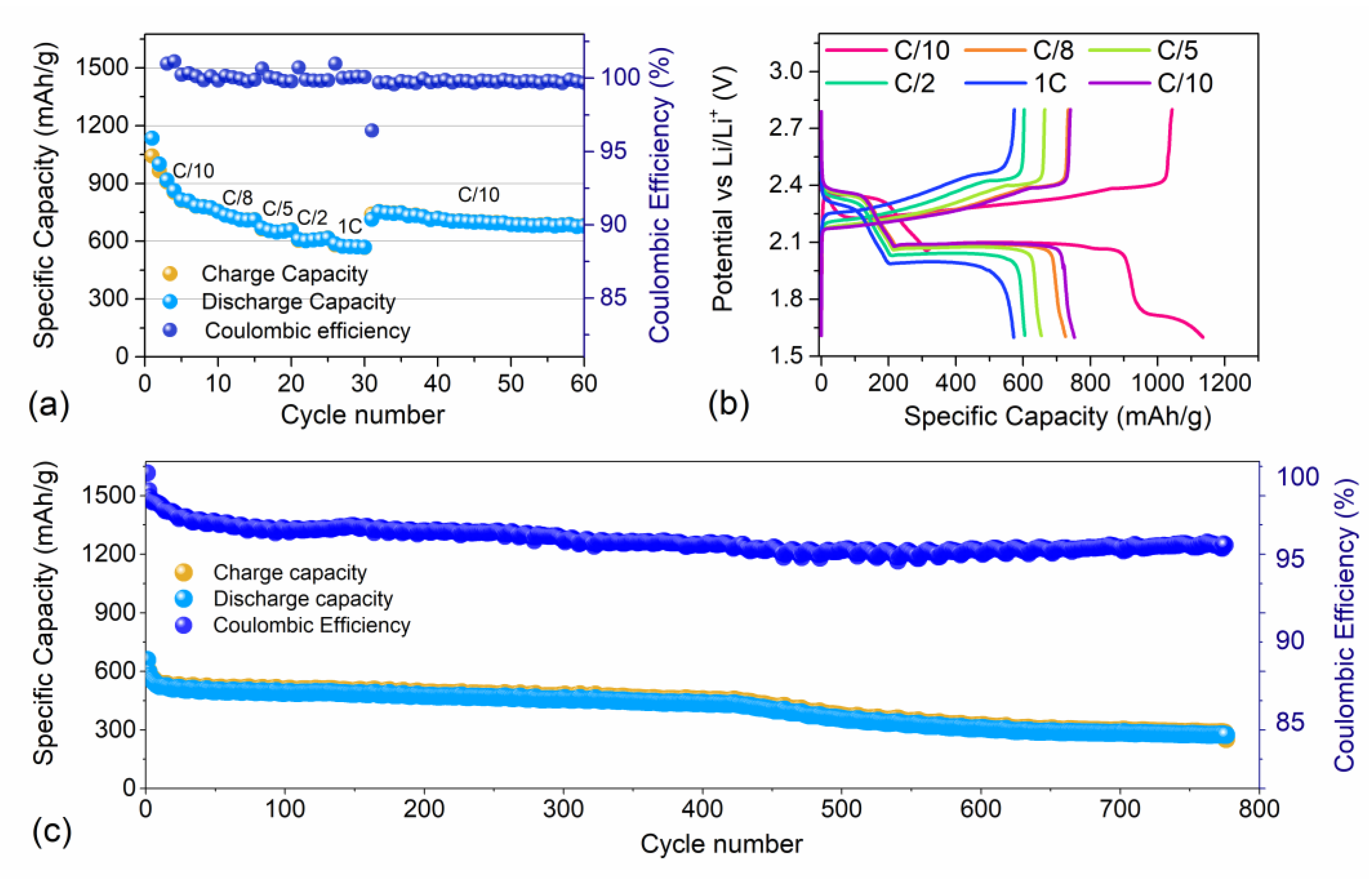
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, C.; Yang, X.; Zeng, P.; Mao, J.; Dai, K.; Zhang, L.; Sun, X. Recent Progress of Functional Separators with Catalytic Effects for High-Performance Lithium-Sulfur Batteries. Nano Energy 2021, 84, 105928. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Liu, X.; Zhang, Y.; Zhao, W.; Li, Y. Journal of Colloid and Interface Science High Specific Surface Area Bimodal Porous Carbon Derived from Biomass Reed Flowers for High Performance Lithium-Sulfur Batteries. J. Colloid Interface Sci. 2020, 569, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Del Rio Castillo, A.E.; Kumar Panda, J.; Pugliese, G.; Scarpellini, A.; Bonaccorso, F.; Pellegrini, V. High-Sulfur-Content Graphene-Based Composite through Ethanol Evaporation for High-Energy Lithium-Sulfur Battery. ChemSusChem 2020, 13, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Structural Design of Lithium–Sulfur Batteries: From Fundamental Research to Practical Application; Springer: Singapore, 2018; Volume 1, ISBN 0123456789. [Google Scholar] [CrossRef] [Green Version]
- Salama, M.; Rosy; Attias, R.; Yemini, R.; Gofer, Y.; Aurbach, D.; Noked, M. Metal-Sulfur Batteries: Overview and Research Methods. ACS Energy Lett. 2019, 4, 436–446. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, J.; Shang, C. N- and S-Doped Ordered Mesoporous Carbon Tubes with Ef Fi Cient S Con Fi Nement for High-Performance Li e S Batteries. Mater. Today Chem. 2022, 24, 100907. [Google Scholar] [CrossRef]
- Li, T.; Bai, X.; Gulzar, U.; Bai, Y.J.; Capiglia, C.; Deng, W.; Zhou, X.; Liu, Z.; Feng, Z.; Zaccaria, R.P. A Comprehensive Understanding of Lithium–Sulfur Battery Technology. Adv. Funct. Mater. 2019, 29, 1901730. [Google Scholar] [CrossRef]
- Schön, P.; Krewer, U. Revealing the Complex Sulfur Reduction Mechanism Using Cyclic Voltammetry Simulation. Electrochim. Acta 2021, 373, 137523. [Google Scholar] [CrossRef]
- Wu, F.; Lee, J.T.; Fan, F.; Nitta, N.; Kim, H.; Zhu, T.; Yushin, G. A Hierarchical Particle-Shell Architecture for Long-Term Cycle Stability of Li2S Cathodes. Adv. Mater. 2015, 27, 5579–5586. [Google Scholar] [CrossRef]
- Benveniste, G.; Sánchez, A.; Rallo, H.; Corchero, C.; Amante, B. Comparative Life Cycle Assessment of Li-Sulphur and Li-Ion Batteries for Electric Vehicles. Resour. Conserv. Recycl. Adv. 2022, 15, 200086. [Google Scholar] [CrossRef]
- Chung, S.H.; Chang, C.H.; Manthiram, A. A Core-Shell Electrode for Dynamically and Statically Stable Li-S Battery Chemistry. Energy Environ. Sci. 2016, 9, 3188–3200. [Google Scholar] [CrossRef]
- Knoop, J.E.; Ahn, S. Recent Advances in Nanomaterials for High-Performance Li—S Batteries. J. Energy Chem. 2020, 47, 86–106. [Google Scholar] [CrossRef]
