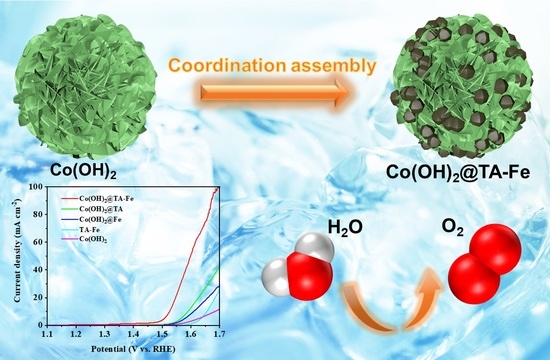
Coupling Plant Polyphenol Coordination Assembly with Co(OH)2 to Enhance Electrocatalytic Performance towards Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Co(OH)2
2.3. Synthesis of Co(OH)2@TA-Fe
2.4. Synthesis of Co(OH)2@TA, Co(OH)2@Fe and TA-Fe
2.5. Materials Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.J.; Kim, H.Y.; Joo, J.; Joo, S.H.; Lim, J.S.; Lee, J.; Huang, H.; Shao, M.; Hu, J.; Kim, J.Y.; et al. Recent advances in non-precious group metal-based catalysts for water electrolysis and beyond. J. Mater. Chem. A 2022, 10, 50–88. [Google Scholar] [CrossRef]
- Tang, T.; Ding, L.; Yao, Z.C.; Pan, H.R.; Hu, J.S.; Wan, L.J. Synergistic Electrocatalysts for Alkaline Hydrogen Oxidation and Evolution Reactions. Adv. Funct. Mater. 2021, 32, 2107479. [Google Scholar] [CrossRef]
- Yu, M.; Budiyanto, E.; Tuysuz, H. Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-Based Oxides for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2022, 61, e202103824. [Google Scholar] [PubMed]
- Tavella, F.; Giusi, D.; Ampelli, C. Nitrogen reduction reaction to ammonia at ambient conditions: A short review analysis of the critical factors limiting electrocatalytic performance. Curr. Opin. Green Sustain. Chem. 2022, 35, 100604. [Google Scholar] [CrossRef]
- Tang, W.; Li, B.; Teng, K.; Wang, X.; Liu, R.; Wu, M.; Zhang, L.; Ren, P.; Zhang, J.; Feng, M. Advanced noble-metal-free bifunctional electrocatalysts for metal-air batteries. J. Mater. 2022, 8, 454–474. [Google Scholar] [CrossRef]
- Gao, L.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8469. [Google Scholar] [CrossRef]
- Singh, B.; Yadav, A.; Indra, A. Realizing electrochemical transformation of a metal–organic framework precatalyst into a metal hydroxide–oxy(hydroxide) active catalyst during alkaline water oxidation. J. Mater. Chem. A 2022, 10, 3843–3868. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Liu, S.; Xu, W.; Wu, L.; Hsieh, Y.-C.; Liu, P.; Zhu, Y.; Sasaki, K.; Renner, J.N.; et al. Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@IrO2 core-shell nanocatalysts. J. Electroanal. Chem. 2018, 819, 296–305. [Google Scholar] [CrossRef]
- Escalera-López, D.; Czioska, S.; Geppert, J.; Boubnov, A.; Röse, P.; Saraçi, E.; Krewer, U.; Grunwaldt, J.-D.; Cherevko, S. Phase- and Surface Composition-Dependent Electrochemical Stability of Ir-Ru Nanoparticles during Oxygen Evolution Reaction. ACS Catal. 2021, 11, 9300–9316. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, L.; Hu, Y.; Liu, F.; Liu, Z.; Xue, Y.; Zhang, J.; Liu, H.; Tang, C. Interfacial modification of Co(OH)2/Co3O4 nanosheet heterostructure arrays for the efficient oxygen evolution reaction. Catal. Sci. Technol. 2021, 11, 3706–3714. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Ramakrishnan, S.; Sathiskumar, C.; Yoo, D.J.; Balamurugan, J.; Noh, H.S.; Kwon, D.; Kim, Y.H.; Lee, H. MOF-derived CoP-nitrogen-doped carbon@NiFeP nanoflakes as an efficient and durable electrocatalyst with multiple catalytically active sites for OER, HER, ORR and rechargeable zinc-air batteries. Chem. Eng. J. 2022, 428, 131115. [Google Scholar] [CrossRef]
- Yao, N.; Wang, G.; Jia, H.; Yin, J.; Cong, H.; Chen, S.; Luo, W. Intermolecular Energy Gap-Induced Formation of High-Valent Cobalt Species in CoOOH Surface Layer on Cobalt Sulfides for Efficient Water Oxidation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117178. [Google Scholar] [CrossRef]
