Exploring Material Properties and Device Output Performance of a Miniaturized Flexible Thermoelectric Generator Using Scalable Synthesis of Bi2Se3 Nanoflakes
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
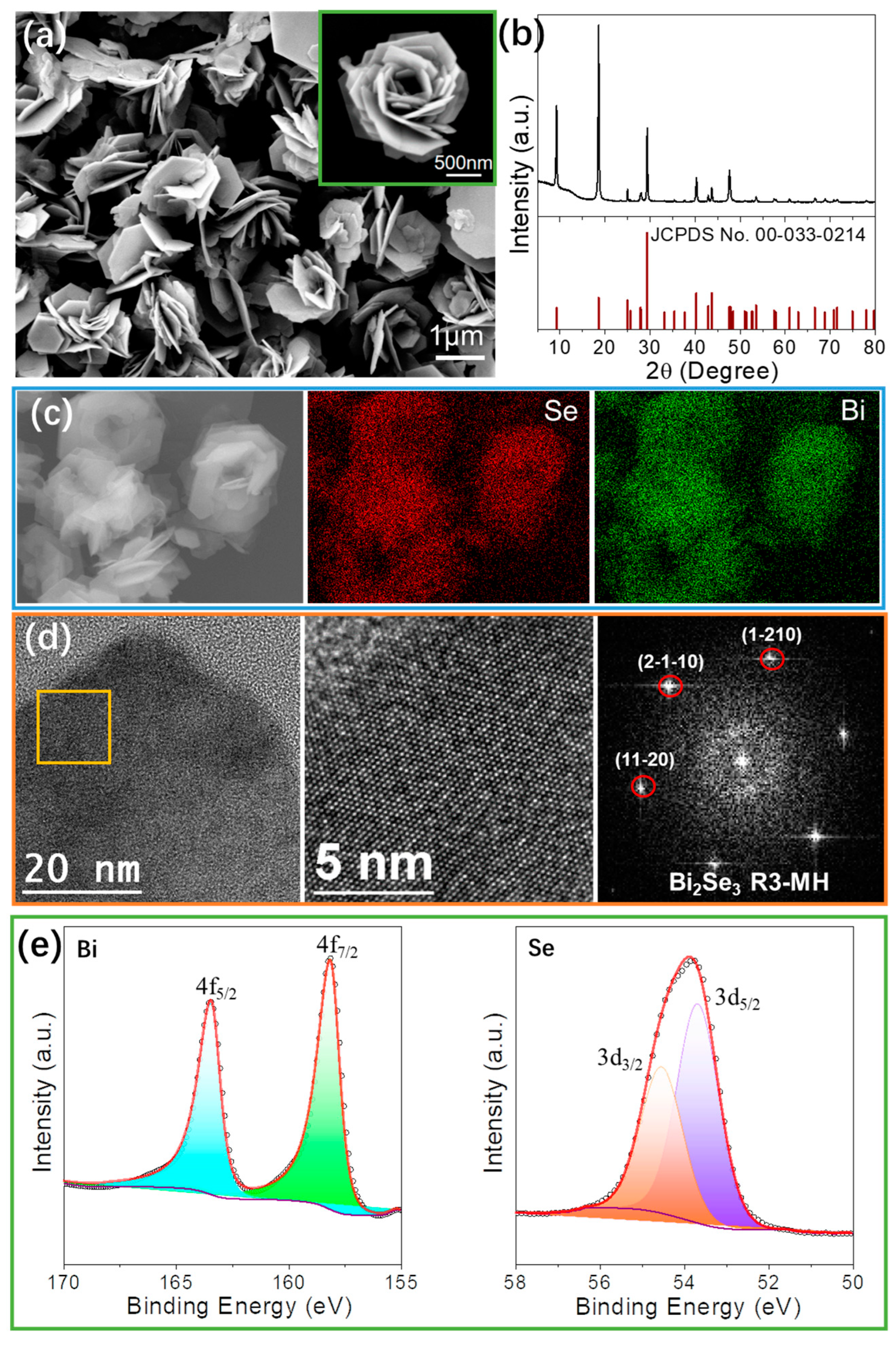
3.1. Nanomaterial
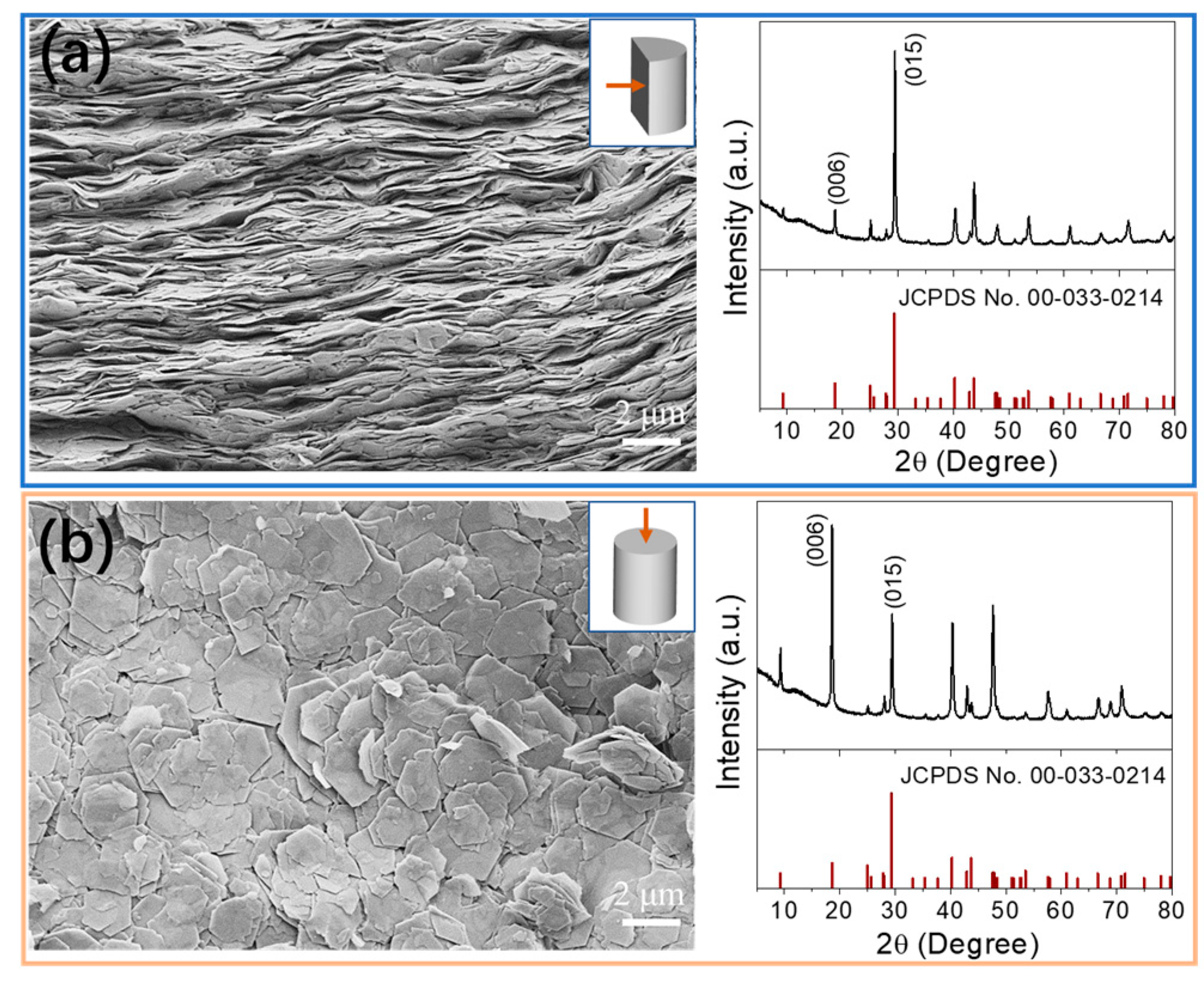
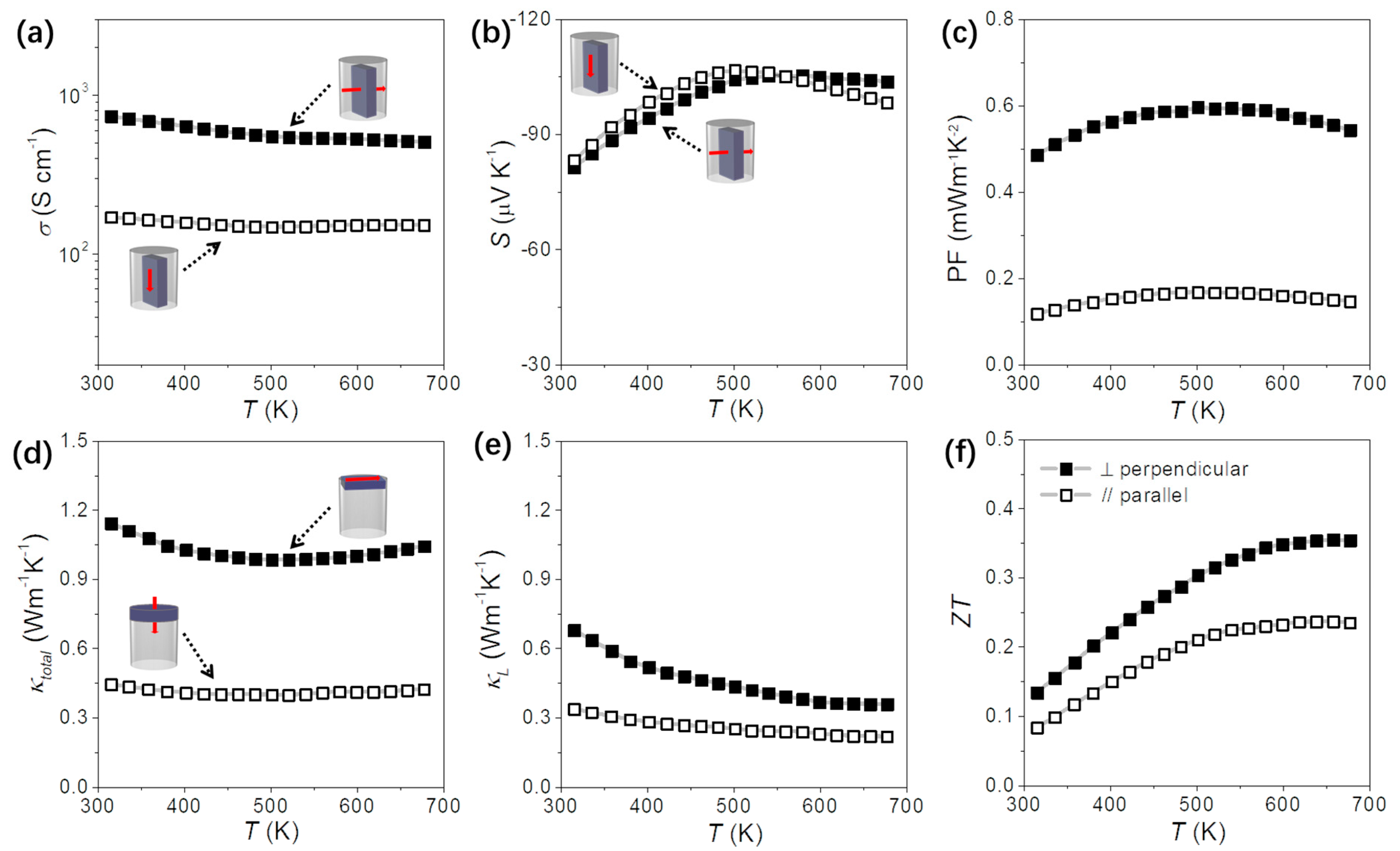
3.2. Consolidation of Bi2Se3 Nanoflakes
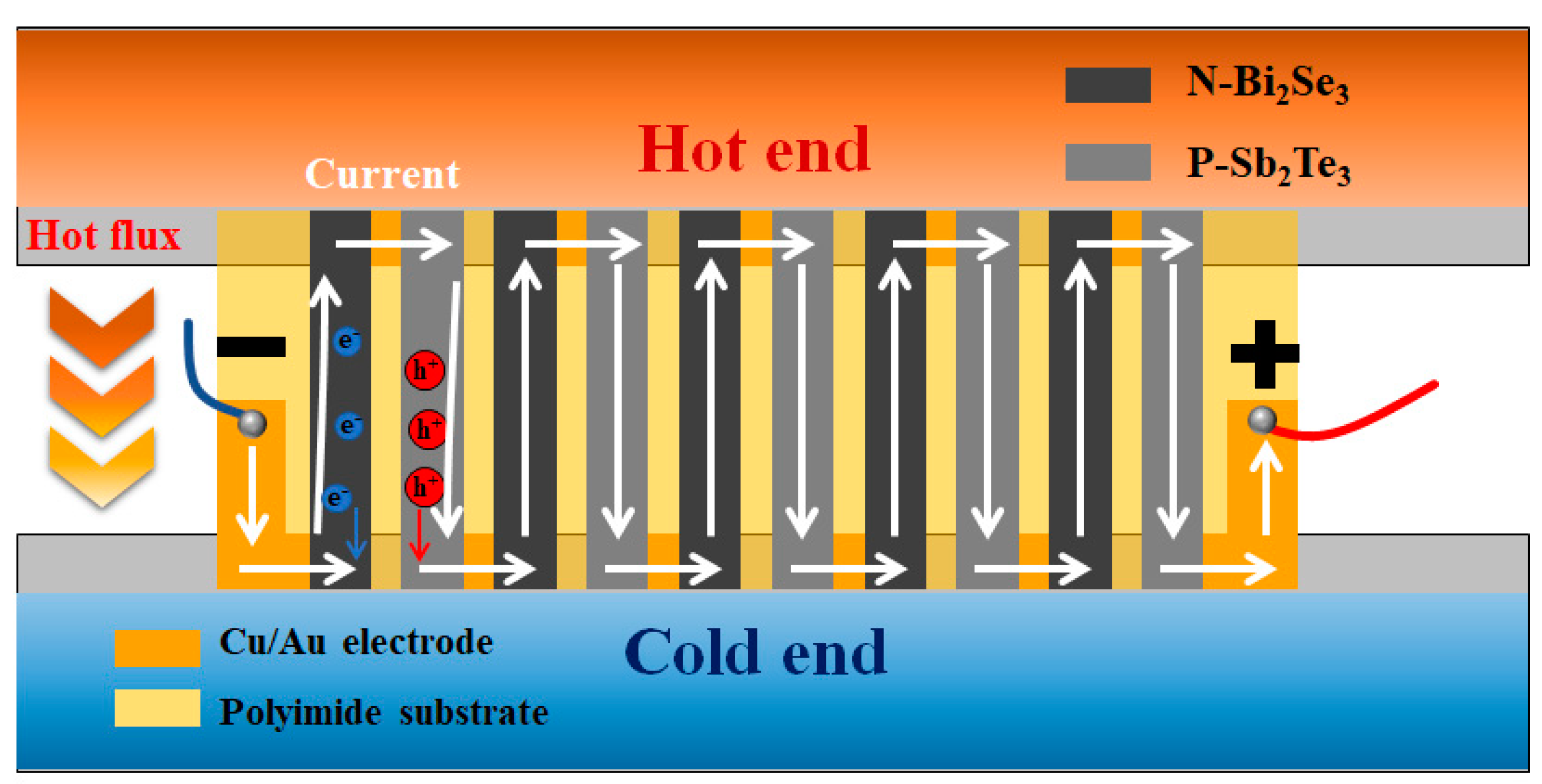
3.3. Flexible TE Generator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, M.; Chen, Z.G.; Yang, L.; Han, G.; Zou, J. Enhanced Thermoelectric Performance of Ultrathin Bi2Se3 Nanosheets through Thickness Control. Adv. Electron. Mater. 2015, 1, 1500025. [Google Scholar] [CrossRef]
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up Engineering of Thermoelectric Nanomaterials and Devices from Solution-Processed Nanoparticle Building Blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ibáñez, M. Tidying up the Mess. Science 2021, 371, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Li, T.; Luo, D.; Yu, L.; Zou, Q.; Huang, D.; Xiao, L.; Gu, H.; Ren, Z.; Ding, F. High-Performance Ag-Modified Bi0.5Sb1.5Te3 Films for the Flexible Thermoelectric Generator. ACS Appl. Mater. Interfaces 2020, 12, 7358–7365. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, Y.F.; Liu, H.B.; Meng, J.H.; Chen, W.H.; Wang, X.D. A New Configuration Design of Thermoelectric Cooler Driven by Thermoelectric Generator. Appl. Eng. 2019, 160, 114087. [Google Scholar] [CrossRef]
- Kim, S.J.; We, J.H.; Cho, B.J. A Wearable Thermoelectric Generator Fabricated on a Glass Fabric. Energy Environ. Sci. 2014, 7, 1959. [Google Scholar] [CrossRef]
