Rational Design of Fluorinated Phthalonitrile/Hollow Glass Microsphere Composite with Low Dielectric Constant and Excellent Heat Resistance for Microelectronic Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of HGM with APTES (HGM-NH2)
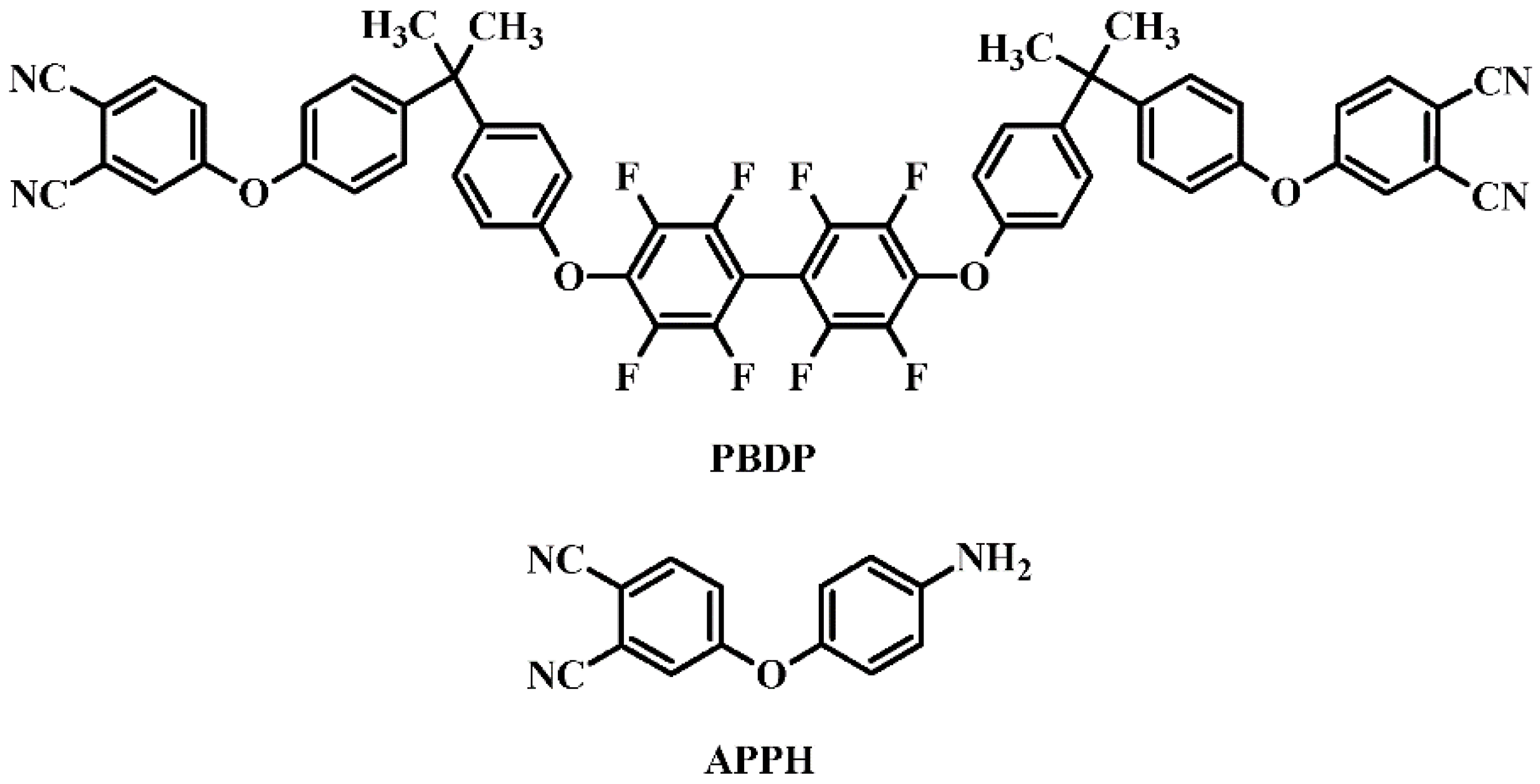
2.3. Preparation of Phthalonitrile Monomer as Matrix of Composite Materials
2.4. Preparation of the PBDP/xHGM Composites
2.5. Instrumentation
3. Results and Discussion
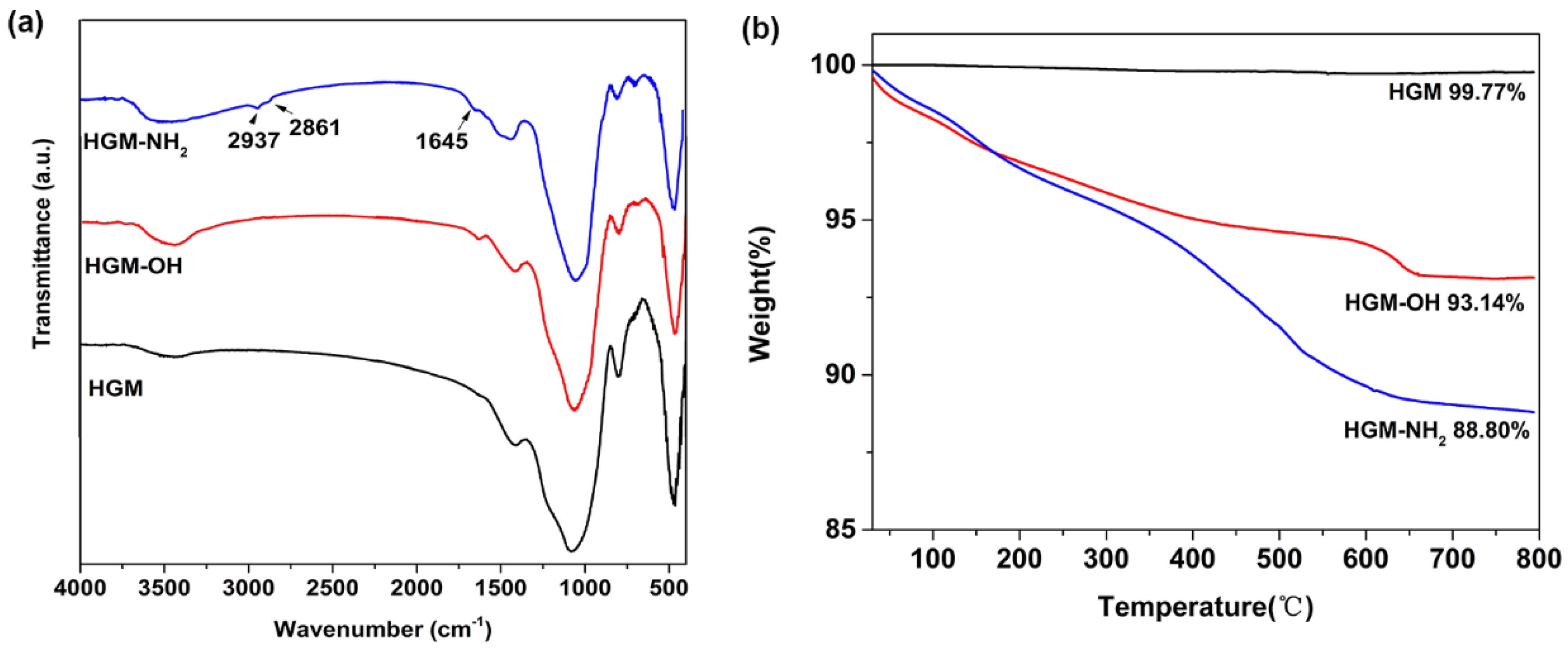
3.1. Characterization of HGM before and after Functionalization
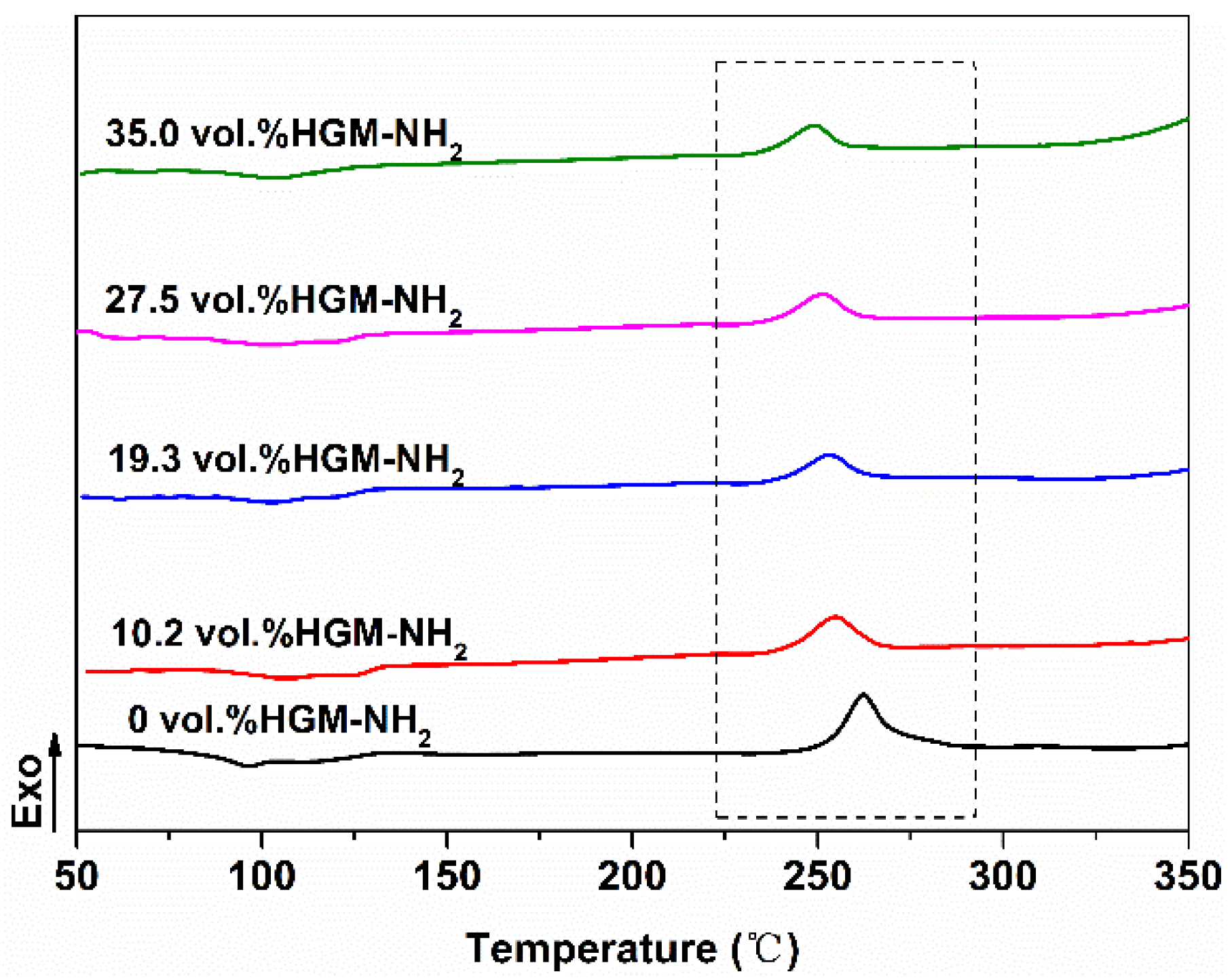
3.2. Cure Studies on PBDP/HGM-NH2 Blends
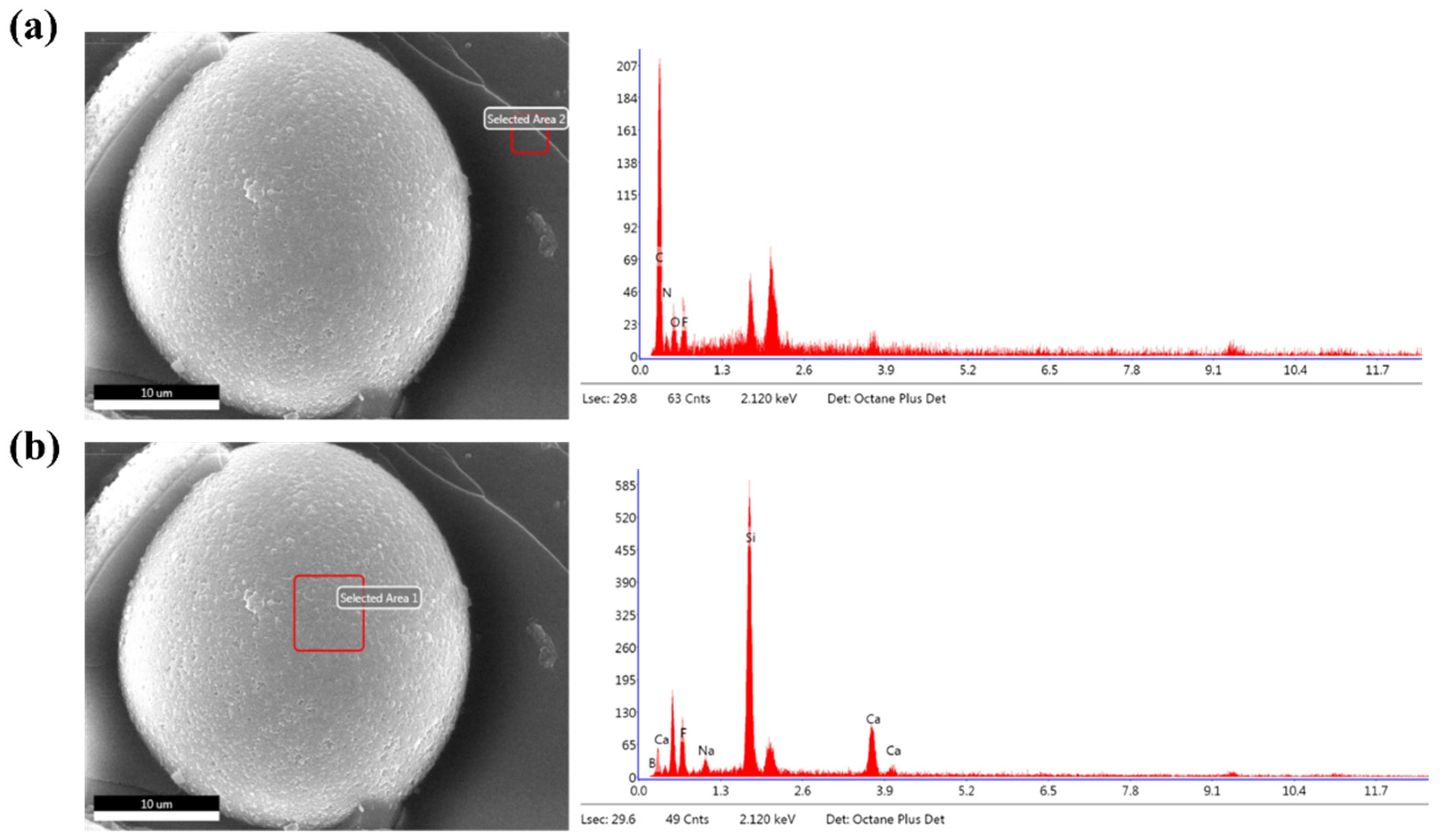
3.3. Microstructure of the Composites
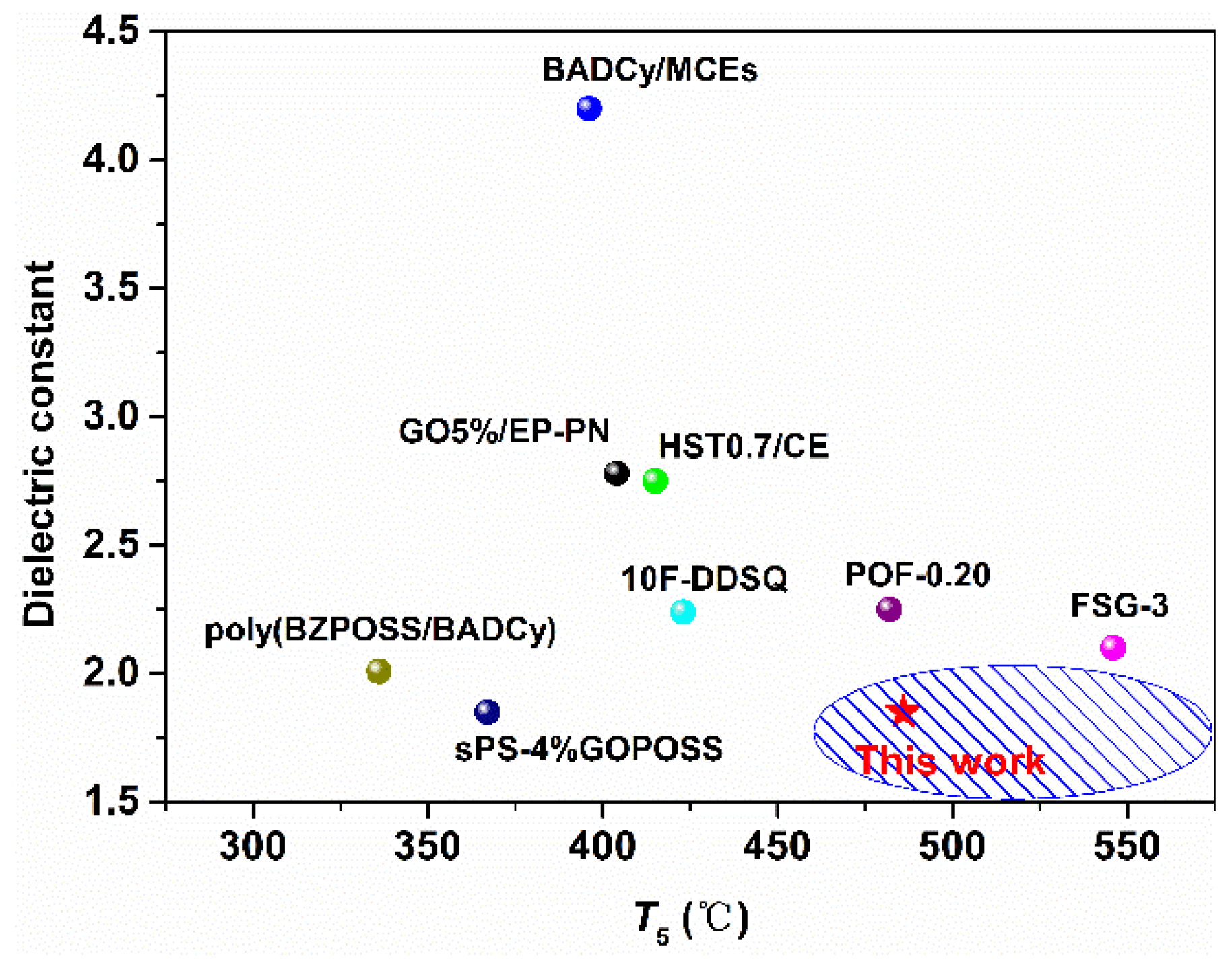
3.4. Dielectric Properties
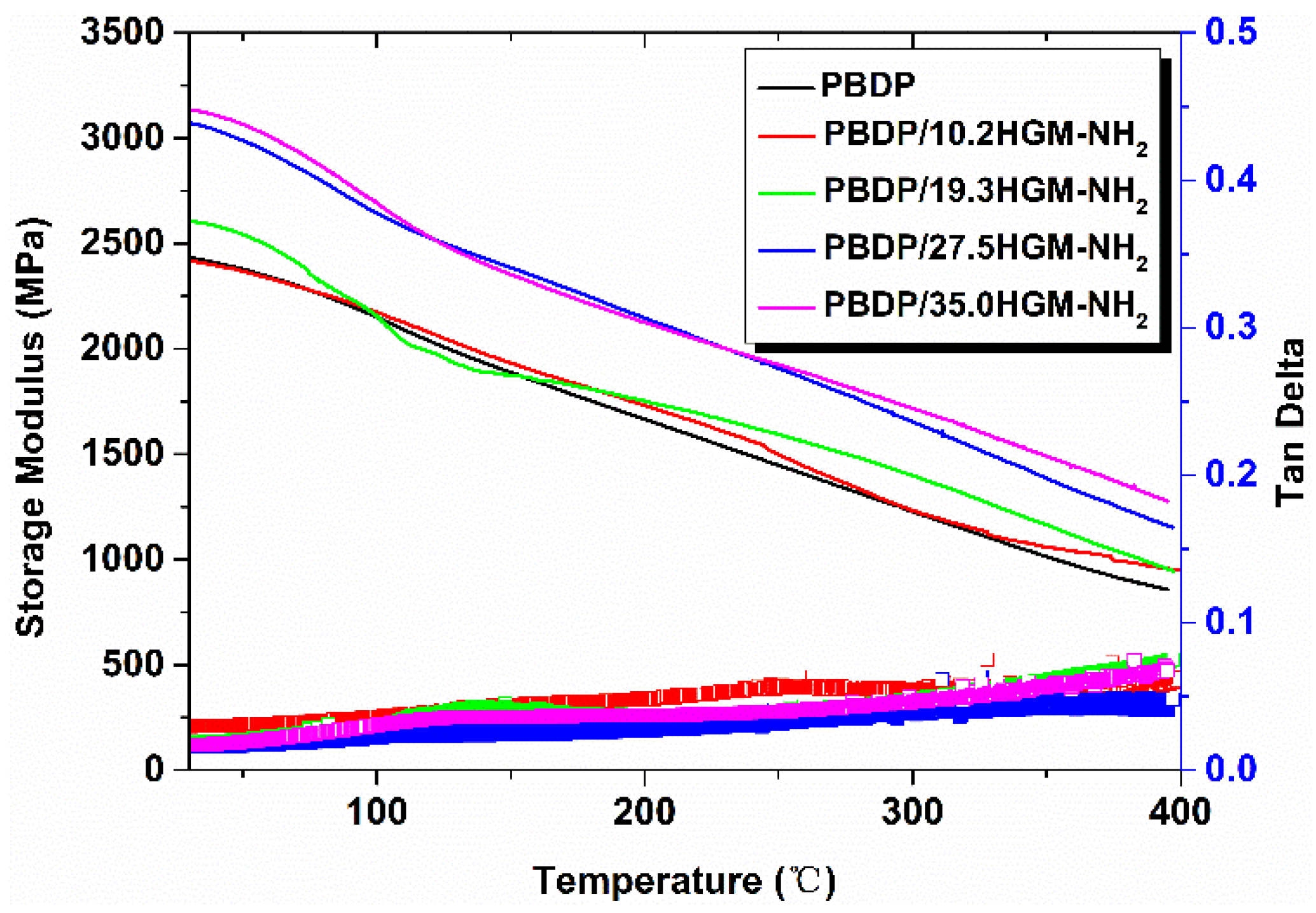
3.5. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, B.; Verma, R.; Baur, C.; Bykova, J.; Mabry, J.M.; Smith, D.W. Ultra low dielectric, self-cleansing and highly oleophobic POSS-PFCP aryl ether polymer composites. J. Mater. Chem. C 2013, 1, 7222–7227. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Lee, C.Y.; Hung, W.J.; Chen, G.S.; Fang, J.S. Comparison of various low dielectric constant materials. Thin Solid Film. 2018, 660, 871–878. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Liu, C.; Zhou, X.; Liu, C.; Cheng, M.; Wei, R.; Liu, X. Ultralow dielectric constant polyarylene ether nitrile foam with excellent mechanical properties—ScienceDirect. Chem. Eng. J. 2020, 384, 123231. [Google Scholar] [CrossRef]
- Zhu, B.L.; Wang, J.; Zheng, H.; Ma, J.; Wu, J.; Wu, R. Investigation of thermal conductivity and dielectric properties of LDPE-matrix composites filled with hybrid filler of hollow glass microspheres and nitride particles. Compos. Part B 2015, 69, 496–506. [Google Scholar] [CrossRef]