- Park, H.; Bae, J.W.; Lee, T.H.; Park, I.J.; Kim, C.; Lee, M.G.; Lee, S.A.; Yang, J.W.; Choi, M.J.; Hong, S.H.; et al. Surface-Tailored Medium Entropy Alloys as Radically Low Overpotential Oxygen Evolution Electrocatalysts. Small 2022, 18, e2105611. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Cai, N.; Wang, M.; Li, H.; Yu, F. High topological tri-metal phosphide of CoP@FeNiP toward enhanced activities in oxygen evolution reaction. Nanoscale 2021, 13, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Peng, D.; Yao, Z.; Xia, J.; Zhang, B.; Liu, H.; Chen, Z.; Wu, F.; Wu, J.; Huang, Y. Cathodic plasma driven self-assembly of HEAs dendrites by pure single FCC FeCoNiMnCu nanoparticles as high efficient electrocatalysts for OER. Chem. Eng. J. 2021, 425, 131533. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Zhang, Y.; Luo, X.; Chen, L.; Shi, Y. N,S-Doped hollow carbon nanosheet-encapsulated Co9S8 nanoparticles as a highly efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Dalton Trans 2022, 51, 12630–12640. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Han, C.; Gao, J.; Pan, L.; Wu, J.; Zhu, X.-D.; Zou, J.-J. NiCo-Based Electrocatalysts for the Alkaline Oxygen Evolution Reaction: A Review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Martinez, E.Y.; Zhu, K.; Li, C.W. Influence of the Defect Stability on n-Type Conductivity in Electron-Doped alpha- and beta-Co(OH)2 Nanosheets. Inorg. Chem. 2021, 60, 6950–6956. [Google Scholar] [CrossRef]
- Huang, C.; Zhong, Y.; Chen, J.; Li, J.; Zhang, W.; Zhou, J.; Zhang, Y.; Yu, L.; Yu, Y. Fe induced nanostructure reorganization and electronic structure modulation over CoNi (oxy)hydroxide nanorod arrays for boosting oxygen evolution reaction. Chem. Eng. J. 2021, 403, 126304. [Google Scholar] [CrossRef]
- McAteer, D.; Godwin, I.J.; Ling, Z.; Harvey, A.; He, L.; Boland, C.S.; Vega-Mayoral, V.; Szydłowska, B.; Rovetta, A.A.; Backes, C.; et al. Liquid Exfoliated Co(OH)2 Nanosheets as Low-Cost, Yet High-Performance, Catalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1702965. [Google Scholar] [CrossRef]
- Gu, L.-F.; Li, C.-F.; Zhao, J.-W.; Xie, L.-J.; Wu, J.-Q.; Ren, Q.; Li, G.-R. Dual modulation of lattice strain and charge polarization induced by Co(OH)2/Ni(OH)2 interfaces for efficient oxygen evolution catalysis. J. Mater. Chem. A 2021, 9, 13279–13287. [Google Scholar] [CrossRef]
- Duraivel, M.; Nagappan, S.; Park, K.H.; Prabakar, K. Hierarchical 3D flower like cobalt hydroxide as an efficient bifunctional electrocatalyst for water splitting. Electrochim. Acta 2022, 411, 140071. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Wang, Z.; Bu, D.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. N and Mn dual-doped cactus-like cobalt oxide nanoarchitecture derived from cobalt carbonate hydroxide as efficient electrocatalysts for oxygen evolution reactions. J. Colloid Interface Sci. 2021, 597, 361–369. [Google Scholar] [CrossRef]
- Du, X.; Guo, J.; Chen, M.; Cheong, W.-C.; Chen, Y.; Liu, D.; Chen, S.; Wang, X.; Lo, K.H.; Hu, J.-S.; et al. Surface reconstruction on silver nanoparticles decorated trimetallic hydroxide nanosheets to generate highly active oxygen-deficient (oxy)hydroxide layer for high-efficient water oxidation. Chem. Eng. J. 2021, 425, 131662. [Google Scholar] [CrossRef]
- Song, X.-Z.; Zhang, N.; Liu, F.; Wang, Z.-H.; Zhu, W.-Y.; Zhang, G.-Z.; Niu, Z.-Y.; Wang, X.-F.; Tan, Z. Spontaneously engineering heterogeneous interface of silver nanoparticles on α-Co(OH)2 for boosting electrochemical oxygen evolution. J. Alloy. Compd. 2021, 873, 159766. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, Y.L.; Zhao, Y.H.; Ni, J.C.; Pan, Y.; Dai, Y.; Tan, Z.; Wang, X.F.; Song, X.Z. Boosting the oxygen evolution electrocatalysis of high-entropy hydroxides by high-valence nickel species regulation. Chem. Commun. 2022, 58, 7682–7685. [Google Scholar] [CrossRef]