- He, W.; Zhang, G.; Zhang, X.; Ji, J.; Li, G.; Zhao, X. Recent Development and Application of Thermoelectric Generator and Cooler. Appl. Energy 2015, 143, 1–25. [Google Scholar] [CrossRef]
- Yan, J.; Liao, X.; Yan, D.; Chen, Y. Review of Micro Thermoelectric Generator. J. Micro electromech. Syst. 2018, 27, 1–18. [Google Scholar] [CrossRef]
- Pourkiaei, S.M.; Ahmadi, M.H.; Sadeghzadeh, M.; Moosavi, S.; Pourfayaz, F.; Chen, L.; Pour Yazdi, M.A.; Kumar, R. Thermoelectric Cooler and Thermoelectric Generator Devices: A Review of Present and Potential Applications, Modeling and Materials. Energy 2019, 186, 115849. [Google Scholar] [CrossRef]
- Harish, S.; Sivaprahasam, D.; Jayachandran, B.; Gopalan, R.; Sundararajan, G. Performance of Bismuth Telluride Modules under Thermal Cycling in an Automotive Exhaust Thermoelectric Generator. Energy Convers Manag. 2021, 232, 113900. [Google Scholar] [CrossRef]
- Ren, W.; Sun, Y.; Zhao, D.; Aili, A.; Zhang, S.; Shi, C.; Zhang, J.; Geng, H.; Zhang, J.; Zhang, L.; et al. High-Performance Wearable Thermoelectric Generator with Self-Healing, Recycling, and Lego-like Reconfiguring Capabilities. Sci. Adv. 2021, 7, eabe0586. [Google Scholar] [CrossRef]
- Myta, C.K.; Tzounis, L.; Karalis, G.; Formanek, P.; Paipetis, A.S. High-Power All-Carbon Fully Printed and Wearable SWCNT-Based Organic Thermoelectric Generator. ACS Appl. Mater. Interfaces 2021, 13, 11151–11165. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, W.; Jin, W.; Di, C.A.; Zhu, D. Advanced Thermoelectric Materials for Flexible Cooling Application. Adv. Funct. Mater. 2021, 31, 2010695. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, G.; Lai, N.