- Han, X.; Guo, X.; Xu, M.; Pang, H. Clean Utilization of Palm Kernel Shell: Sustainable and Naturally Heteroatom-Doped Porous Activated Carbon for Lithium–Sulfur Batteries. Rare Met. 2020, 39, 1099–1106. [Google Scholar] [CrossRef]
- Fan, X.; Sun, W.; Meng, F.; Xing, A.; Liu, J. Advanced Chemical Strategies for Lithium–Sulfur Batteries: A Review. Green Energy Environ. 2018, 3, 2–19. [Google Scholar] [CrossRef]
- He, Q.; Gorlin, Y.; Patel, M.U.M.; Gasteiger, H.A.; Lu, Y.-C. Unraveling the Correlation between Solvent Properties and Sulfur Redox Behavior in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A4027–A4033. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Chen, W.; Zhang, Y.; Zheng, Y. New Insight into the “Shuttle Mechanism” of Rechargeable Lithium-Sulfur Batteries. ChemElectroChem 2019, 6, 2782–2787. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. High-Performance Lithium-Sulfur Batteries with a Self-Supported, 3D Li2S-Doped Graphene Aerogel Cathodes. Adv. Energy Mater. 2016, 6, 1501355. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Zhang, S.; Liang, G.; Kang, F.; Chen, G.; Li, B. Fe3O4-Decorated Porous Graphene Interlayer for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 26264–26273. [Google Scholar] [CrossRef]
- Gupta, A.; Bhargav, A.; Manthiram, A. Highly Solvating Electrolytes for Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1803096. [Google Scholar] [CrossRef]
- McOwen, D.W.; Seo, D.M.; Borodin, O.; Vatamanu, J.; Boyle, P.D.; Henderson, W.A. Concentrated Electrolytes: Decrypting Electrolyte Properties and Reassessing Al Corrosion Mechanisms. Energy Environ. Sci. 2014, 7, 416–426. [Google Scholar] [CrossRef]
- Cho, S.H.; Cho, S.M.; Bae, K.Y.; Kim, B.H.; Son, B.D.; Yoon, W.Y. Improving Electrochemical Properties of Lithium–Sulfur Batteries by Adding a Catalyst-Embedded Interlayer. Electrochim. Acta 2019, 315, 33–40. [Google Scholar] [CrossRef]
- Quay, Y.J.; Chung, S.H. Structural and Surfacial Modification of Carbon Nanofoam as an Interlayer for Electrochemically Stable Lithium-Sulfur Cells. Nanomaterials 2021, 11, 3342. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cao, B.; Zhao, F.; Zhang, L.; Qu, Y.; Chen, Y. Electrochimica Acta A Mulberry-like Hollow Carbon Cluster Decorated by Al-Doped ZnO Particles for Advanced Lithium-Sulfur Cathode. Electrochim. Acta 2019, 304, 62–69. [Google Scholar] [CrossRef]
- Niu, S.; Hu, C.; Liu, Y.; Zhao, Y.; Yin, F. Nanoporous Co and N-Codoped Carbon Composite Derived from Zif-67 for High-Performance Lithium-Sulfur Batteries. Nanomaterials 2021, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Hwa, Y.; Cairns, E.J. Nanostructured Sulfur and Sulfides for Advanced Lithium/Sulfur Cells. ChemElectroChem 2020, 7, 3927–3942. [Google Scholar] [CrossRef]