- Kitano, S.; Noguchi, T.G.; Nishihara, M.; Kamitani, K.; Sugiyama, T.; Yoshioka, S.; Miwa, T.; Yoshizawa, K.; Staykov, A.; Yamauchi, M. Heterointerface Created on Au-Cluster-Loaded Unilamellar Hydroxide Electrocatalysts as a Highly Active Site for the Oxygen Evolution Reaction. Adv. Mater. 2022, 34, e2110552. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhang, N.; Wang, X.F.; Tan, Z. Recent advances of metal-organic frameworks and their composites toward oxygen evolution electrocatalysis. Mater. Today Energy 2021, 19, 100597. [Google Scholar] [CrossRef]
- Gong, C.; Li, W.; Lei, Y.; He, X.; Chen, H.; Du, X.; Fang, W.; Wang, D.; Zhao, L. Interfacial engineering of ZIF-67 derived CoSe/Co(OH)2 catalysts for efficient overall water splitting. Compos. Part B Eng. 2022, 236, 109823. [Google Scholar] [CrossRef]
- Devi, B.; Koner, R.R.; Kurungot, S. Recent advances in the metal-organic framework-based electrocatalysts for trifunctional electrocatalysis. Dalton Trans. 2022, 51, 13573–13590. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jang, H.; Wang, J.; Wu, Z.; Liu, X.; Cho, J. Cobalt-Tannin-Framework-Derived Amorphous Co-P/Co-N-C on N, P Co-Doped Porous Carbon with Abundant Active Moieties for Efficient Oxygen Reactions and Water Splitting. ChemSusChem 2019, 12, 830–838. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Z.; Zeng, C.; Jiang, J.; Gao, H.; Ai, L. Synergistically boosting oxygen evolution performance of iron-tannic electrocatalyst via localized photothermal effect. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128248. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Zhao, S.; Chen, Q.; Zhang, J. Interfacial coordination assembly of tannic acid with metal ions on three-dimensional nickel hydroxide nanowalls for efficient water splitting. J. Mater. Chem. A 2020, 8, 15845–15852. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. MOFs-Derived Carbon-Based Metal Catalysts for Energy-Related Electrocatalysis. Small 2021, 17, e2004398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, H.; Yuan, W.; Li, C.M. Tannic Acid-Mediated In Situ Controlled Assembly of NiFe Alloy Nanoparticles on Pristine Graphene as a Superior Oxygen Evolution Catalyst. ACS Appl. Energy Mater. 2020, 3, 3966–3977. [Google Scholar] [CrossRef]
- Li, H.; Shu, X.; Tong, P.; Zhang, J.; An, P.; Lv, Z.; Tian, H.; Zhang, J.; Xia, H. Fe-Ni Alloy Nanoclusters Anchored on Carbon Aerogels as High-Efficiency Oxygen Electrocatalysts in Rechargeable Zn-Air Batteries. Small 2021, 17, e2102002. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhu, W.Y.; Ni, J.C.; Zhao, Y.H.; Zhang, T.; Tan, Z.; Liu, L.Z.; Wang, X.F. Boosting Hydrogen Evolution Electrocatalysis via Regulating the Electronic Structure in a Crystalline-Amorphous CoP/CeOx p-n Heterojunction. ACS Appl. Mater Interfaces 2022, 14, 33151–33160. [Google Scholar] [CrossRef]
- Fang, H.; Chen, G.; Wang, L.; Yan, J.; Zhang, L.; Gao, K.; Zhang, Y.; Wang, L. Facile fabrication of hierarchical film composed of Co(OH)2@Carbon nanotube core/sheath nanocables and its capacitive performance. RSC Adv. 2018, 8, 38550–38555. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, H.; Chen, L.; Wei, X.; Shi, J.; He, M. Facile synthesis of Cu doped cobalt hydroxide (Cu–Co(OH)2) nano-sheets for efficient electrocatalytic oxygen evolution. J. Mater. Chem. A 2017, 5, 22568–22575. [Google Scholar] [CrossRef]