- Pan, S.; Wang, T.; Jin, K.; Cai, X. Understanding and designing metal matrix nanocomposites with high electrical conductivity: A review. J. Mater. Sci. 2022, 57, 6487–6523. [Google Scholar] [CrossRef]
- Cho, J.; Boccaccini, A.R.; Shaffer, M.S. Ceramic matrix composites containing carbon nanotubes. J. Mater. Sci. 2009, 44, 1934–1951. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, J.; Cheng, W.; Zou, J.; Zhao, D. Progress on polymer composites with low dielectric constant and low dielectric loss for high-frequency signal transmission. Front. Mater. 2021, 8, 774843. [Google Scholar] [CrossRef]
- Wang, B.; Shang, Y.R.; Zhe, M.; Pan, L.; Li, Y.S. Non-porous ultra low dielectric constant materials based on novel silicon-containing cycloolefin copolymers with tunable performance. Polymer 2017, 116, 105–112. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Tkachenko, I.M.; Bliznyuk, V.N.; Shevchenko, V.V. Fluorinated polybenzoxazines as advanced phenolic resins for leading-edge applications. React. Funct. Polym. 2018, 133, 71–92. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Cui, Z.; Gu, J.; Lin, S.; Zhuang, Q. Design and development of HMS@ZIF-8/fluorinated polybenzoxazole composite films with excellent low-k performance, mechanical properties and thermal stability. J. Mater. Chem. C 2020, 8, 7476–7484. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, D.M.; Song, G.L.; Dang, G.D.; Chen, C.H.; Zhou, H.W.; Zhao, X.G. Novel soluble polyimides derived from 2,2′-bis 4-(5-amino-2-pyridinoxy)phenyl hexafluoropropane: Preparation, characterization, and optical, dielectric properties. Polymer 2014, 55, 3634–3641. [Google Scholar] [CrossRef]
- Tao, L.; Yang, H.; Liu, J.; Lin, F.; Yang, S. Synthesis and characterization of highly optical transparent and low dielectric constant fluorinated polyimides. Polymer 2009, 50, 6009–6018. [Google Scholar] [CrossRef]
