Development and Efficacy Evaluation of a Novel Nano-Emulsion Adjuvant for a Foot-and-Mouth Disease Virus-like Particles Vaccine Based on Squalane
Abstract
:1. Introduction
2. Materials and Methods
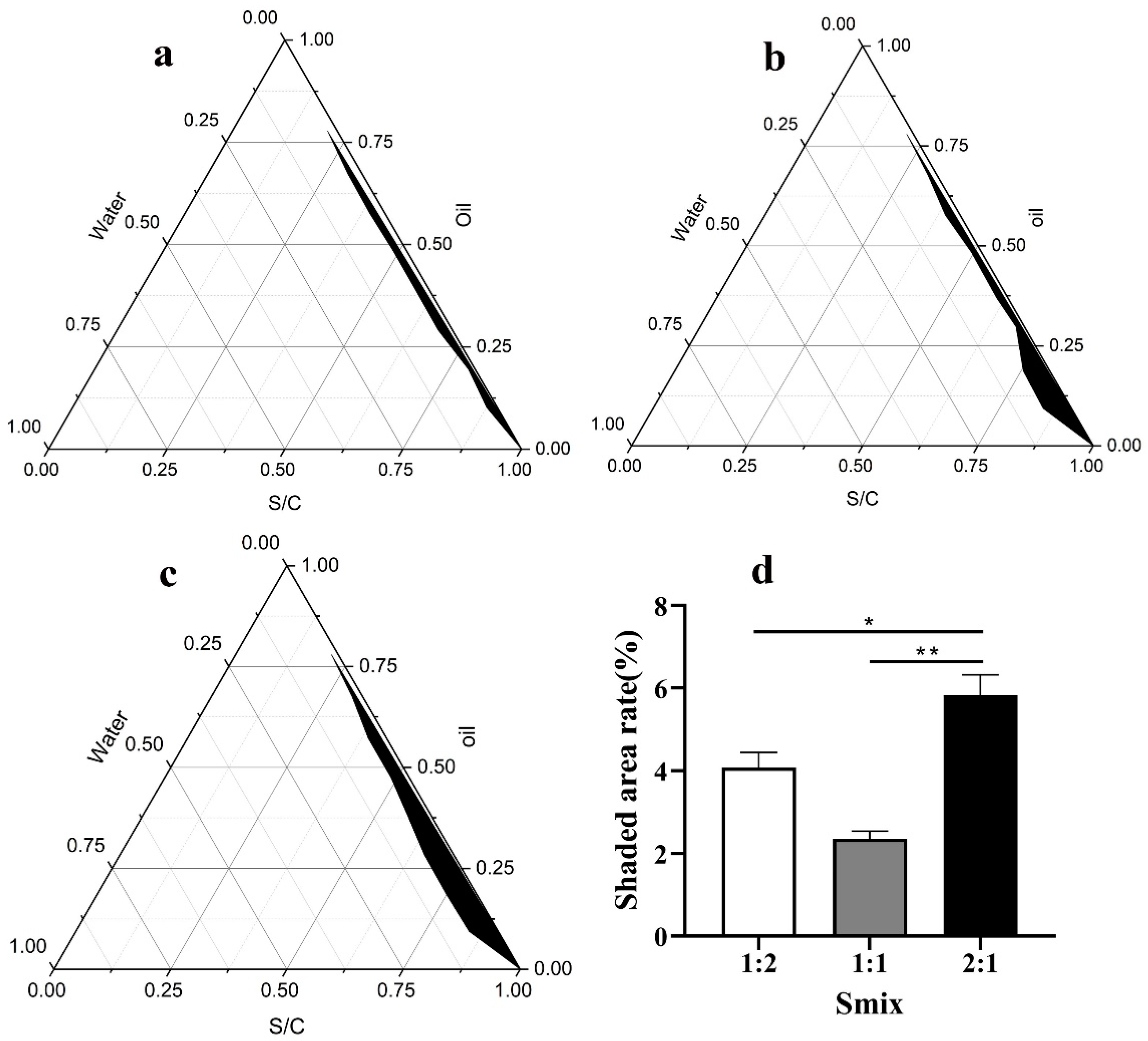
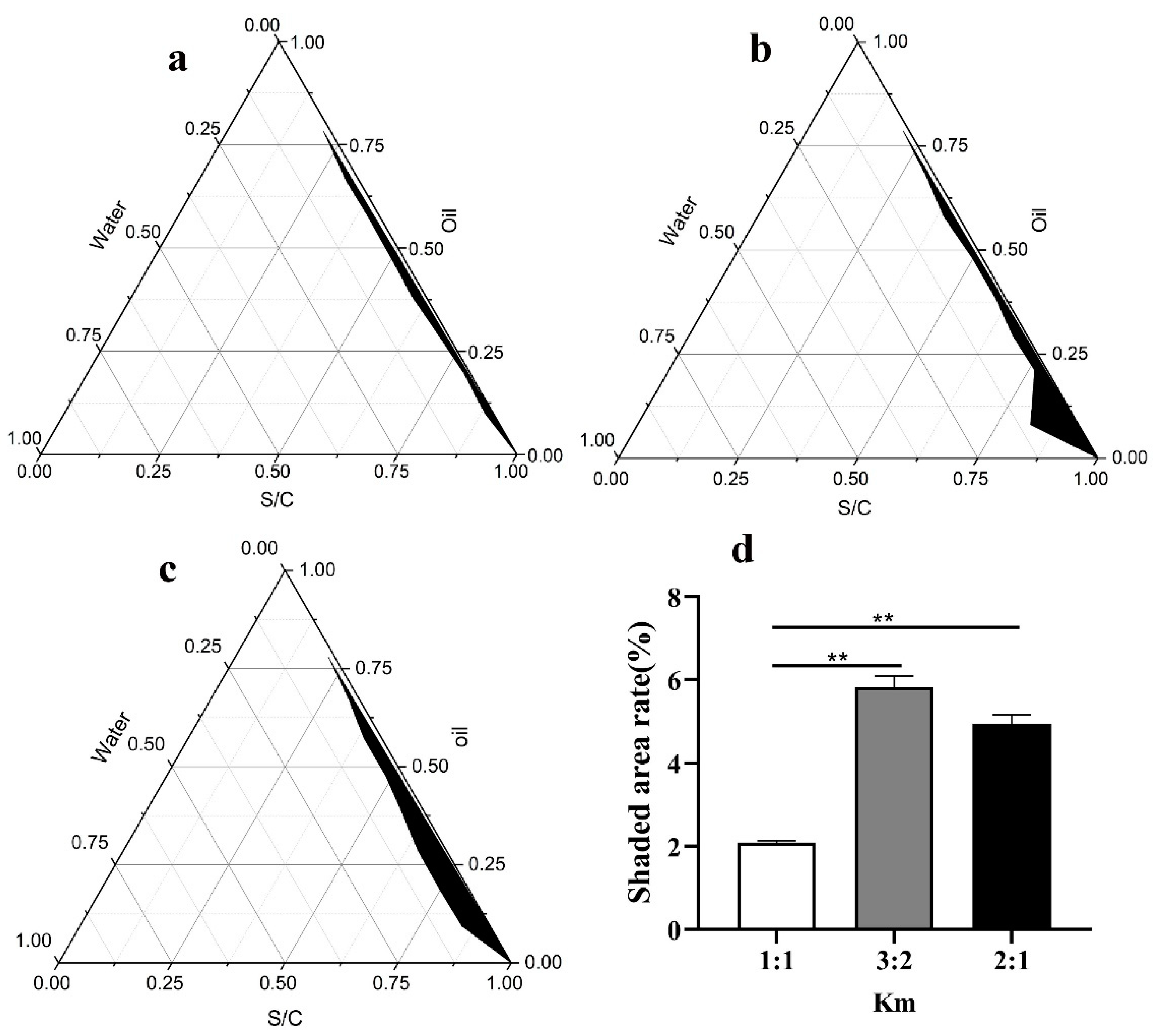
2.1. Selection of the Surfactant and Cosurfactant
2.2. Preparation of the SNA
2.3. Characterization of the SNA
2.4. Stability Assessment of the SNA
2.4.1. High-Speed Centrifuge Stability and Thermodynamic Stability
2.4.2. Long-Term Stability Test
2.5. Biocompatibility Evaluation of the SNA
2.5.1. Tissue Toxicity
2.5.2. Cytotoxicity
2.6. Preparation of the FMD-VLP Vaccine with SNA
2.7. Immunization Studies in BALB/c Female Mice
2.8. Guinea Pig Immunization with FMD-VLP Vaccine with SNA
2.8.1. Animal Vaccination
2.8.2. Detection of Specific Antibodies and Cytokine Levels
2.8.3. Detection of the Neutralizing Antibodies
2.8.4. T-Cell Proliferation Assay
2.8.5. Challenge Protocols
2.9. Statistical Analysis
3. Results
3.1. Preparation of the SNA
3.2. Characterization of the SNA
3.3. Stability of the SNA
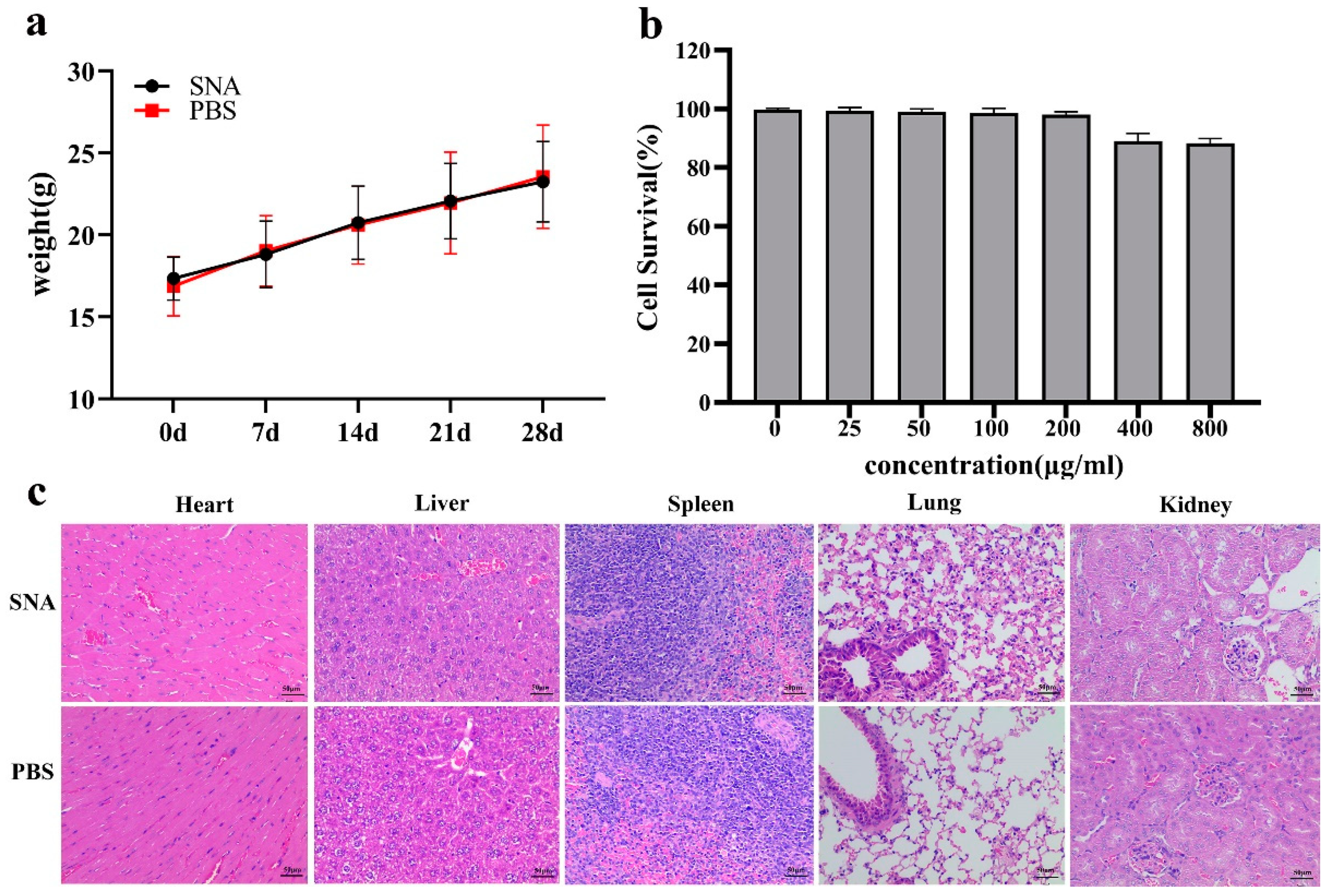
3.4. Safety Evaluation of the SNA
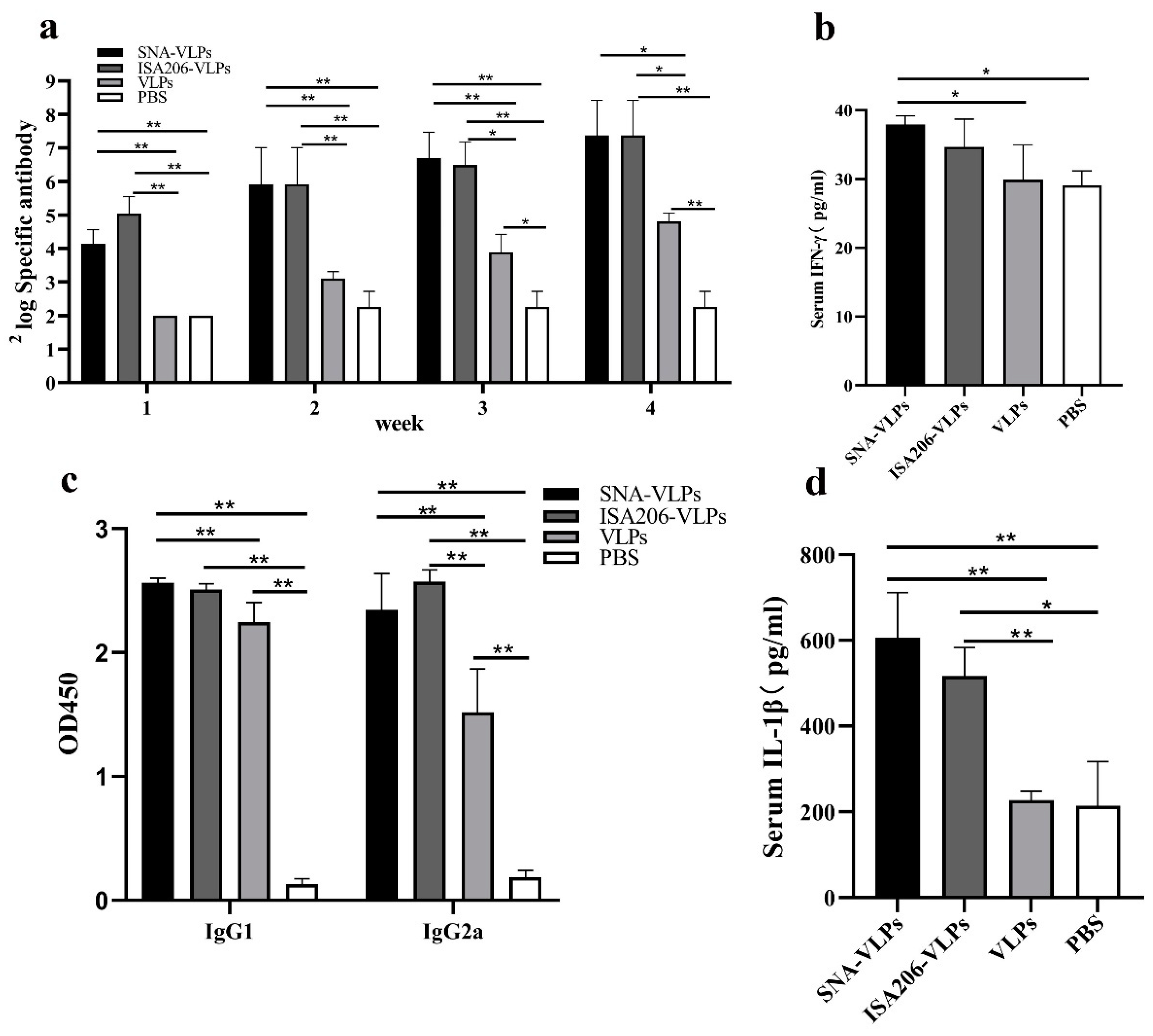
3.5. Effects of the FMDV-VLP Vaccine with SNA on the Antibody Production in BALB/c Mice
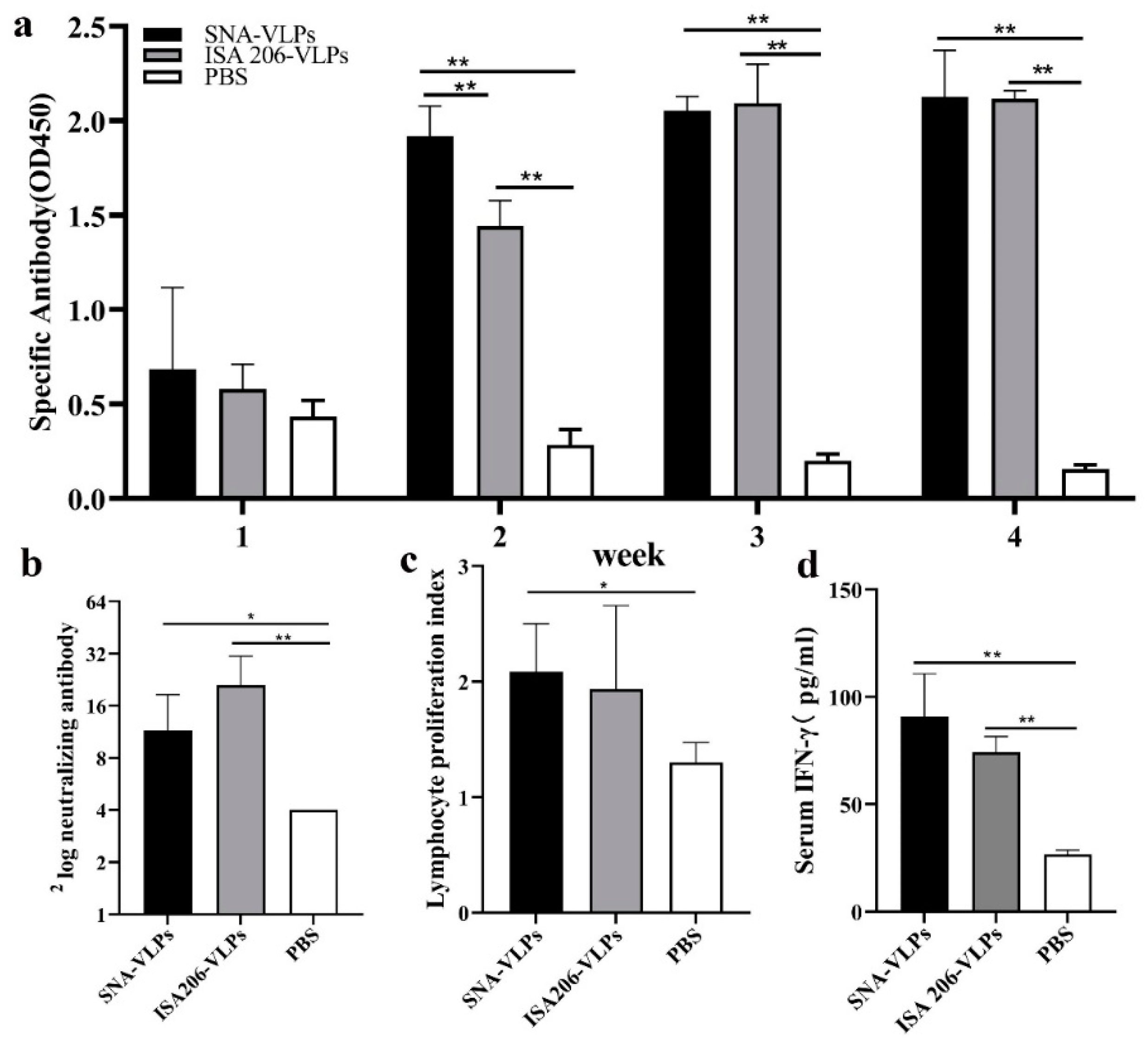
