Enhancing the Mechanical Stability of 2D Fullerene with a Graphene Substrate and Encapsulation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Monolayer Fullerene
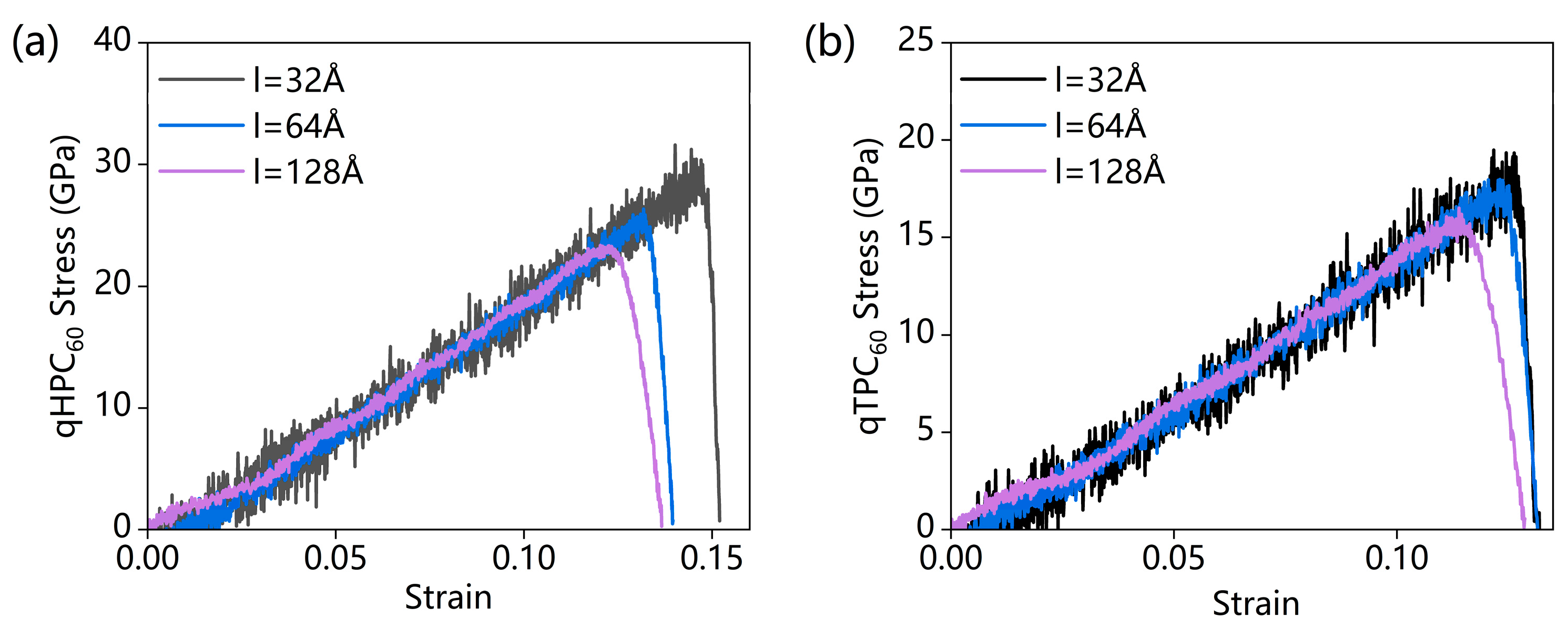
3.1.1. Effect of System Size
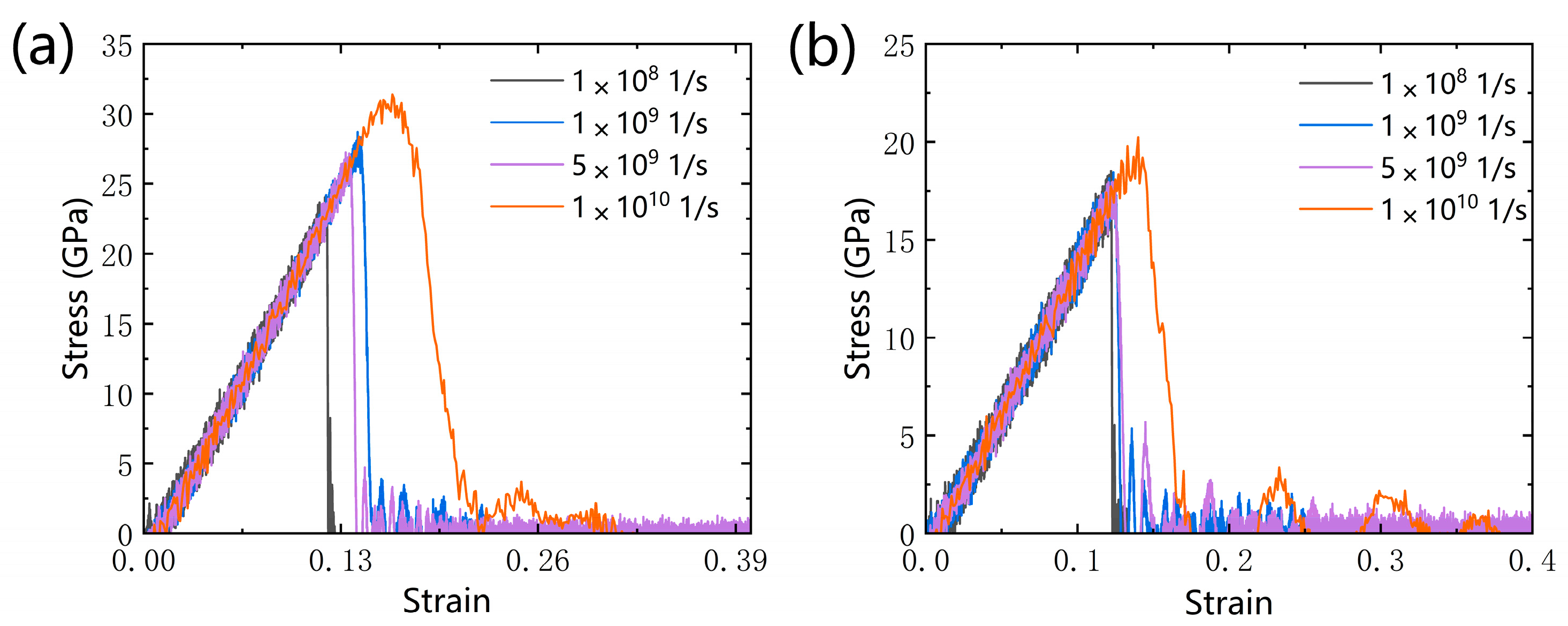
3.1.2. Effect of Strain Rate
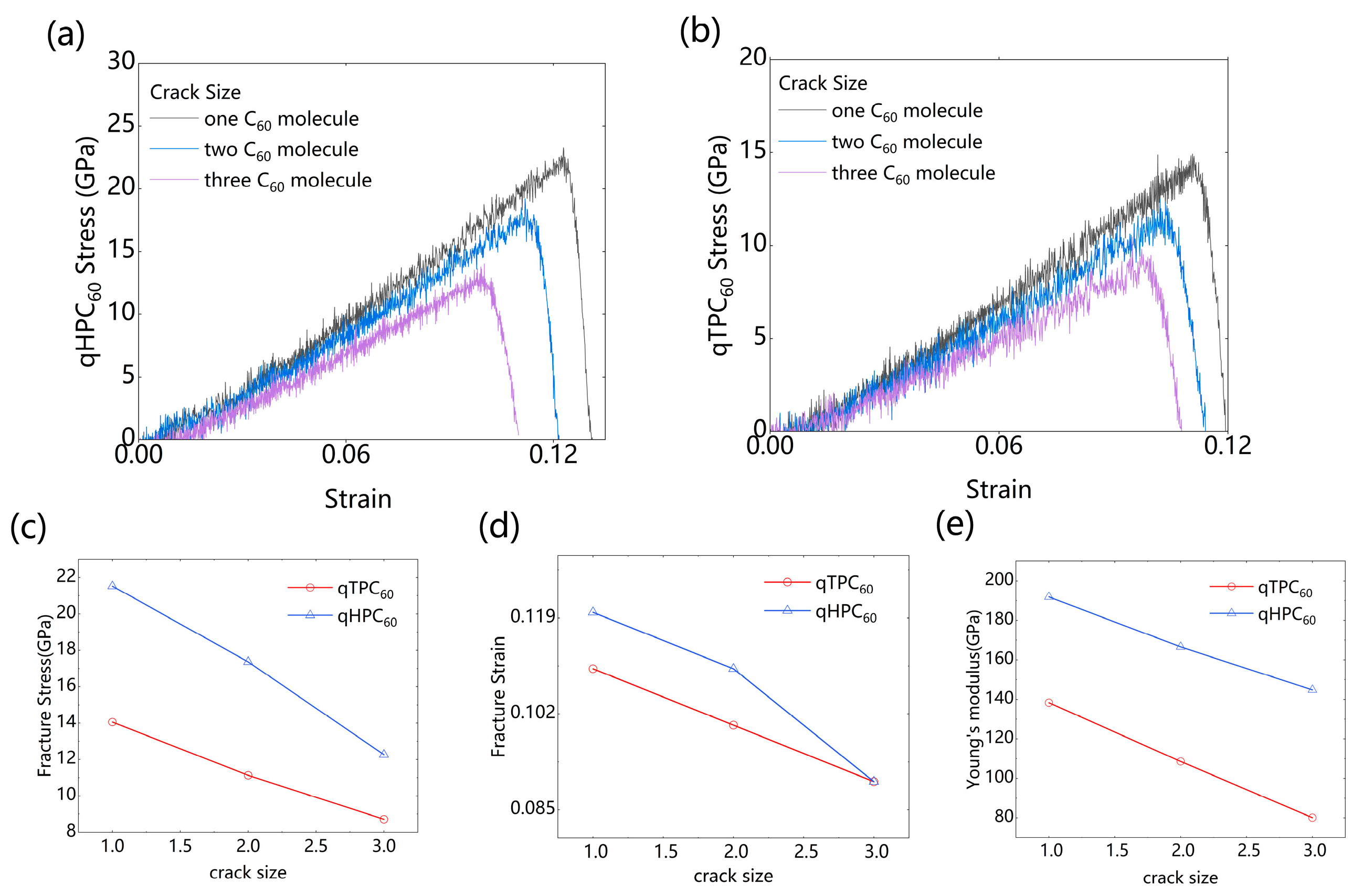
3.1.3. Presence of Cracks
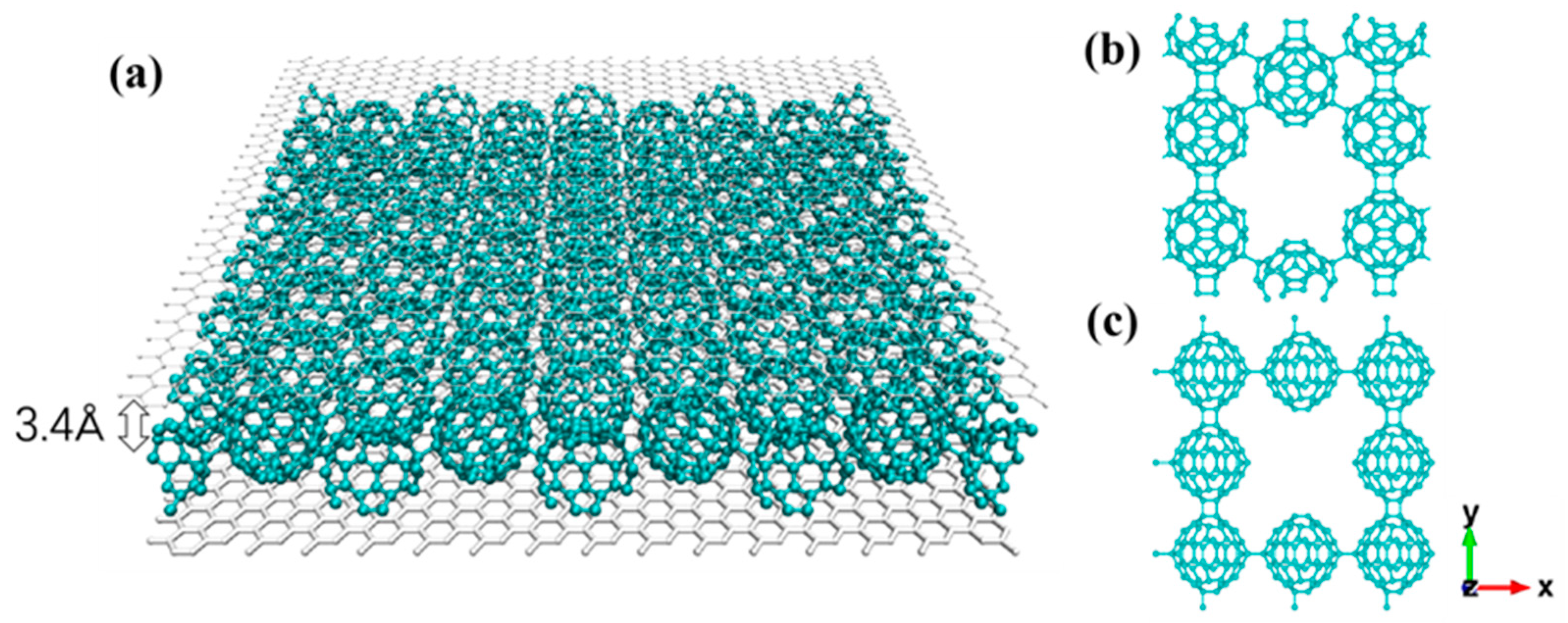
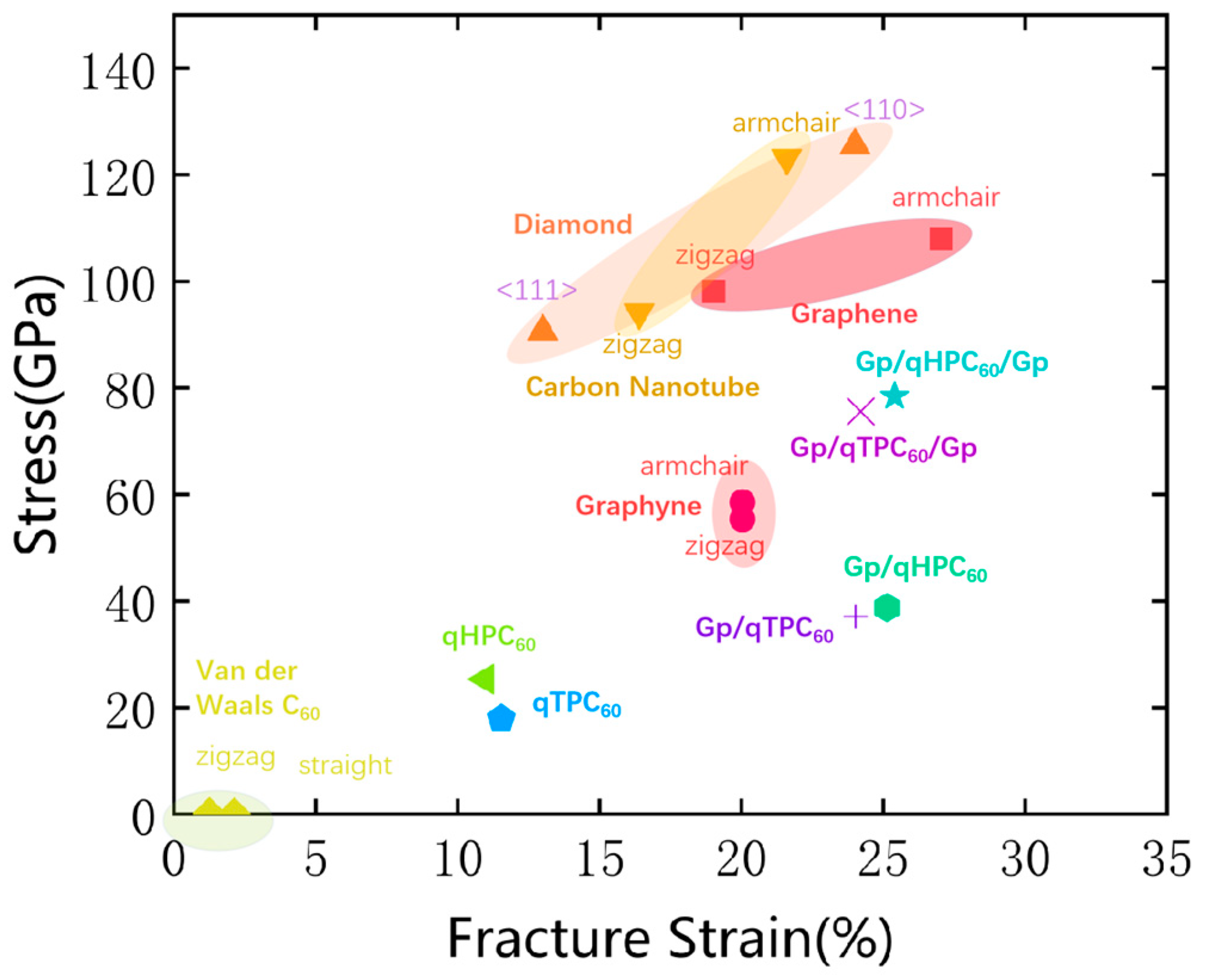
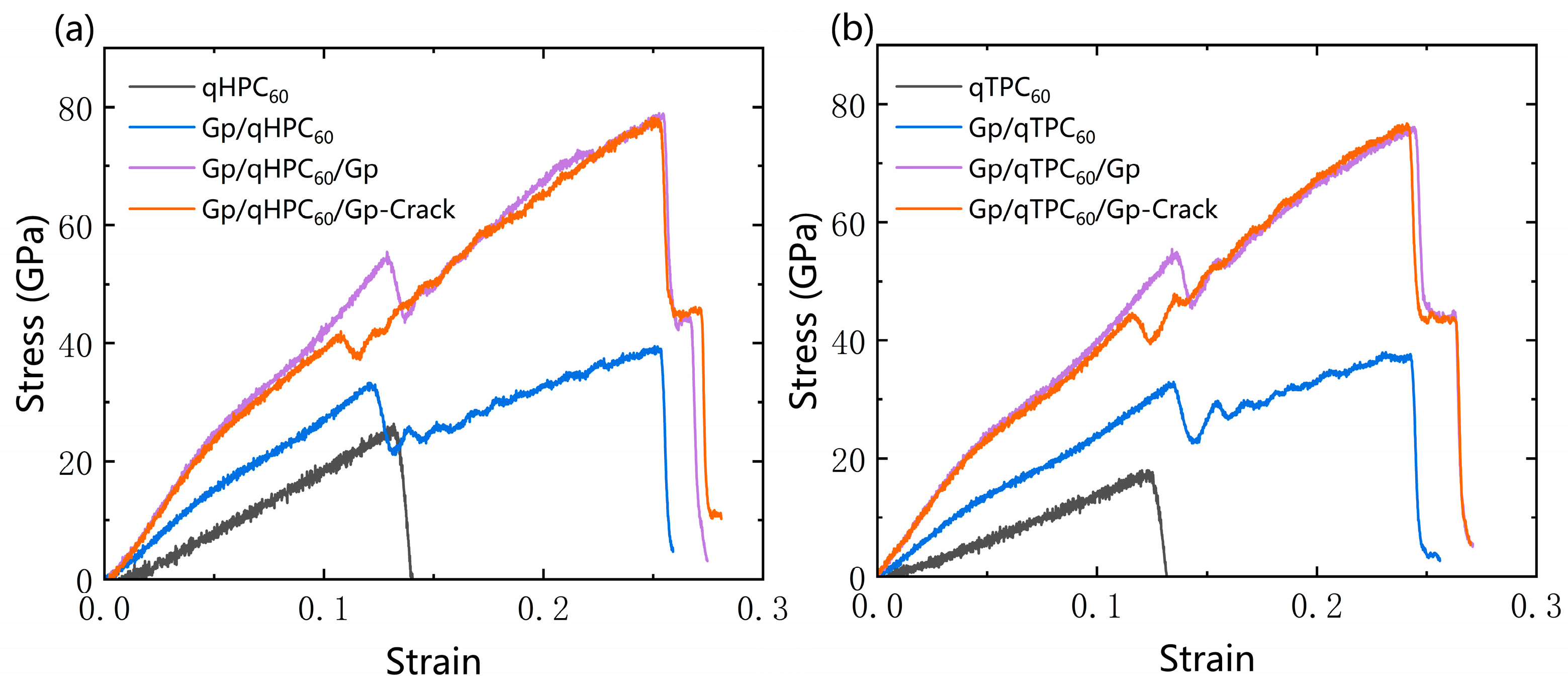
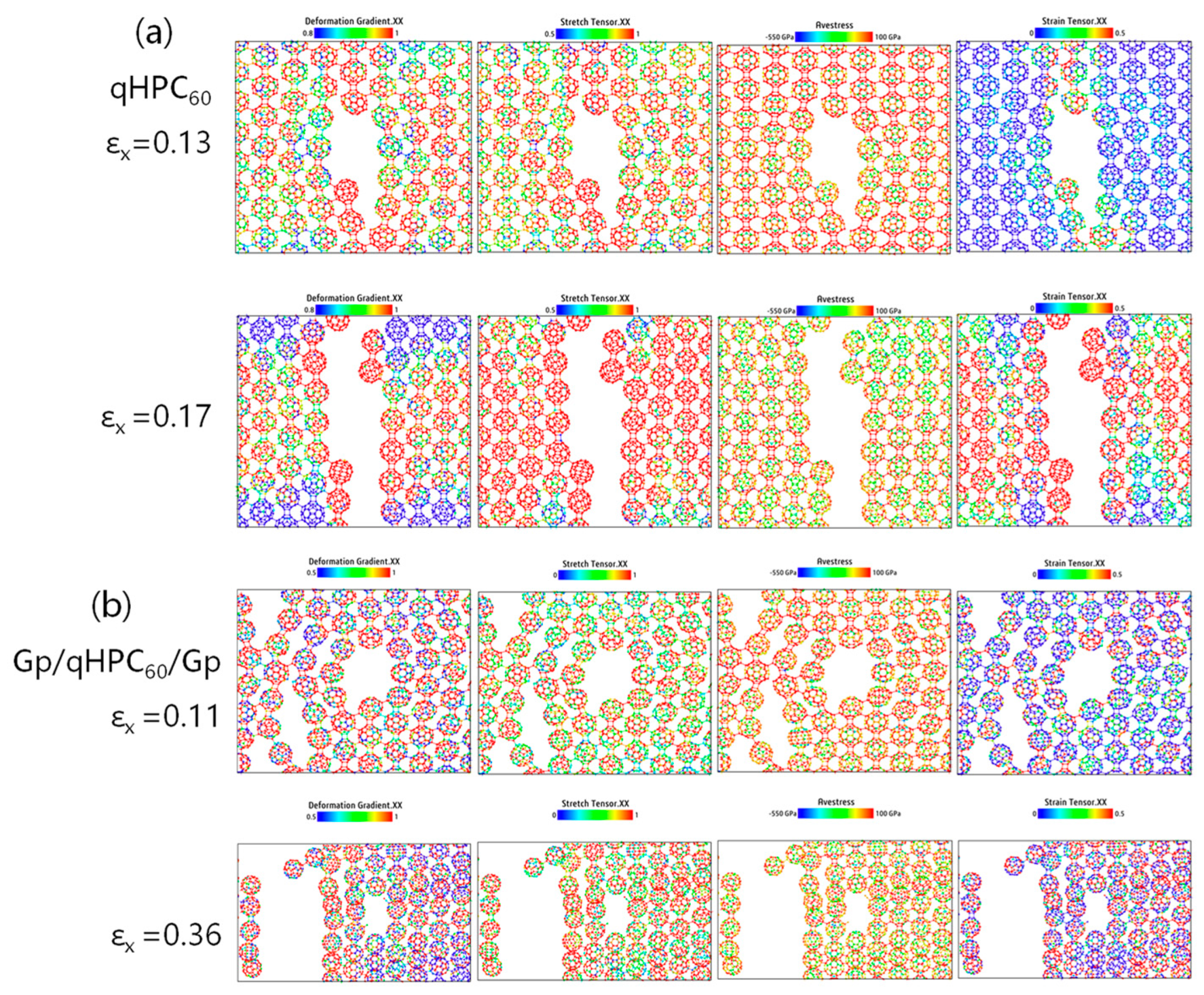
3.2. qHPC60 and qTPC60 with Graphene Substrate
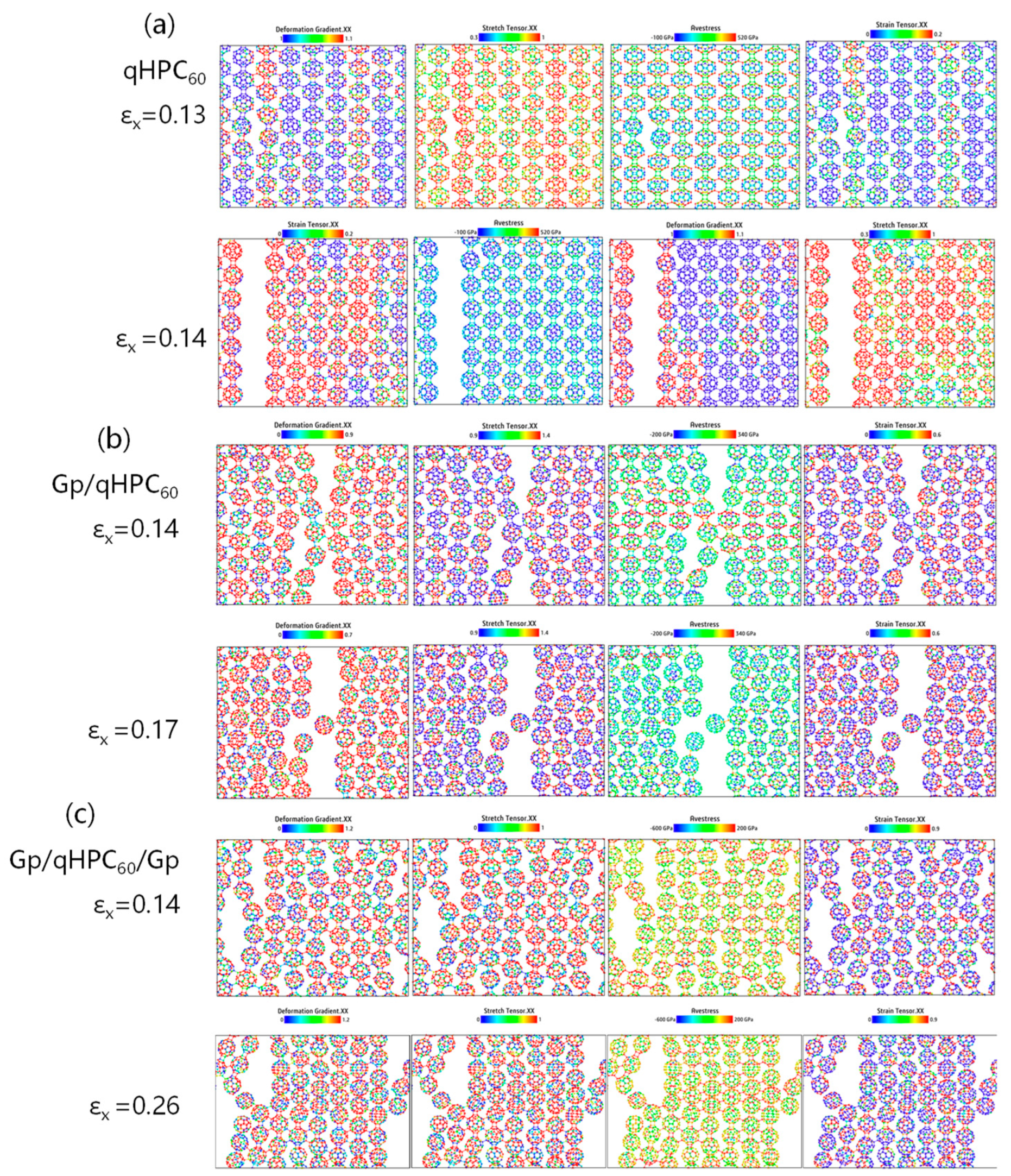
3.3. Analysis of Internal Atom Stress
3.3.1. Without Defects