- Tkachenko, I.; Kononevich, Y.; Kobzar, Y.; Purikova, O.; Yakovlev, Y.; Khalakhan, I.; Muzafarov, A.; Shevchenko, V. Low dielectric constant silica-containing cross-linked organic-inorganic materials based on fluorinated poly(arylene ether)s. Polymer 2018, 157, 131–138. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Jia, C.C. High-performance cyanate ester composites with plasma-synthesized MgSiO3-SiO2-hBN powders for thermally conductive and dielectric properties. Ceram. Int. 2019, 45, 6491–6498. [Google Scholar] [CrossRef]
- Tong, L.F.; Jia, K.; Liu, X.B. Novel phthalonitrile-terminated polyarylene ether nitrile with high glass transition temperature and enhanced thermal stability. Mater. Lett. 2014, 128, 267–270. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Wang, G.X.; Xu, S.S.; Han, Y.; Liu, X.; Luo, Z.H.; Ye, L.; Zhou, H.; Zhao, T. Preparation and characterization of a self-catalyzed fluorinated novolac-phthalonitrile resin. Polym. Adv. Technol. 2018, 29, 2936–2942. [Google Scholar] [CrossRef]
- Sun, Y.T.; Krishtab, M.; Struyf, H.; Verdonck, P.; De Feyter, S.; Baklanov, M.R.; Armini, S. Impact of Plasma Pretreatment and Pore Size on the Sealing of Ultra-Low-k Dielectrics by Self-Assembled Monolayers. Langmuir 2014, 30, 3832–3844. [Google Scholar] [CrossRef]
- Jin, C.; Lin, S.; Wetzel, J.T. Evaluation of ultra-low-k dielectric materials for advanced interconnects. J. Electron. Mater. 2001, 30, 284–289. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Tkachenko, I.M.; Bliznyuk, V.N.; Lobko, E.V.; Shekera, O.V.; Shevchenko, V.V. Synthesis and characterization of fluorinated isomeric polybenzoxazines from core-fluorinated diamine-based benzoxazines. Polymer 2018, 145, 62–69. [Google Scholar] [CrossRef]
- Zhu, B.L.; Zheng, H.; Wang, J.; Ma, J.; Wu, J.; Wu, R. Tailoring of thermal and dielectric properties of LDPE-matrix composites by the volume fraction, density, and surface modification of hollow glass microsphere filler. Compos. Part B-Eng. 2014, 58, 91–102. [Google Scholar] [CrossRef]