- Xu, Q.; Jiu, H.; Zhang, L.; Song, W.; Wei, H.; Wang, C.; Yang, J.; Guo, F.; Gao, T. Structure design of CuO/Cu2O@C heterostructure polyhedron accumulated by hollow microspheres for high-performance lithium storage. J. Alloy. Compd. 2021, 887, 161417. [Google Scholar] [CrossRef]
- Deng, G.; Wang, T.; Alshehri, A.A.; Alzahrani, K.A.; Wang, Y.; Ye, H.; Luo, Y.; Sun, X. Improving the electrocatalytic N2 reduction activity of Pd nanoparticles through surface modification. J. Mater. Chem. A 2019, 7, 21674–21677. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, Y.; Liang, Y.; Du, Y.; Zhang, B. In Situ Electrochemical Conversion of an Ultrathin Tannin Nickel Iron Complex Film as an Efficient Oxygen Evolution Reaction Electrocatalyst. Angew. Chem. Int. Ed. 2019, 58, 3769–3773. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Han, S.; Wang, J.; Zhu, Y. MIL-100-Fe derived N-doped Fe/Fe3C@C electrocatalysts for efficient oxygen reduction reaction. Appl. Surf. Sci. 2018, 434, 1266–1273. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhu, C.; Liu, Z.; Zhou, W.; Zhu, C.; Guan, C.; Fang, G. Rational design of iron single atom anchored on nitrogen doped carbon as a high-performance electrocatalyst for all-solid-state flexible zinc-air batteries. Chem. Eng. J. 2021, 405, 125956. [Google Scholar] [CrossRef]
- Peng, H.; Zhou, K.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H. Hierarchical nanostructure with ultrafine MoO3 particles-decorated Co(OH)2 nanosheet array on Ag nanowires for promoted hydrogen evolution reaction. Chem. Eng. J. 2022, 429, 132477. [Google Scholar] [CrossRef]
- Xu, Z.; Zuo, W.; Shi, T.; Liu, X.; Li, H.; Zhao, P.; Cheng, G. An Fe-doped Co-oxide electrocatalyst synthesized through a post-modification method toward advanced water oxidation. Dalton Trans. 2022, 51, 3137–3145. [Google Scholar] [CrossRef]
- Jia, X.; Wu, J.; Lu, K.; Li, Y.; Qiao, X.; Kaelin, J.; Lu, S.; Cheng, Y.; Wu, X.; Qin, W. Organic–inorganic hybrids of Fe–Co polyphenolic network wrapped Fe3O4 nanocatalysts for significantly enhanced oxygen evolution. J. Mater. Chem. A 2019, 7, 14302–14308. [Google Scholar] [CrossRef]
- Cheng, J.; Yue, X.; Chen, C.; Shen, X.; Zeng, S.; Ji, Z.; Yuan, A.; Zhu, G. Template-assisted synthesis of accordion-like CoFe(OH) nanosheet clusters on GO sheets for electrocatalytic water oxidation. J. Electroanal. Chem. 2022, 905, 115957. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Fan, R.Y.; Cao, Y.N.; Wang, H.Y.; Dong, B.; Zhao, H.Y.; Wang, F.L.; Yu, J.F.; Chai, Y.M. Oriented and robust anchoring of Fe via anodic interfacial coordination assembly on ultrathin Co hydroxides for efficient water oxidation. Nanoscale 2021, 13, 13463–13472. [Google Scholar] [CrossRef]
- Sung, M.-C.; Lee, G.-H.; Kim, D.-W. CeO2/Co(OH)2 hybrid electrocatalysts for efficient hydrogen and oxygen evolution reaction. J. Alloys Compd. 2019, 800, 450–455. [Google Scholar] [CrossRef]
- Ge, R.; Ren, X.; Ji, X.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X.; Chen, L. Benzoate Anion-Intercalated Layered Cobalt Hydroxide Nanoarray: An Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemSusChem 2017, 10, 4004–4008. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Peng, H.; Wang, X.; Hu, L.; Peng, M.; Liu, Z.; Jiang, G. Nitrate reduction by nanoscale zero valent iron (nFe0)-based Systems: Mechanism, reaction pathway and strategy for enhanced N2 formation. Chem. Eng. J. 2022, 430, 133133. [Google Scholar] [CrossRef]