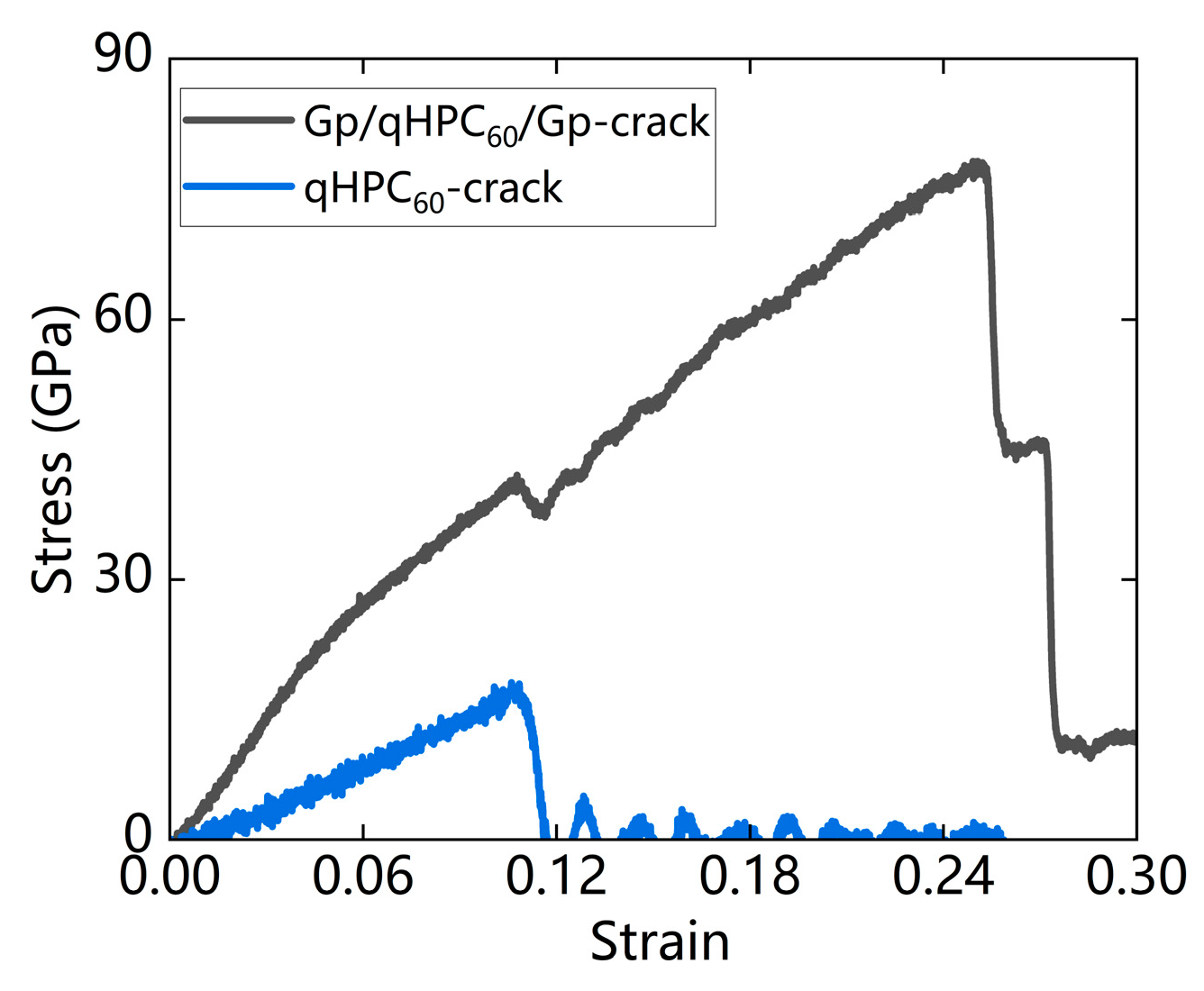
3.3.2. With Defects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Joanni, E.; Singh, R.K.; Singh, D.P.; Moshkalev, S.A. Recent Advances in the Synthesis and Modification of Carbon-Based 2D Materials for Application in Energy Conversion and Storage. Prog. Energy Combust. Sci. 2018, 67, 115–157. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; Adhikari, A.D.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317. [Google Scholar] [CrossRef]
- Lu, H.; Li, S.-D. Two-Dimensional Carbon Allotropes from Graphene to Graphyne. J. Mater. Chem. 2013, 1, 3677. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, F.; Shen, B.; Zhang, R.J.; Zheng, Y.X.; Chen, L.Y.; Wang, S.Y.; Wang, C.Z.; Ho, K.M.; Fan, Y.