- Weret, M.A.; Su, W.N.; Hwang, B.J. Strategies towards High Performance Lithium-Sulfur Batteries. Batter. Supercaps 2022, 5, e202200059. [Google Scholar] [CrossRef]
- Fan, K.; Huang, H. Two-Dimensional Host Materials for Lithium-Sulfur Batteries: A Review and Perspective. Energy Storage Mater. 2022, 50, 696–717. [Google Scholar] [CrossRef]
- Liang, X.; Nazar, L.F. In Situ Reactive Assembly of Scalable Core-Shell Sulfur-MnO2 Composite Cathodes. ACS Nano 2016, 10, 4192–4198. [Google Scholar] [CrossRef]
- Doñoro, Á.; Muñoz-Mauricio, Á.; Etacheri, V. High-Performance Lithium Sulfur Batteries Based on Multidimensional Graphene-CNT-Nanosulfur Hybrid Cathodes. Batteries 2021, 7, 26. [Google Scholar] [CrossRef]
- Ma, Z.; Jing, F.; Fan, Y.; Li, J.; Zhao, Y.; Shao, G. High Electrical Conductivity of 3D Mesporous Carbon Nanocage as an Efficient Polysulfide Buffer Layer for High Sulfur Utilization in Lithium-Sulfur Batteries. J. Alloys Compd. 2019, 789, 71–79. [Google Scholar] [CrossRef]
- Laverde, J.; Rosero-Navarro, N.C.; Miura, A.; Buitrago-Sierra, R.; Tadanaga, K.; López, D. Impact of Sulfur Infiltration Time and Its Content in an N-Doped Mesoporous Carbon for Application in Li-S Batteries. Batteries 2022, 8, 58. [Google Scholar] [CrossRef]
- Kang, H.J.; Rafiqul Bari, G.A.K.M.; Lee, T.G.; Khan, T.T.; Park, J.W.; Hwang, H.J.; Cho, S.Y.; Jun, Y.S. Microporous Carbon Nanoparticles for Lithium-Sulfur Batteries. Nanomaterials 2020, 10, 2012. [Google Scholar] [CrossRef]
- Zhou, K.; Fan, X.J.; Wei, X.F.; Liu, J.H. The Strategies of Advanced Cathode Composites for Lithium-Sulfur Batteries. Sci. China Technol. Sci. 2017, 60, 175–185. [Google Scholar] [CrossRef]
- Rajkumar, P.; Diwakar, K.; Subadevi, R.; Gnanamuthu, R.M.; Sivakumar, M. Sulfur Cloaked with Different Carbonaceous Materials for High Performance Lithium Sulfur Batteries. Curr. Appl. Phys. 2019, 19, 902–909. [Google Scholar] [CrossRef]
- Jeoun, Y.; Kim, M.; Lee, S.; Hyun, J.; Sung, Y.; Yu, S. Lean-Electrolyte Lithium-Sulfur Batteries: Recent Advances in the Design of Cell Components. Chem. Eng. J. 2022, 450, 138209. [Google Scholar] [CrossRef]
- Han, L.; Li, Z.; Feng, Y.; Wang, L.; Li, B.; Lei, Z.; Wang, W.; Huang, W. Biomass-Derived Carbon/Sulfur Composite Cathodes with Multiwalled Carbon Nanotube Coatings for Li-S Batteries. Processes 2022, 10, 136. [Google Scholar] [CrossRef]
- Bandow, S.; Kokai, F.; Takahashi, K.; Yudasaka, M.; Qin, L.C.; Iijima, S. Interlayer Spacing Anomaly of Single-Wall Carbon Nanohorn Aggregate. Chem. Phys. Lett. 2000, 321, 514–519. [Google Scholar] [CrossRef]