- Joseph, A.M.; Nagendra, B.; Surendran, K.P.; Gowd, E.B. Syndiotactic Polystyrene/Hybrid Silica Spheres of POSS Siloxane Composites Exhibiting Ultra low Dielectric Constant. ACS Appl. Mater. Interfaces 2015, 7, 19474–19483. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Ning, Y. Oligosilylarylnitrile: The Thermoresistant Thermosetting Resin with High Comprehensive Properties. ACS Appl. Mater. Interfaces 2018, 10, 11933–11940. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Wang, J.; Guo, H.M.; Chen, X.G.; Yu, X.Y.; Ma, Y.H.; Ji, P.G.; Naito, K.; Zhang, Z.J.; Zhang, Q.X. A novel high temperature vinylpyridine-based phthalonitrile polymer with a low melting point and good mechanical properties. Polym. Chem. 2018, 9, 976–983. [Google Scholar] [CrossRef]
- Laskoski, M.; Clarke, J.S.; Neal, A.; Harvey, B.G.; Ricks-Laskoski, H.L.; Hervey, W.J.; Daftary, M.N.; Shepherd, A.R.; Keller, T.M. Sustainable High-Temperature Phthalonitrile Resins Derived from Resveratrol and Dihydroresveratrol. Chemistryselect 2016, 1, 3423–3427. [Google Scholar] [CrossRef]
- Wang, G.X.; Han, Y.; Guo, Y.; Wang, S.K.; Sun, J.S.; Zhou, H.; Zhao, T. Phthalonitrile-Terminated Silicon-Containing Oligomers: Synthesis, Polymerization, and Properties. Ind. Eng. Chem. Res. 2019, 58, 9921–9930. [Google Scholar] [CrossRef]
- Wu, M.J.; Xu, J.J.; Bai, S.N.; Chen, X.G.; Yu, X.Y.; Naito, K.; Zhang, Z.J.; Zhang, Q.X. A high-performance functional phthalonitrile resin with a low melting point and a low dielectric constant. Soft Matter 2020, 16, 1888–1896. [Google Scholar] [CrossRef]
- Yung, K.C.; Zhu, B.L.; Yue, T.M.; Xie, C.S. Preparation and properties of hollow glass microsphere-filled epoxy-matrix composites. Compos. Sci. Technol. 2009, 69, 260–264. [Google Scholar] [CrossRef]