3.6. Evaluation of Immunization Effect after Immunization of Guinea Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, B.D.; Rich, K.M. Poverty impacts of foot-and-mouth disease and the poverty reduction implications of its control. Vet. Record. 2007, 160, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, Z.W.; Lv, J.L.; Zhou, P.; Hu, W.F.; Fang, Y.Z.; Chen, H.T.; Liu, X.S.; Shao, J.J.; Zhao, F.R.; et al. Induction of mucosal immune responses and protection of cattle against direct-contact challenge by intranasal delivery with foot-and-mouth disease virus antigen mediated by nanoparticles. Int. J. Nanomed. 2014, 9, 5603–5618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.M. Adjuvants for foot-and-mouth disease virus vaccines: Recent progress. Expert Rev. Vaccines 2014, 13, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Jiang, X. Subviral particle as vaccine and vaccine platform. Curr. Opin. Virol. 2014, 6, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Qian, C.Y.; Liu, X.L.; Xu, Q.; Wang, Z.P.; Chen, J.; Li, T.T.; Zheng, Q.B.; Yu, H.; Gu, Y.; Li, S.W.; et al. Recent Progress on the Versatility of Virus-Like Particles. Vaccines 2020, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.C.; Sun, S.Q.; Jin, Y.; Yang, S.L.; Wei, Y.Q.; Sun, D.H.; Yin, S.H.; Ma, J.W.; Liu, Z.X.; Guo, J.H.; et al. Foot-and-mouth disease virus-like particles produced by a SUMO fusion protein system in Escherichia coli induce potent protective immune responses in guinea pigs, swine and cattle. Vet. Res. 2013, 44, 48. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.R.; O’Hagan, D.T.; Amiji, M.M.; Brito, L.A. The impact of size on particulate vaccine adjuvants. Nanomedicine 2014, 9, 2671–2681. [Google Scholar] [CrossRef]
- Xing, Q.; Song, J.; You, X.H.; Dong, L.; Wang, K.X.; Song, J.Q.; Guo, Q.; Li, P.Y.; Wu, C.B.; Hu, H.Y. Microemulsions containing long-chain oil ethyl oleate improve the oral bioavailability of piroxicam by increasing drug solubility and lymphatic transportation simultaneously. Int. J. Pharm. 2016, 11, 709–718. [Google Scholar] [CrossRef]
- Popa, O.; Băbeanu, N.E.; Popa, I.; Sultana, N.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. Biomed Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef]
- Schmidt, R.; Holznagel, E.; Neumann, B.; Alex, N.; Sawatsky, B.; Enkirch, T.; Pfeffermann, K.; Kruip, C.; Messling, V.V.; Wagner, R. Squalene-containing licensed adjuvants enhance strain-specific antibody responses against the influenza hemagglutinin and induce subtype-specific antibodies against the neuraminidase. Vaccine 2016, 34, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Contant, P.; Sangster, M.Y.; Topham, D.J. Squalene-Based Influenza Vaccine Adjuvants and Their Impact on the Hemagglutinin-Specific B Cell Response. Pathogens 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Gregor, C.; Ulrich, V. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Sun, H.W.; Liu, K.Y.; Liu, W.; Wang, W.X.; Guo, C.L.; Tang, B.; Gu, J.; Zhang, J.Y.; Li, H.B.; Mao, X.H.; et al. Development and characterization of a novel nanoemulsion drug-delivery system for potential application in oral delivery of protein drugs. Int. J. Nanomed. 2012, 7, 5529–5530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.W.; Wei, C.; Liu, B.S.; Jing, H.M.; Feng, Q.; Tong, Y.N.; Yang, Y.; Yang, L.Y.; Zuo, Q.F.; Zhang, Y.; et al. Induction of systemic and mucosal immunity against methicillin-resistant Staphylococcus aureus infection by a novel nanoemulsion adjuvant vaccine. Int. J. Nanomed. 2015, 10, 7275–7290. [Google Scholar] [CrossRef] [Green Version]
- Myc, A.; Kukowska-Latallo, J.F.; Bielinska, A.U.; Cao, P.; Myc, P.P.; Janczak, K.; Sturm, T.R.; Grabinski, M.S.; Landers, J.J.; Young, S.K.; et al. Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemulsion. Vaccine 2003, 21, 3801–3814. [Google Scholar] [CrossRef]
- Teng, Z.D.; Sun, S.Q.; Luo, X.; Zhang, Z.H.; Seo, H.; Xu, X.Y.; Huang, J.; Dong, H.; Mu, S.Y.; Du, P.; et al. Bi-functional gold nanocages enhance specific immunological responses of foot-and-mouth disease virus-like particles vaccine as a carrier and adjuvant. Nanomedicine 2021, 33, 102358. [Google Scholar] [CrossRef]
- Teng, Z.D.; Sun, S.Q.; Chen, H.; Huang, J.; Du, P.; Dong, H.; Xu, X.Y.; Mu, S.Y.; Zhang, Z.J.; Guo, C.H. Golden-star nanoparticles as adjuvant effectively promotes immune response to foot-and-mouth disease virus-like particles vaccine. Vaccine 2018, 36, 6752–6760. [Google Scholar] [CrossRef]
- Bidart, J.; Mignaqui, A.; Kornuta, C.; Lupi, G.; Gammella, M.; Soria, I.; Galarza, R.; Ferella, A.; Cardillo, S.; Langellotti, C.; et al. FMD empty capsids combined with the Immunostant Particle Adjuvant -ISPA or ISA206 induce protective immunity against foot and mouth disease virus. Virus. Res. 2021, 297, 198339. [Google Scholar] [CrossRef]
- Suresh Kumar, R.S.; Shiny, P.J.; Anjali, C.H.; Jerobin, J.; Goshen, K.M.; Mukherjee, A.; Mukherjee, A.; Chandrasekaran, N. Distinctive effects of nano-sized permethrin in the environment. Environ. Sci. Pollut. Res. Int. 2013, 20, 2593–2602. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, G.L.; Wu, J.R.; Zhang, Y.L.; Liu, J.; Luo, H.Y.; Shao, L.Q. Insights into the angiogenic effects of nanomaterials: Mechanisms involved and potential applications. J. Nanobiotechnol. 