- Kasuya, D.; Yudasaka, M.; Takahashi, K.; Kokai, F.; Iijima, S. Selective Production of Single-Wall Carbon Nanohorn Aggregates and Their Formation Mechanism. J. Phys. Chem. B 2002, 106, 4947–4951. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Synthesis of Single-Wall Carbon Nanohorns by Arc-Discharge in Air and Their Formation Mechanism. Carbon 2010, 48, 1580–1585. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M. Nano-Aggregates of Single-Walled Graphitic Carbon Nano-Horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Takikawa, H.; Ikeda, M.; Hirahara, K.; Hibi, Y.; Tao, Y.; Ruiz, P.A.; Sakakibara, T.; Itoh, S.; Iijima, S. Fabrication of Single-Walled Carbon Nanotubes and Nanohorns by Means of a Torch Arc in Open Air. Phys. B Condens. Matter 2002, 323, 277–279. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Y.; Wu, C.; Guan, L. Single-Walled Carbon Nanohorns with Unique Horn-Shaped Structures as a Scaffold for Lithium-Sulfur Batteries. RSC Adv. 2014, 4, 28636–28639. [Google Scholar] [CrossRef]
- Gulzar, U.; Li, T.; Bai, X.; Colombo, M.; Ansaldo, A.; Marras, S.; Prato, M.; Goriparti, S.; Capiglia, C.; Zaccaria, R.P. Nitrogen-Doped Single-Walled Carbon Nanohorns as a Cost-Effective Carbon Host toward High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5551–5559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Ding, Y.; Guan, L. Single-Walled Carbon Nanohorns Coated with Fe2O3 as a Superior Anode Material for Lithium Ion Batteries. Chem. Commun. 2011, 47, 7416–7418. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Z.; Xu, J.; Zhao, Y. Therapeutic Applications of Low-Toxicity Spherical Nanocarbon Materials. NPG Asia Mater. 2014, 6, e84. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, G. Single-Walled Carbon Nanohorns and Their Applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef]
- Nan, Y.; He, Y.; Zhang, Z.; Wei, J.; Zhang, Y. Controllable Synthesis of N-Doped Carbon Nanohorns: Tip from Closed to Half-Closed, Used as Efficient Electrocatalysts for Oxygen Evolution Reaction. RSC Adv. 2021, 11, 35463–35471. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, S.; Wang, C.; Li, J.; Xu, G. Single-Walled Carbon Nanohorns for Energy Applications. Nanomaterials 2015, 5, 1732–1755. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Choi, W.; Shin, H.C.; Choi, J.Y.; Kim, J.M.; Park, M.S.; Yoon, W.S. (How to Read CV for Batteries) Applications of Voltammetry in Lithium Ion Battery Research. J. Electrochem. Sci. Technol. 2020, 11, 14–25. [Google Scholar] [CrossRef]