- Sheng, H.T.; Peng, X.G.; Guo, H.; Yu, X.Y.; Naito, K.; Qu, X.W.; Zhang, Q.X. Synthesis of high performance bisphthalonitrile resins cured with self-catalyzed 4-aminophenoxy phthalonitrile. Thermochim. Acta 2014, 577, 17–24. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.; Wang, Q.; Liu, M.L.; Du, C.Y.; Li, B.A.; Feng, L.F. High thermal conductive poly(vinylidene fluoride)-based composites with well-dispersed carbon nanotubes/graphene three-dimensional network structure via reduced interfacial thermal resistance. Compos. Sci. Technol. 2019, 181, 107713. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.J.; Min, P.; Sui, G.X. BN@PPS core-shell structure particles and their 3D segregated architecture composites with high thermal conductivities. Compos. Sci. Technol. 2017, 144, 63–69. [Google Scholar] [CrossRef]
- Kaliavaradhan, K.; Rukmanikrishnan, B.; Muthusamy, S. Investigation of the effect of graphene oxide on the properties of epoxy-phthalonitrile nanocomposites. Adv. Polym. Tech. 2018, 37, 1256–1267. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Chen, X.G.; Yu, X.Y.; Zhang, Q.X. Improvement of thermal conductivities and mechanical properties for polyphthalonitrile nanocomposites via incorporating functionalized h-BN fillers. High Perform. Polym. 2019, 31, 294–303. [Google Scholar] [CrossRef]
- Huang, Z.X.; Zhao, J.Q.; Yuan, Y.C.; Yan, S.J.; Liu, S.M.; Zan, X.T. Preparation and characterization of polyimide/pure silica zeolite hybrid films. Polym. Adv. Technol. 2013, 24, 600–608. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.C.; Zhang, Y.; Li, Z.Y.; Ji, T.Z.; Wu, G.L. Investigation of the dielectric and thermal conductive properties of core-shell structured HGM@hBN/PTFE composites. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2018, 238, 61–70. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhi, C.Y.; Jiang, P.K.