-J.; et al. Electronic and Optical Properties of Novel Carbon Allotropes. Carbon N. Y. 2016, 101, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.-K.; Laksono, E.; Rodrigues, J.N.B.; Sengupta, P.; Assaad, F.F.; Adam, S. Interaction-Driven Metal-Insulator Transition in Strained Graphene. Phys. Rev. Lett. 2015, 115, 186602. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.H.; Hu, F.M.; Tang, H.K.; Lin, H.Q. Magnetic Impurity in the Vicinity of a Vacancy in Bilayer Graphene. Int. J. Mod. Phys. B 2013, 27, 1362039. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.; Tang, H.-K.; Shao, L.; Han, K.; Shen, X. Phonon Thermal Transport in Silicene/Graphene Heterobilayer Nanostructures: Effect of Interlayer Interactions. ACS Omega 2022, 7, 5844–5852. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Ivanovskii, A.L. Graphene Allotropes. Phys. Status Solidi 2011, 248, 1879–1883. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.-F.; Zhang, X.; Zhu, Q.; Dong, H.; Zhao, M.; Oganov, A.R. Phagraphene: A Low-Energy Graphene Allotrope Composed of 5–6–7 Carbon Rings with Distorted Dirac Cones. Nano Lett. 2015, 15, 6182–6186. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yang, B.; Chen, H.; Ruckenstein, E. Popgraphene: A New 2D Planar Carbon Allotrope Composed of 5–8–5 Carbon Rings for High-Performance Lithium-Ion Battery Anodes from Bottom-up Programming. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 6815–6821. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wu, X.; Yang, J. Me-Graphene: A Graphene Allotrope with near Zero Poisson’s Ratio, Sizeable Band Gap, and High Carrier Mobility. Nanoscale 2020, 12, 19359–19366. [Google Scholar] [CrossRef] [PubMed]
- Karaush, N.N.; Baryshnikov, G.V.; Minaev, B.F. DFT Characterization of a New Possible Graphene Allotrope. Chem. Phys. Lett. 2014, 612, 229–233. [Google Scholar] [CrossRef]
- Zhang, R.-S.; Jiang, J.-W. The Art of Designing Carbon Allotropes. Front. Phys. 2018, 14, 13401. [Google Scholar] [CrossRef]
- Berber, S.; Osawa, E.; Tománek, D. Rigid Crystalline Phases of Polymerized Fullerenes. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 70, 085417. [Google Scholar] [CrossRef] [Green Version]
- Alsayoud, A.Q.; Manga, V.R.; Muralidharan, K.; Vita, J.; Bringuier, S.; Runge, K.; Deymier, P. Atomistic Insights into the Effect of Polymerization on the Thermophysical Properties of 2-D C60 Molecular Solids. Carbon N. Y. 2018, 133, 267–274. [Google Scholar] [CrossRef]
- Fan, Q.; Yan, L.; Tripp, M.W.; Krejci, O.; Dimosthenous, S.; Kachel, S.R.; Chen, M.; Foster, A.S.; Koert, U.; Liljeroth, P.; et al. Biphenylene Sheet: A Nonbenzenoid Carbon Allotrope 2020. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/60c753300f50db26b2397b25 (accessed on 20 June 2023).