- BOUKAMP, B. A Nonlinear Least Squares Fit Procedure for Analysis of Immittance Data of Electrochemical Systems. Solid State Ionics 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Sun, Y.; Wu, Y.; Li, J.; Huang, W.; Shi, K.; Lin, Y.; Dong, H.; Liu, Q. Yolk-Double Shells Hierarchical N-Doped Carbon Nanosphere as an Electrochemical Nanoreactor for High Performance Lithium-Sulfur Batteries. Carbon 2022, 198, 80–90. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Wang, Z.; Zhang, Y.; Tan, T.; Wang, G.; Bakenov, Z. Three-Dimensionally Hierarchical Graphene Based Aerogel Encapsulated Sulfur as Cathode for Lithium/Sulfur Batteries. Nanomaterials 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Gorlin, Y.; Patel, M.U.M.; Freiberg, A.; He, Q.; Piana, M.; Tromp, M.; Gasteiger, H.A. Understanding the Charging Mechanism of Lithium-Sulfur Batteries Using Spatially Resolved Operando X-Ray Absorption Spectroscopy. J. Electrochem. Soc. 2016, 163, A930–A939. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.F.; Lau, M.Y.L.; Ong, W.J. Lithium–Sulfur Battery Cathode Design: Tailoring Metal-Based Nanostructures for Robust Polysulfide Adsorption and Catalytic Conversion. Adv. Mater. 2021, 33, 2008654. [Google Scholar] [CrossRef]
- Kaiser, M.R.; Han, Z.; Liang, J.; Dou, S.X.; Wang, J. Lithium Sulfide-Based Cathode for Lithium-Ion/Sulfur Battery: Recent Progress and Challenges. Energy Storage Mater. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Krauss, F.T.; Pantenburg, I.; Roling, B. Transport of Ions, Molecules, and Electrons across the Solid Electrolyte Interphase: What Is Our Current Level of Understanding? Adv. Mater. Interfaces 2022, 9, 2101891. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Clark, J.A.; Soliman, J.; Derr, J.B.; Morales, M.; Vullev, V.I. Practical Aspects of Cyclic Voltammetry: How to Estimate Reduction Potentials When Irreversibility Prevails. J. Electrochem. Soc. 2019, 166, H3175–H3187. [Google Scholar] [CrossRef]
- Di Lecce, D.; Brescia, R.; Scarpellini, A.; Prato, M.; Hassoun, J. A High Voltage Olivine Cathode for Application in Lithium-Ion Batteries. ChemSusChem 2016, 9, 223–230. [Google Scholar] [CrossRef]
- Huang, X.; Luo, B.; Knibbe, R.; Hu, H.; Lyu, M.; Xiao, M.; Sun, D.; Wang, S.; Wang, L. An Integrated Strategy towards Enhanced Performance of the Lithium–Sulfur Battery and Its Fading Mechanism. Chem. A Eur. J. 2018, 24, 18544–18550. [Google Scholar] [CrossRef]
- Pozio, A.; Di Carli, M.; Aurora, A.; Falconieri, M.; Della Seta, L.; Prosini, P.P. Hard Carbons for Use as Electrodes in Li-S and Li-Ion Batteries. Nanomaterials 2022, 12, 1349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X.; Li, X.Y.; Li, B.Q.; Huang, J.Q. An Organodiselenide Comediator to Facilitate Sulfur Redox Kinetics in Lithium–Sulfur Batteries. Adv. Mater. 2021, 33, e2007298. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, B.; Su, H.; Jiang, C.; Li, X.; Qiao, S.; Zhang, H. Co9S8 Nanotube Wrapped with Graphene Oxide as Sulfur Hosts with Ultra-High Sulfur Content for Lithium-Sulfur Battery. Ceram. Int. 2021, 47, 2686–2693. [Google Scholar] [CrossRef]