-C.; Wang, X.; Lu, Y.-C.; Prezhdo, O.V.; Xu, D. Chemically Switchable N-Type and p-Type Conduction in Bismuth Selenide Nanoribbons for Thermoelectric Energy Harvesting. ACS Nano 2021, 15, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, U.J.; Lee, J.W.; Kim, F.; Kim, Y.; Lee, G.; Son, J.S.; Baik, J.M. Triboelectric Charge-Driven Enhancement of the Output Voltage of BiSbTe-Based Thermoelectric Generators. ACS Energy Lett. 2021, 6, 1095–1103. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Cai, K.; Gao, M.; Zhao, W.; He, J.; Wei, P. Enhanced-Performance PEDOT:PSS/Cu2Se-Based Composite Films for Wearable Thermoelectric Power Generators. ACS Appl. Mater. Interfaces 2021, 13, 631–638. [Google Scholar] [CrossRef]
- Dashevsky, Z.; Mamykin, S.; Dzundza, B.; Auslender, M.; Shneck, R.Z. A Review of Nanocrystalline Film Thermoelectrics on Lead Chalcogenide Semiconductors: Progress and Application. Energies 2023, 16, 3774. [Google Scholar] [CrossRef]
- Cui, H.; Liu, H.; Li, X.; Wang, J.; Han, F.; Zhang, X.; Boughton, R.I. Synthesis of Bi2Se3 Thermoelectric Nanosheets and Nanotubes through Hydrothermal Co-Reduction Method. J. Solid. State Chem. 2004, 177, 4001–4006. [Google Scholar] [CrossRef]
- Janíček, P.; Drašar, Č.; Beneš, L.; Lošták, P. Thermoelectric Properties of Tl-Doped Bi2Se3 Single Crystals. Cryst. Res. Technol. 2009, 44, 505–510. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Z.; Soni, A.; Zhao, Y.; Xiong, Y.; Peng, B.; Wang, J.; Dresselhaus, M.S.; Xiong, Q. Raman Spectroscopy of Few-Quintuple Layer Topological Insulator Bi2Se3 Nanoplatelets. Nano Lett. 2011, 11, 2407–2414. [Google Scholar] [CrossRef]