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral Oligosilsesquioxane-Modified Boron Nitride Nanotube Based Epoxy Nanocomposites: An Ideal Dielectric Material with High Thermal Conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Todd, M.G.; Shi, F.G. Characterizing the interphase dielectric constant of polymer composite materials: Effect of chemical coupling agents. J. Appl. Phys. 2003, 94, 4551–4557. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.H.; Li, X.D.; Fan, H.J.; Ran, Q.C.; Fu, Q.; Gu, Y. A novel ultra low-k nanocomposites of benzoxazinyl modified polyhedral oligomeric silsesquioxane and cyanate ester. Eur. Polym. J. 2018, 103, 124–132. [Google Scholar] [CrossRef]
- Yu, W.Q.; Fu, J.F.; Dong, X.; Chen, L.Y.; Shi, L.Y. A graphene hybrid material functionalized with POSS: Synthesis and applications in low-dielectric epoxy composites. Compos. Sci. Technol. 2014, 92, 112–119. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, F.F.; Chen, D.D.; Zong, L.S.; Wang, J.Y.; Jian, X.G. Wave-transparent composites based on phthalonitrile resins with commendable thermal properties and dielectric performance. Polymer 2020, 198, 122490. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zhang, J.X.; Li, Q.; Chen, W.; Zhang, X. The Influence of High Content Nano-Al2O3 on the Properties of Epoxy Resin Composites. Polym. Plast. Technol. Eng. 2009, 48, 384–388. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Q.; Dang, J.; Xie, C. Thermal conductivity epoxy resin composites filled with boron nitride. Polym. Adv. Technol. 2012, 23, 1025–1028. [Google Scholar] [CrossRef]
- Gu, J.W.; Lv, Z.Y.; Wu, Y.L.; Guo, Y.Q.; Tian, L.D.; Qiu, H.; Li, W.Z.; Zhang, Q.Y. Dielectric thermally conductive boron nitride/polyimide composites with outstanding thermal stabilities via in-situ polymerization-electrospinning-hot press method. Compos. Part A-Appl. Sci. Manuf. 2017, 94, 209–216. [Google Scholar] [CrossRef]