- Liang, X.; Yun, J.; Xu, K.; Xiang, H.; Wang, Y.; Sun, Y.; Yu, Y. A Multi-Layered Ti3C2/Li2S Composite as Cathode Material for Advanced Lithium-Sulfur Batteries. J. Energy Chem. 2019, 39, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Choi, Y.; Jeong, E.D.; Lee, S.; Kim, H.G.; Chung, J.M.; Kim, J.; Lee, S.; Bae, J. Synthesis and Electrochemical Performance of Microporous Hollow Carbon from Milkweed Pappus as Cathode Material of Lithium–Sulfur Batteries. Nanomaterials 2022, 12, 3605. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, J.; Yang, X.; Liu, M.; Chang, J.; Shao, Y.; Liu, B. Mass Production of 3d Connective Graphene Networks by Fluidized Bed Chemical Vapor Deposition and Its Application in High Performance Lithium–Sulfur Battery. Nanomaterials 2022, 12, 150. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chung, C.H.; Hsieh, T.H.; Lin, H.P. Green and Highly-Efficient Microwave Synthesis Route for Sulfur/Carbon Composite for Li-s Battery. Int. J. Mol. Sci. 2022, 23, 39. [Google Scholar] [CrossRef]
- Kim, E.; Lee, A.S.; Lee, T.; Seo, H.J.; Chae, S.; Kim, K.; Park, J.W.; Lee, S.G.; Lee, J.H. Organic Dye-Derived n, s Co-Doped Porous Carbon Hosts for Effective Lithium Polysulfide Confinement in Lithium–Sulfur Batteries. Nanomaterials 2021, 11, 2954. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.H.; Wang, Z.L.; Wang, A.B.; Zhang, H.; Wang, W.K.; Jin, Z.Q.; Fan, L.Z. Synergistic Adsorption-Catalytic Sites Tin/Ta2 O5 with Multidimensional Carbon Structure to Enable High-Performance Li-s Batteries. Nanomaterials 2021, 11, 2882. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Zhao, Y.; Zeng, Q.; Zhou, G.; Jin, M. Three-Dimensionally Ordered Macro/Mesoporous Nb2O5/Nb4N5 Heterostructure as Sulfur Host for High-Performance Lithium/Sulfur Batteries. Nanomaterials 2021, 11, 1531. [Google Scholar] [CrossRef]
- Benítez, A.; Amaro-Gahete, J.; Esquivel, D.; Romero-Salguero, F.J.; Morales, J.; Caballero, Á. MIL-88A Metal-Organic Framework as a Stable Sulfur-Host Cathode for Long-Cycle Li-S Batteries. Nanomaterials 2020, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; Liu, J.; Gong, Z.; Wang, S.; Li, X.; Yuan, J.; Mei, X.; Luo, Z. Graphene Coated and Nitrogen-Rich Porous Carbon Composites with Sulfur Encapusulation as Cathode for Lithium-Sulfur Batteries. Mater. Lett. 2022, 316, 132050. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Tan, L.; Zhou, Q.; Zhang, J.; Hou, P.; Jin, X.; Lv, T.; Zhao, Z.; Zeng, Z.; et al. Fabrication of Cubic and Porous Carbon Cages with In-Situ-Grown Carbon Nanotube Networks and Cobalt Phosphide for High-Capacity and Stable Lithium-Sulfur Batteries. ACS Sustain. Chem. Eng. 2022, 10, 10223–10233. [Google Scholar] [CrossRef]