- Liu, W.; Lukas, K.C.; McEnaney, K.; Lee, S.; Zhang, Q.; Opeil, C.P.; Chen, G.; Ren, Z. Studies on the Bi2Te3-Bi2Se3-Bi2S3 System for Mid-Temperature Thermoelectric Energy Conversion. Energy Environ. Sci. 2013, 6, 552–560. [Google Scholar] [CrossRef]
- Min, Y.; Roh, J.W.; Yang, H.; Park, M.; Kim, S.I.; Hwang, S.; Lee, S.M.; Lee, K.H.; Jeong, U. Surfactant-Free Scalable Synthesis of Bi2Te3 and Bi2Se3 Nanoflakes and Enhanced Thermoelectric Properties of Their Nanocomposites. Adv. Mater. 2013, 25, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Y.; Yang, J.; Duan, B.; Wu, L.; Zhang, W.; Yang, J. High Thermoelectric Performance in Te-Free (Bi,Sb)2Se3: Via Structural Transition Induced Band Convergence and Chemical Bond Softening. Energy Environ. Sci. 2016, 9, 3436–3447. [Google Scholar] [CrossRef]
- Sassi, S.; Candolfi, C.; Delaizir, G.; Migot, S.; Ghanbaja, J.; Gendarme, C.; Dauscher, A.; Malaman, B.; Lenoir, B. Crystal Structure and Transport Properties of the Homologous Compounds (PbSe)5(Bi2Se3)3m (m = 2, 3). Inorg. Chem. 2018, 57, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, D.; Wu, H.; Wang, J.; Zhang, Y.; Wang, G.; Pennycook, S.J.; Zhao, L.D. Intrinsically Low Thermal Conductivity in BiSbSe3: A Promising Thermoelectric Material with Multiple Conduction Bands. Adv. Funct. Mater. 2019, 29, 1806558. [Google Scholar] [CrossRef]
- Samanta, M.; Pal, K.; Pal, P.; Waghmare, U.V.; Biswas, K. Localized Vibrations of Bi Bilayer Leading to Ultralow Lattice Thermal Conductivity and High Thermoelectric Performance in Weak Topological Insulator N-Type BiSe. J. Am. Chem. Soc. 2018, 140, 5866–5872. [Google Scholar] [CrossRef]