- Jin, C.; Hou, M.; Li, X.; Liu, D.; Qu, D.; Dong, Y.; Xie, Z.; Zhang, C. Rapid electrodeposition of Fe-doped nickel selenides on Ni foam as a bi-functional electrocatalyst for water splitting in alkaline solution. J. Electroanal. Chem. 2022, 906, 116014. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Sun, F.; Li, L.; Wang, G.; Lin, Y. Iron incorporation affecting the structure and boosting catalytic activity of β-Co(OH)2: Exploring the reaction mechanism of ultrathin two-dimensional carbon-free Fe3O4-decorated β-Co(OH)2 nanosheets as efficient oxygen evolution electrocatalysts. J. Mater. Chem. A 2017, 5, 6849–6859. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Zhou, M. Fabrication of hierarchical Co(OH)2@Ni(OH)2 core-shell nanosheets on carbon cloth as an advanced electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2019, 479, 1270–1276. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, R.; Xie, H.; Zhang, D.; Liu, X.; Si, Y.; Li, N. Electronic structure tuning of FeCo nanoparticles embedded in multi-dimensional carbon matrix for enhanced bifunctional oxygen electrocatalysis. J. Alloys Compd. 2021, 853, 157070. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Z.; Jin, Z.; Li, X.; Chen, Y. The cobalt carbide/bimetallic CoFe phosphide dispersed on carbon nanospheres as advanced bifunctional electrocatalysts for the ORR, OER, and rechargeable Zn-air batteries. J. Colloid Interface Sci. 2021, 590, 321–329. [Google Scholar] [CrossRef]
- Adamson, W.; Jia, C.; Li, Y.; Zhao, C. Vanadium-induced fragmentation of crystalline CoFe hydr(oxy)oxide electrocatalysts for enhanced oxygen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 35230–35238. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chen, Y.-P.; Cao, Y.; Zhang, L.; Feng, J.-J.; Wang, A.-J. Aminouracil-assisted synthesis of CoFe decorated bougainvillea-like N-doped carbon nanoflowers for boosting Zn–air battery and water electrolysis. J. Power Sources 2022, 521, 230926. [Google Scholar] [CrossRef]
- Lei, Z.; Tan, Y.; Zhang, Z.; Wu, W.; Cheng, N.; Chen, R.; Mu, S.; Sun, X. Defects enriched hollow porous Co-N-doped carbons embedded with ultrafine CoFe/Co nanoparticles as bifunctional oxygen electrocatalyst for rechargeable flexible solid zinc-air batteries. Nano Res. 2020, 14, 868–878. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Zhang, Z.; Cui, B.; Chen, Y.; Deng, Y.; Hu, W. Nano-manufacturing of Co(OH)2@NC for efficient oxygen evolution/reduction reactions. J. Mater. Sci. Technol. 2021, 81, 131–138. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.-Z.; Zhao, Y.-H.; Zhang, F.; Ni, J.-C.; Zhang, Z.; Tan, Z.; Wang, X.-F.; Li, Y. Coupling Plant Polyphenol Coordination Assembly with Co(OH)2 to Enhance Electrocatalytic Performance towards Oxygen Evolution Reaction. Nanomaterials 2022, 12, 3972. https://doi.org/10.3390/nano12223972
Song X-Z, Zhao Y-H, Zhang F, Ni J-C, Zhang Z, Tan Z, Wang X-F, Li Y. Coupling Plant Polyphenol Coordination Assembly with Co(OH)2 to Enhance Electrocatalytic Performance towards Oxygen Evolution Reaction. Nanomaterials. 2022; 12(22):3972. https://doi.org/10.3390/nano12223972
Chicago/Turabian StyleSong, Xue-Zhi, Yu-Hang Zhao, Fan Zhang, Jing-Chang Ni, Zhou Zhang, Zhenquan Tan, Xiao-Feng Wang, and Yanqiang Li. 2022. "Coupling Plant Polyphenol Coordination Assembly with Co(OH)2 to Enhance Electrocatalytic Performance towards Oxygen Evolution Reaction" Nanomaterials 12, no. 22: 3972. https://doi.org/10.3390/nano12223972