2020, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Simon, J.K.; Baker, J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Joyappa, D.H.; Kumar, C.A.; Banumathi, N.; Reddy, G.R.; Suryanarayana, V.V.S. Calcium phosphate nanoparticle prepared with foot and mouth disease virus P1-3CD gene construct protects mice and guinea pigs against the challenge virus. Vet. Microbiol. 2009, 139, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Huang, C.Y.; Lin, S.C.; Chen, J.H.; Ku, C.C.; Chou, A.H.; Liu, S.J.; Chen, H.W.; Chong, P.; Leng, C.H. Enhancement of potent antibody and T-cell responses by a single-dose, novel nanoemulsion-formulated pandemic influenza vaccine. Microbes Infect. 2009, 11, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Taccone, M.; Monaci, E.; Brito, L.A.; Bonci, A.; O’Hagan, D.T.; Amiji, M.M.; Seubert, A. The droplet size of emulsion adjuvants has significant impact on their potency, due to differences in immune cell-recruitment and activation. Sci. Rep. 2019, 9, 11520. [Google Scholar] [CrossRef] [Green Version]
- Anjali, C.H.; Sharma, Y.; Mukherjee, A.; Chandrasekaran, N. Neem oil (Azadirachta indica) nanoemulsion-a potent larvicidal agent against Culex quinquefasciatus. Pest Manag. Sci. 2012, 68, 158–163. [Google Scholar] [CrossRef]