- Maurya, V.K.; Dong, C.L.; Chen, C.L.; Asokan, K.; Patnaik, S. High Spin State Driven Magnetism and Thermoelectricity in Mn Doped Topological Insulator Bi2Se3. J. Magn. Magn. Mater. 2018, 456, 1–5. [Google Scholar] [CrossRef]
- Zong, P.A.; Zhang, P.; Yin, S.; Huang, Y.; Wang, Y.; Wan, C. Fabrication and Characterization of a Hybrid Bi2Se3/Organic Superlattice for Thermoelectric Energy Conversion. Adv. Electron. Mater. 2019, 5, 1800842. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Cai, S.; Hao, S.; Bailey, T.P.; Hu, X.; Hanus, R.; Ma, R.; Tan, G.; Chica, D.G.; Snyder, G.J.; et al. Ultralow Thermal Conductivity and High-Temperature Thermoelectric Performance in n-Type K2.5Bi8.5Se14. Chem. Mater. 2019, 31, 5943–5952. [Google Scholar] [CrossRef]
- Jia, F.; Liu, Y.Y.; Zhang, Y.F.; Shu, X.; Chen, L.; Wu, L.M. Bi8Se7: Delocalized Interlayer π-Bond Interactions Enhancing Carrier Mobility and Thermoelectric Performance near Room Temperature. J. Am. Chem. Soc. 2020, 142, 12536–12543. [Google Scholar] [CrossRef]
- Bauer, C.; Veremchuk, I.; Kunze, C.; Benad, A.; Dzhagan, V.M.; Haubold, D.; Pohl, D.; Schierning, G.; Nielsch, K.; Lesnyak, V.; et al. Heterostructured Bismuth Telluride Selenide Nanosheets for Enhanced Thermoelectric Performance. Small Sci. 2021, 1, 2000021. [Google Scholar] [CrossRef]
- Min, Y.; Park, G.; Kim, B.; Giri, A.; Zeng, J.; Roh, J.W.; Kim, S.I.; Lee, K.H.; Jeong, U. Synthesis of Multishell Nanoplates by Consecutive Epitaxial Growth of Bi2Se3 and Bi2Te3 Nanoplates and Enhanced Thermoelectric Properties. ACS Nano 2015, 9, 6843–6853. [Google Scholar] [CrossRef]