- Gu, J.W.; Dong, W.C.; Xu, S.; Tang, Y.S.; Ye, L.; Kong, J. Development of wave-transparent, light-weight composites combined with superior dielectric performance and desirable thermal stabilities. Compos. Sci. Technol. 2017, 144, 185–192. [Google Scholar] [CrossRef]
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
- Tschke, P.P.; Fornes, T.D.; Paul, D.R. Rheological behavior of multiwalled carbon nanotube/polycarbonate composites. Polymer 2002, 43, 3247–3255. [Google Scholar]
- Kim, K.; Ju, H.; Kim, J. Surface modification of BN/Fe3O4 hybrid particle to enhance interfacial affinity for high thermal conductive material. Polymer 2016, 91, 74–80. [Google Scholar] [CrossRef]
- Luo, K.J.; Song, G.C.; Wang, Y.; Yu, J.R.; Zhu, J.; Hu, Z.M. Low-k and Recyclable High-Performance POSS/Polyamide Composites Based on Diels-Alder Reaction. ACS Appl. Polym. Mater. 2019, 1, 944–952. [Google Scholar] [CrossRef]
- Joseph, A.M.; Nagendra, B.; Surendran, K.P.; Gowd, E.B. Sustainable in Situ Approach to Covalently Functionalize Graphene Oxide with POSS Molecules Possessing Extremely Low Dielectric Behavior. Langmuir 2019, 35, 4672–4681. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.Y.; Wang, W.M.; Ren, M.M.; Li, B.Y.; Fan, C.; Liu, X.Y. Fluorographene with High Fluorine/Carbon Ratio: A Nanofiller for Preparing LOW-kappa Polyimide Hybrid Films. ACS Appl. Mater. Interfaces 2014, 6, 16182–16188. [Google Scholar] [CrossRef]
- Jiang, Q.Y.; Zhang, W.H.; Hao, J.M.; Wei, Y.F.; Mu, J.X.; Jiang, Z.H. A unique “cage-cage” shaped hydrophobic fluoropolymer film derived from a novel double-decker structural POSS with a low dielectric constant. J. Mater. Chem. C 2015, 3, 11729–11734. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, G.Z.; Gu, A.J. The thermal and dielectric properties of high performance cyanate ester resins/microcapsules composites. Polym. Degrad. Stab. 2011, 96, 84–90. [Google Scholar] [CrossRef]