- Kyu, D.; Won, C.; Lee, J.W. Electrochimica Acta Electrostatic Self-Assembly of 2-Dimensional MXene-Wrapped Sulfur Composites for Enhancing Cycle Performance of Lithium–Sulfur Batteries. Electrochim. Acta 2022, 402, 139539. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Bao, X.; Qing, C.; Zhu, T.; Wang, H. Macro / Mesoporous Carbon / Defective TiO2 Composite as a Functional Host for Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2022, 5, 2573–2579. [Google Scholar] [CrossRef]
- Fan, X.; Chen, R.; Lin, Y.; Chen, F.; Li, L.; Ye, B.; Yang, K.; Zhan, L.; Zhang, Y. Oxygen-Defective MnO2 Decorated Carbon Nanotube as an Effective Sulfur Host for High Performance Lithium Sulfur Battery. Adv. Powder Technol. 2022, 33, 103396. [Google Scholar] [CrossRef]

| Cathode | Initial Discharge Capacity (mAh/g1) at Low C-Rate | Cycle Life (Cycles) | C-Rate | Specific Discharge Capacity after Cycling (mAh/g) | Reference |
|---|---|---|---|---|---|
| S80SWCNH20 | 1135 | 775 | C/4 | 300 | This work |
| Ti3C2Tx@S | ~1250 | 200 | C/2 | 429 | [65] |
| MPC–6S | 931 | 400 | C/2 | ~200 | [66] |
| Biomass-derived carbon/sulfur | 1067 | 500 | C/10 | 254 | [37] |
| 3DGNs/S | 790 | 280 | 1C | ~400 | [67] |
| S/MPC | ~1600 | 70 | 0.2 A·g−1 | ~500 | [68] |
| Sulfur/MBGO20 | ~900 | 100 | C/5 | ~400 | [69] |
| Ta2O5@C/S | ~1250 | 300 | C/2 | ~400 | [70] |
| S/ZIF-67 | 968 | 1000 | 1C | 237 | [25] |
| S-Nb4N5 | ~1200 | 400 | 1C | ~300 | [71] |
| MIL-88A@S | 600 | 1000 | C/2 | ~300 | [72] |
| S/RGO@NPC | ~1200 | 400 | C/2 | 600 | [73] |
| CoP-CNT@C/S | 1457 | 750 | C/2 | 474 | [74] |
| S@d-MXene | 769 | 500 | 1C | 506 | [75] |
| MMC@S | 1124 | 60 | C/5 | 616 | [76] |
| NT/MnO2-S | 704 | 300 | C/2 | 429 | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venezia, E.; Salimi, P.; Chauque, S.; Proietti Zaccaria, R. Sustainable Synthesis of Sulfur-Single Walled Carbon Nanohorns Composite for Long Cycle Life Lithium-Sulfur Battery. Nanomaterials 2022, 12, 3933. https://doi.org/10.3390/nano12223933
Venezia E, Salimi P, Chauque S, Proietti Zaccaria R. Sustainable Synthesis of Sulfur-Single Walled Carbon Nanohorns Composite for Long Cycle Life Lithium-Sulfur Battery. Nanomaterials. 2022; 12(22):3933. https://doi.org/10.3390/nano12223933
Chicago/Turabian StyleVenezia, Eleonora, Pejman Salimi, Susana Chauque, and Remo Proietti Zaccaria. 2022. "Sustainable Synthesis of Sulfur-Single Walled Carbon Nanohorns Composite for Long Cycle Life Lithium-Sulfur Battery" Nanomaterials 12, no. 22: 3933. https://doi.org/10.3390/nano12223933