- Dun, C.; Hewitt, C.A.; Huang, H.; Xu, J.; Zhou, C.; Huang, W.; Cui, Y.; Zhou, W.; Jiang, Q.; Carroll, D.L. Flexible N-Type Thermoelectric Films Based on Cu-Doped Bi2Se3 Nanoplate and Polyvinylidene Fluoride Composite with Decoupled Seebeck Coefficient and Electrical Conductivity. Nano Energy 2015, 18, 306–314. [Google Scholar] [CrossRef]
- Xu, B.; Feng, T.; Agne, M.T.; Tan, Q.; Li, Z.; Imasato, K.; Zhou, L.; Bahk, J.-H.; Ruan, X.; Snyder, G.J.; et al. Manipulating Band Structure through Reconstruction of Binary Metal Sulfide for High-Performance Thermoelectrics in Solution-Synthesized Nanostructured Bi13S18I2. Angew. Chem. 2018, 130, 2437–2442. [Google Scholar] [CrossRef]
- Adam, A.M.; Elshafaie, A.; Mohamed, A.E.M.A.; Petkov, P.; Ibrahim, E.M.M. Thermoelectric Properties of Te Doped Bulk Bi2Se3 System. Mater. Res. Express 2018, 5, 035514. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, Y.; Zhou, W.; Tang, D. Temperature-Dependent Raman Scattering of Large Size Hexagonal Bi2Se3 Single-Crystal Nanoplates. Appl. Sci. 2018, 8, 1794. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, Y.; Zhang, T.; Zuo, Y.; Xiao, K.; Arbiol, J.; Llorca, J.; Liu, Y.; Cabot, A. Enhanced Thermoelectric Performance of N-Type Bi2se3 Nanosheets through Sn Doping. Nanomaterials 2021, 11, 1827. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ortega, S.; Ibáñez, M.; Lim, K.H.; Grau-Carbonell, A.; Martí-Sánchez, S.; Ng, K.M.; Arbiol, J.; Kovalenko, M.V.; et al. Crystallographically Textured Nanomaterials Produced from the Liquid Phase Sintering of BixSb2-XTe3Nanocrystal Building Blocks. Nano Lett. 2018, 18, 2557–2563. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.; Lim, K.H.; Ibáñez, M.; Ortega, S.; Li, M.; David, J.; Martí-Sánchez, S.; Ng, K.M.; Arbiol, J.; et al. High Thermoelectric Performance in Crystallographically Textured N-Type Bi2Te3-xSex Produced from Asymmetric Colloidal Nanocrystals. ACS Nano 2018, 12, 7174–7184. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Tang, X.; Liu, Y.; Xu, Z.; Liu, K.; Li, J.; Zhang, Z.; Wang, H. Improving the Performance of a Screen-Printed Micro-Radioisotope Thermoelectric Generator through Stacking Integration. J. Power Sources 2019, 414, 509–516. [Google Scholar] [CrossRef]
- Yuan, Z.; Tang, X.; Xu, Z.; Li, J.; Chen, W.; Liu, K.; Liu, Y.; Zhang, Z. Screen-Printed Radial Structure Micro Radioisotope Thermoelectric Generator. Appl. Energy 2018, 225, 746–754. [Google Scholar] [CrossRef]
- Le, P.H.; Wu, K.H.; Luo, C.W.; Leu, J. Growth and Characterization of Topological Insulator Bi2Se3 Thin Films on SrTiO3 Using Pulsed Laser Deposition. Thin. Solid Film. 2013, 534, 659–665. [Google Scholar] [CrossRef]
- Yan, H.; Lin, S.; Zhang, R.; Li, H.; Fu, B.; Liu, J.; Liu, L.; Kunsági-Máté, S.; An, Y. Facile Synthesis of High-Crystalline Bi2Se3 Nanoribbons without Se Vacancies and Their Properties. J. Mater. Sci. 2020, 55, 5145–5155. [Google Scholar] [CrossRef]
- Buks, K.; Andzane, J.; Smits, K.; Zicans, J.; Bitenieks, J.; Zarins, A.; Erts, D. Growth Mechanisms and Related Thermoelectric Properties of Innovative Hybrid Networks Fabricated by Direct Deposition of Bi2Se3 and Sb2Te3 on Multiwalled Carbon Nanotubes. Mater. Today Energy 2020, 18, 100526. [Google Scholar] [CrossRef]
- Andzane, J.; Buks, K.; Bitenieks, J.; Bugovecka, L.; Kons, A.; Merijs-Meri, R.; Svirksts, J.; Zicans, J.; Erts, D. P-Type PVA/MWCNT-Sb2Te3 Composites for Application in Different Types of Flexible Thermoelectric Generators in Combination with n-Type PVA/MWCNT-Bi2Se3 Composites. Polymers 2022, 14, 5130. [Google Scholar] [CrossRef]