- Zhuo, D.X.; Gu, A.J.; Liang, G.Z.; Hu, J.T.; Yuan, L. Preparation and properties of hollow silica tubes/cyanate ester hybrids for high-frequency copper-clad laminates. J. Mater. Sci. 2011, 46, 1571–1580. [Google Scholar] [CrossRef]



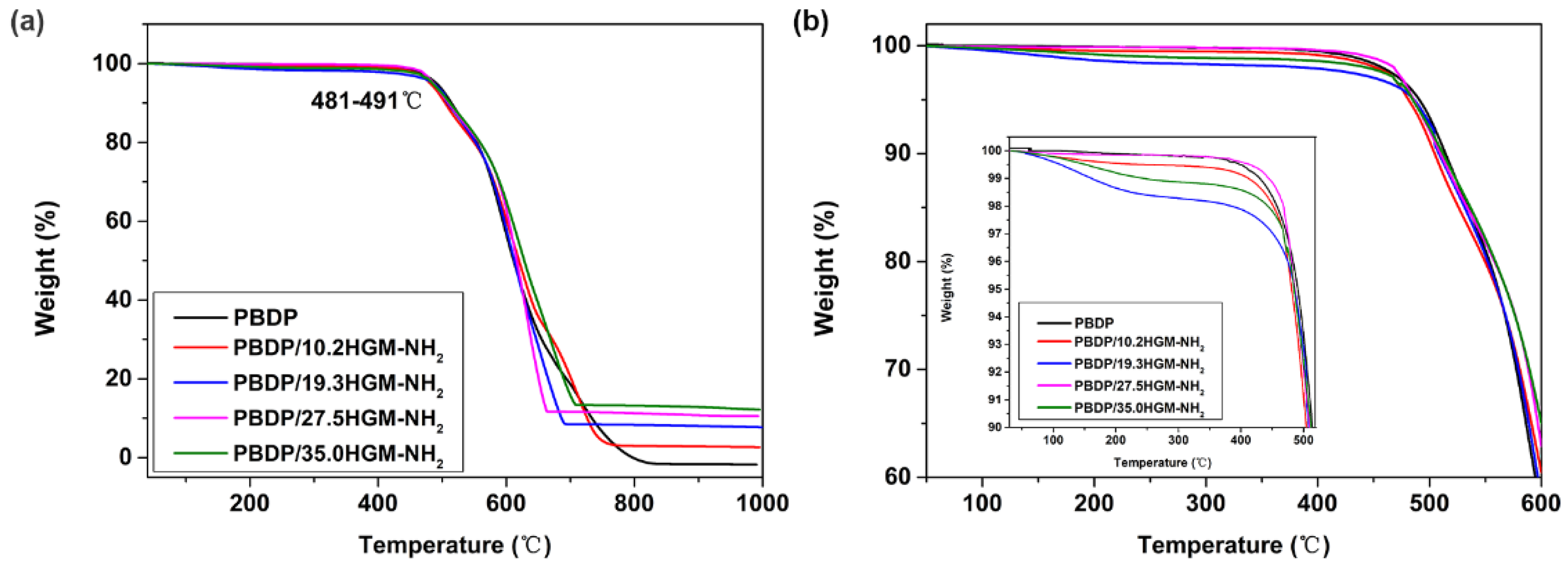
| Samples | T5 (°C) | T10 (°C) | T30 (°C) | THRI (°C) | YC (%) |
|---|---|---|---|---|---|
| PBDP | 490.8 | 514.1 | 577.6 | 266.0 | 0 |
| PBDP/10.2HGM-NH2 | 481.1 | 504.6 | 580.7 | 265.0 | 2.8 |
| PBDP/19.3HGM-NH2 | 484.3 | 503.5 | 579.7 | 265.3 | 8.0 |
| PBDP/27.5HGM-NH2 | 485.7 | 509.7 | 588.3 | 268.1 | 10.7 |
| PBDP/35.0HGM-NH2 | 484.9 | 511.9 | 589.5 | 268.4 | 12.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Han, W.; Zhang, C.; Zhang, S.; Zhang, X.; Chen, X.; Naito, K.; Yu, X.; Zhang, Q. Rational Design of Fluorinated Phthalonitrile/Hollow Glass Microsphere Composite with Low Dielectric Constant and Excellent Heat Resistance for Microelectronic Packaging. Nanomaterials 2022, 12, 3973. https://doi.org/10.3390/nano12223973
Wu M, Han W, Zhang C, Zhang S, Zhang X, Chen X, Naito K, Yu X, Zhang Q. Rational Design of Fluorinated Phthalonitrile/Hollow Glass Microsphere Composite with Low Dielectric Constant and Excellent Heat Resistance for Microelectronic Packaging. Nanomaterials. 2022; 12(22):3973. https://doi.org/10.3390/nano12223973
Chicago/Turabian StyleWu, Minjie, Wenshuang Han, Chun Zhang, Shuo Zhang, Xinyang Zhang, Xinggang Chen, Kimiyoshi Naito, Xiaoyan Yu, and Qingxin Zhang. 2022. "Rational Design of Fluorinated Phthalonitrile/Hollow Glass Microsphere Composite with Low Dielectric Constant and Excellent Heat Resistance for Microelectronic Packaging" Nanomaterials 12, no. 22: 3973. https://doi.org/10.3390/nano12223973