- Hou, J.; Deng, B.; Zhu, H.; Lan, Y.; Shi, Y.; De, S.; Liu, L.; Chakraborty, P.; Gao, F.; Peng, Q. Magic Auxeticity Angle of Graphene. Carbon N. Y. 2019, 149, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Hou, J.; Zhu, H.; Liu, S.; Liu, E.; Shi, Y.; Peng, Q. The Normal-Auxeticity Mechanical Phase Transition in Graphene. 2D Mater. 2017, 4, 021020. [Google Scholar] [CrossRef]
- Yu, T.; Li, J.; Yang, Z.; Li, H.; Peng, Q.; Tang, H.-K. Effects of Crack Formation on the Mechanical Properties of Bilayer Graphene: A Comparative Analysis. Crystals 2023, 13, 584. [Google Scholar] [CrossRef]
- Qi, P.; Zhu, H.; Borodich, F.; Peng, Q. A Review of the Mechanical Properties of Graphene Aerogel Materials: Experimental Measurements and Computer Simulations. Materials 2023, 16, 1800. [Google Scholar] [CrossRef]
- Shoaib, H.; Peng, Q.; Alsayoud, A.Q. Atomic Insights into Fracture Characteristics of Twisted Tri-Layer Graphene. Crystals 2021, 11, 1202. [Google Scholar] [CrossRef]
- Liu, A.; Peng, Q. A Molecular Dynamics Study of the Mechanical Properties of Twisted Bilayer Graphene. Micromachines 2018, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Peng, Q.; Jiao, Z.; van Rooyen, I.; Skerjanc, W.F.; Gao, F. Ab Initio Study of the Stability of Intrinsic and Extrinsic Ag Point Defects in 3C SiC. J. Nucl. Mater. 2018, 510, 596–602. [Google Scholar] [CrossRef]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A Theoretical Analysis of the Effect of the Hydrogenation of Graphene to Graphane on Its Mechanical Properties. Phys. Chem. Chem. Phys. 2013, 15, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Kroto, H.W. ChemInform Abstract: The Stability of Fullerenes Cn, with n = 24, 28, 32, 36, 50, 60, and 70. ChemInform 1988, 19. [Google Scholar] [CrossRef]
- Kroto, H. ChemInform Abstract: C60, Fullerenes, Giant Fullerenes, and Soot. ChemInform 1990, 21. [Google Scholar] [CrossRef]
- Goroff, N.S. Mechanism of Fullerene Formation. Acc. Chem. Res. 1996, 29, 77–83. [Google Scholar] [CrossRef]
- Hou, L.; Cui, X.; Guan, B.; Wang, S.; Li, R.; Liu, Y.; Zhu, D.; Zheng, J. Synthesis of a Monolayer Fullerene Network. Nature 2022, 606, 507–510. [Google Scholar] [CrossRef]
- Tromer, R.M.; Ribeiro, L.A.; Galvão, D.S. A DFT Study of the Electronic, Optical, and Mechanical Properties of a Recently Synthesized Monolayer Fullerene Network. Chem. Phys. Lett. 2022, 804, 139925. [Google Scholar] [CrossRef]
- Yu, L.; Xu, J.; Peng, B.; Qin, G.; Su, G. Anisotropic Optical, Mechanical, and Thermoelectric Properties of Two-Dimensional Fullerene Networks. J. Phys. Chem. Lett. 2022, 13, 11622–11629. [Google Scholar] [CrossRef] [PubMed]
- Peng, B. Monolayer Fullerene Networks as Photocatalysts for Overall Water Splitting. J. Am. Chem. Soc. 2022, 144, 19921–19931. [Google Scholar] [CrossRef] [PubMed]
- Ying, P.; Dong, H.; Liang, T.; Fan, Z.; Zhong, Z.; Zhang, J. Atomistic Insights into the Mechanical Anisotropy and Fragility of Monolayer Fullerene Networks Using Quantum Mechanical Calculations and Machine-Learning Molecular Dynamics Simulations. Extreme Mech. Lett. 2023, 58, 101929. [Google Scholar] [CrossRef]
- Dong, H.; Cao, C.; Ying, P.; Fan, Z.; Qian, P.; Su, Y. Anisotropic and High Thermal Conductivity in Monolayer Quasi-Hexagonal Fullerene: A Comparative Study against Bulk Phase Fullerene. Int. J. Heat Mass Transf. 2023, 206, 123943. [Google Scholar] [CrossRef]
- Yuan, D.; Pi, H.; Jiang, Y.; Hu, Y.; Zhou, L.; Jia, Y.; Su, G.; Fang, Z.; Weng, H.; Ren, X.; et al. Highly In-Plane Anisotropic Optical Properties of Fullerene Monolayers. Sci. China. Ser. G Phys. Mech. Astron. 2023, 66, 247211. [Google Scholar] [CrossRef]
- Peng, B. Stability and Strength of Monolayer Polymeric C60. Nano Lett. 2023, 23, 652–658. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, X.; Ni, Y.; Peng, Q.; Wei, Y. Anisotropic Mechanical Response of a 2D Covalently Bound Fullerene Lattice. Carbon N. Y. 2023, 202, 118–124. [Google Scholar] [CrossRef]
- Shen, G.; Li, L.; Tang, S.; Jin, J.; Chen, X.-J.; Peng, Q. Stability and Elasticity of Quasi-Hexagonal Fullerene Monolayer from First-Principles Study. Crystals 2023, 13, 224. [Google Scholar] [CrossRef]
- Ribeiro, L.A.; Pereira, M.L.; Giozza, W.F.; Tromer, R.M.; Galvão, D.S. Thermal Stability and Fracture Patterns of a Recently Synthesized Monolayer Fullerene Network: A Reactive Molecular Dynamics Study. Chem. Phys. Lett. 2022, 807, 140075. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kamiya, S.; Yoshimoto, S.; Suzuki, M.; Kuwahara, D.; Sasaki, N.; Miura, K. Nanocomposite Materials of Alternately StackedC60Monolayer and Graphene. J. Nanomater. 2010, 2010, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mirzayev, R.; Mustonen, K.; Monazam, M.R.A.; Mittelberger, A.; Pennycook, T.J.; Mangler, C.; Susi, T.; Kotakoski, J.; Meyer, J.C. Buckyball Sandwiches. Sci. Adv. 2017, 3, e1700176. [Google Scholar] [CrossRef] [Green Version]