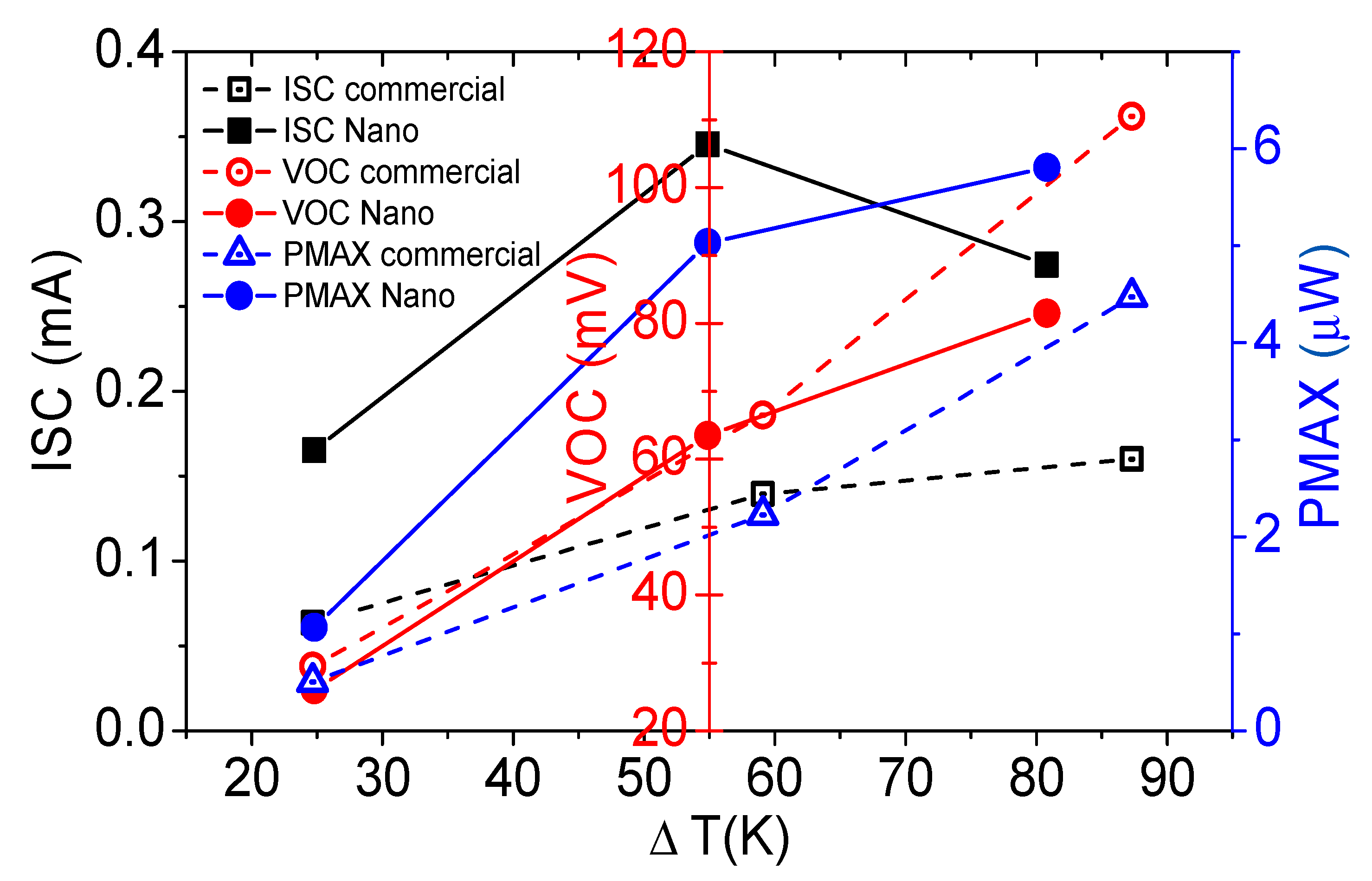
| ΔT (K) | Isc (μA) | Voc (mV) | Pmax (μW) | Rint (Ω) | E (mV·K−1) | Ρ (mW·cm−1·K−1) | |
|---|---|---|---|---|---|---|---|
| Bi2Se3 nanoflakes | 24.8 | 165.27 | 26.02 | 1.06 | 157.44 | 1.05 | 0.0536 |
| 54.9 | 345.37 | 63.51 | 5.03 | 183.87 | 1.16 | 0.1146 | |
| 80.8 | 274.36 | 81.51 | 5.80 | 297.07 | 1.01 | 0.0898 | |
| 109.5 | 340.43 | 111.00 | 9.51 | 326.07 | 1.01 | 0.1086 | |
| Commercial Bi2Se3 | 24.7 | 63.37 | 29.48 | 505.73 | 465.18 | 1.19 | 0.0256 |
| 59.1 | 139.46 | 66.51 | 2.23 | 476.87 | 1.13 | 0.0471 | |
| 87.3 | 160.01 | 110.49 | 4.47 | 690.51 | 1.27 | 0.0640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Zhao, X.; Wang, C.; Hang, S.; Li, M.; Liu, Y. Exploring Material Properties and Device Output Performance of a Miniaturized Flexible Thermoelectric Generator Using Scalable Synthesis of Bi2Se3 Nanoflakes. Nanomaterials 2023, 13, 1937. https://doi.org/10.3390/nano13131937
Yuan Z, Zhao X, Wang C, Hang S, Li M, Liu Y. Exploring Material Properties and Device Output Performance of a Miniaturized Flexible Thermoelectric Generator Using Scalable Synthesis of Bi2Se3 Nanoflakes. Nanomaterials. 2023; 13(13):1937. https://doi.org/10.3390/nano13131937
Chicago/Turabian StyleYuan, Zicheng, Xueke Zhao, Canhui Wang, Shuang Hang, Mengyao Li, and Yu Liu. 2023. "Exploring Material Properties and Device Output Performance of a Miniaturized Flexible Thermoelectric Generator Using Scalable Synthesis of Bi2Se3 Nanoflakes" Nanomaterials 13, no. 13: 1937. https://doi.org/10.3390/nano13131937