- Kumar, S.; Chaturvedi, V.K.; Kumar, B.; Kumar, P.; Sudha Rani Somarajan, S.R.; Mishra, A.K.; Sharma, B. Effect of alum co-adjuvantation of oil adjuvant vaccine on emulsion stability and immune responses against haemorhagic septicaemia in mice. Iran J. Microbiol. 2015, 7, 79–87. [Google Scholar]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Iho, S.; Maeyama, J.; Suzuki, F. CpG oligodeoxynucleotides as mucosal adjuvants. Hum. Vaccine Immunother. 2015, 11, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Kubo, S.; Kobayashi, M.; Masunaga, Y.; Ishii, H.; Hirano, Y.; Takahashi, K.; Shimizu, Y. Cytokine and chemokine expression in cigarette smoke-induced lung injury in Guinea pigs. Eur. Respir. J. 2005, 26, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.B.; Pan, L.; Lv, J.L.; Zhang, Z.W.; Wang, Y.Y.; Hu, W.F.; Liu, X.S.; Zhou, P.; Wang, Y.L.; Zhang, Y.G. Comparison of immune responses in guinea pigs by intranasal delivery with different nanoparticles-loaded FMDV DNA vaccine. Microb. Pathog. 2020, 142, 104061. [Google Scholar] [CrossRef] [PubMed]
- Galarza, J.M.; Latham, T.; Cupo, A. Virus-like particle vaccine conferred complete protection against a lethal influenza virus challenge. Viral. Immunol. 2015, 18, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, D.; Ramya, K.; Nuru, S.; Bedaso, M.; Subodh, K.; Ganesh, K.; Kondabattula, G. Montanide ISATM 201 adjuvanted FMD vaccine induces improved immune responses and protection in cattle. Vaccine 2013, 31, 3327–3332. [Google Scholar] [CrossRef]