- Artyukh, A.A.; Chernozatonskii, L.A. Simulation of the Formation and Mechanical Properties of Layered Structures with Polymerized Fullerene-Graphene Components. JETP Lett. 2020, 111, 109–115. [Google Scholar] [CrossRef]
- Pei, C.; Wang, L. Recent Progress on High-Pressure and High-Temperature Studies of Fullerenes and Related Materials. Matter Radiat. Extrem. 2019, 4, 028201. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Zauco, E.; Sobral, H.; Basiuk, E.V.; Saniger-Blesa, J.M.; Villagrán-Muniz, M. Polymerization of C60 Fullerene Thin Films by UV Pulsed Laser Irradiation. Appl. Surf. Sci. 2005, 248, 243–247. [Google Scholar] [CrossRef]
- Xue, Y.; Park, H.S.; Jiang, J.-W. On/off Switchable Interfacial Thermal Resistance in Graphene/Fullerene/Graphene Heterostructures. Int. J. Heat Mass Transf. 2023, 212, 124222. [Google Scholar] [CrossRef]
- Reddy, C.D.; Gen Yu, Z.; Zhang, Y.-W. Two-Dimensional van Der Waals C60 Molecular Crystal. Sci. Rep. 2015, 5, 12221. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Ji, W.; De, S. Mechanical Properties of Graphyne Monolayers: A First-Principles Study. Phys. Chem. Chem. Phys. 2012, 14, 13385–13391. [Google Scholar] [CrossRef] [Green Version]
- Meo, M.; Rossi, M. Tensile Failure Prediction of Single Wall Carbon Nanotube. Eng. Fract. Mech. 2006, 73, 2589–2599. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Z.; Xu, B.; Yu, D.; Tian, Y.; Wang, H.-T.; He, J. Compressive Strength of Diamond from First-Principles Calculation. J. Phys. Chem. C Nanomater. Interfaces 2010, 114, 17851–17853. [Google Scholar] [CrossRef]
- Liu, F.; Ming, P.; Li, J. Ab Initio Calculation of Ideal Strength and Phonon Instability of Graphene under Tension. Phys. Rev. B Condens. Matter 2007, 76, 064120. [Google Scholar] [CrossRef] [Green Version]
- Cao, K.; Feng, S.; Han, Y.; Gao, L.; Hue Ly, T.; Xu, Z.; Lu, Y. Elastic Straining of Free-Standing Monolayer Graphene. Nat. Commun. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and Analysis of Atomistic Simulation Data with OVITO–the Open Visualization Tool. Modell. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Smith, R.; Jolley, K.; Latham, C.; Heggie, M.; van Duin, A.; van Duin, D.; Wu, H. A ReaXFF Carbon Potential for Radiation Damage Studies. Nucl. Instrum. Methods Phys. Res. B 2017, 393, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF Reactive Force-Field: Development, Applications and Future Directions. npj Comput. Mater. 2016, 2. [Google Scholar] [CrossRef] [Green Version]
- David, W.I.F.; Ibberson, R.M.; Matthewman, J.C.; Prassides, K.; Dennis, T.J.S.; Hare, J.P.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Crystal Structure and Bonding of Ordered C60. Nature 1991, 353, 147–149. [Google Scholar] [CrossRef]
- Michel, K.H.; Verberck, B. Theory of the Elastic Constants of Graphite and Graphene. Phys. Status Solidi B Basic Res. 2008, 245, 2177–2180. [Google Scholar] [CrossRef]
| Substrate | Fracture Stress (GPa) | Strain Energy (J/m3) | Young’s Modulus (GPa) |
|---|---|---|---|
| qHPC60 | 24.5 | 1.6 | 191.6 |
| Gp/qHPC60 | 39.5 | 6.1 | 322.7 |
| Gp/qHP C60/Gp | 78.7 | 11.4 | 531.4 |
| Gp/qHPC60/Gp-Crack | 77.4 | 11.1 | 518.7 |
| Substrate | Fracture Stress (GPa) | Strain Energy (J/m3) | Young’s Modulus (GPa) |
|---|---|---|---|
| qTPC60 | 17.6 | 1.1 | 134.7 |
| Gp/qTPC60 | 37.4 | 5.7 | 295.9 |
| Gp/qTPC60/Gp | 76.7 | 10.7 | 504.1 |
| Gp/qTPC60/Gp-Crack | 75.3 | 10.2 | 489.1 |
| Substrate | Fracture Stress (GPa) | Fracture Strain | Young’s Modulus (GPa) |
|---|---|---|---|
| qHPC60-crack | 17.3 | 0.12 | 170.1 |
| Gp/qHPC60/Gp-crack | 77.4 | 0.36 | 518.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Li, J.; Han, M.; Zhang, Y.; Li, H.; Peng, Q.; Tang, H.-K. Enhancing the Mechanical Stability of 2D Fullerene with a Graphene Substrate and Encapsulation. Nanomaterials 2023, 13, 1936. https://doi.org/10.3390/nano13131936
Yu T, Li J, Han M, Zhang Y, Li H, Peng Q, Tang H-K. Enhancing the Mechanical Stability of 2D Fullerene with a Graphene Substrate and Encapsulation. Nanomaterials. 2023; 13(13):1936. https://doi.org/10.3390/nano13131936
Chicago/Turabian StyleYu, Taotao, Jianyu Li, Mingjun Han, Yinghe Zhang, Haipeng Li, Qing Peng, and Ho-Kin Tang. 2023. "Enhancing the Mechanical Stability of 2D Fullerene with a Graphene Substrate and Encapsulation" Nanomaterials 13, no. 13: 1936. https://doi.org/10.3390/nano13131936