| Group | Guinea Pigs Number | Protection | Rate of Protection (%) |
|---|---|---|---|
| FMD VLPs-SNA | 6 | 5 | 83.3% (5/6) |
| FMD VLPs-ISA206 | 6 | 5 | 83.3% (5/6) |
| PBS | 6 | 0 | 0 (0/6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Yang, K.; Song, H.; Teng, Z.; Zhang, Y.; Ding, W.; Wang, A.; Tan, S.; Dong, H.; Sun, S.; et al. Development and Efficacy Evaluation of a Novel Nano-Emulsion Adjuvant for a Foot-and-Mouth Disease Virus-like Particles Vaccine Based on Squalane. Nanomaterials 2022, 12, 3934. https://doi.org/10.3390/nano12223934
Shi X, Yang K, Song H, Teng Z, Zhang Y, Ding W, Wang A, Tan S, Dong H, Sun S, et al. Development and Efficacy Evaluation of a Novel Nano-Emulsion Adjuvant for a Foot-and-Mouth Disease Virus-like Particles Vaccine Based on Squalane. Nanomaterials. 2022; 12(22):3934. https://doi.org/10.3390/nano12223934
Chicago/Turabian StyleShi, Xiaoni, Kun Yang, Hetao Song, Zhidong Teng, Yun Zhang, Weihao Ding, Aofei Wang, Shuzhen Tan, Hu Dong, Shiqi Sun, and et al. 2022. "Development and Efficacy Evaluation of a Novel Nano-Emulsion Adjuvant for a Foot-and-Mouth Disease Virus-like Particles Vaccine Based on Squalane" Nanomaterials 12, no. 22: 3934. https://doi.org/10.3390/nano12223934






