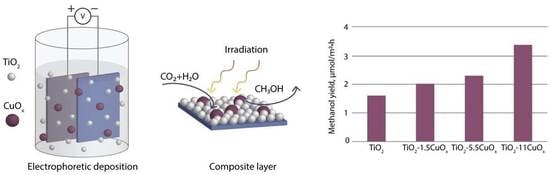
The Composite TiO2–CuOx Layers Formed by Electrophoretic Method for CO2 Gas Photoreduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Electrophoretic Deposition Process
2.2. Material Characterization
- Scanning speed 10 °C·min−1;
- Scan range from 3° to 90°;
- Radiation Cu Kα;
- The voltage on the tube 20 Kv;
- The anode current 7 mA.
2.3. Optical Band Gap Investigation
2.4. Photoelectrochemical Measurements
2.5. Photocatalytic Activity Study
3. Results
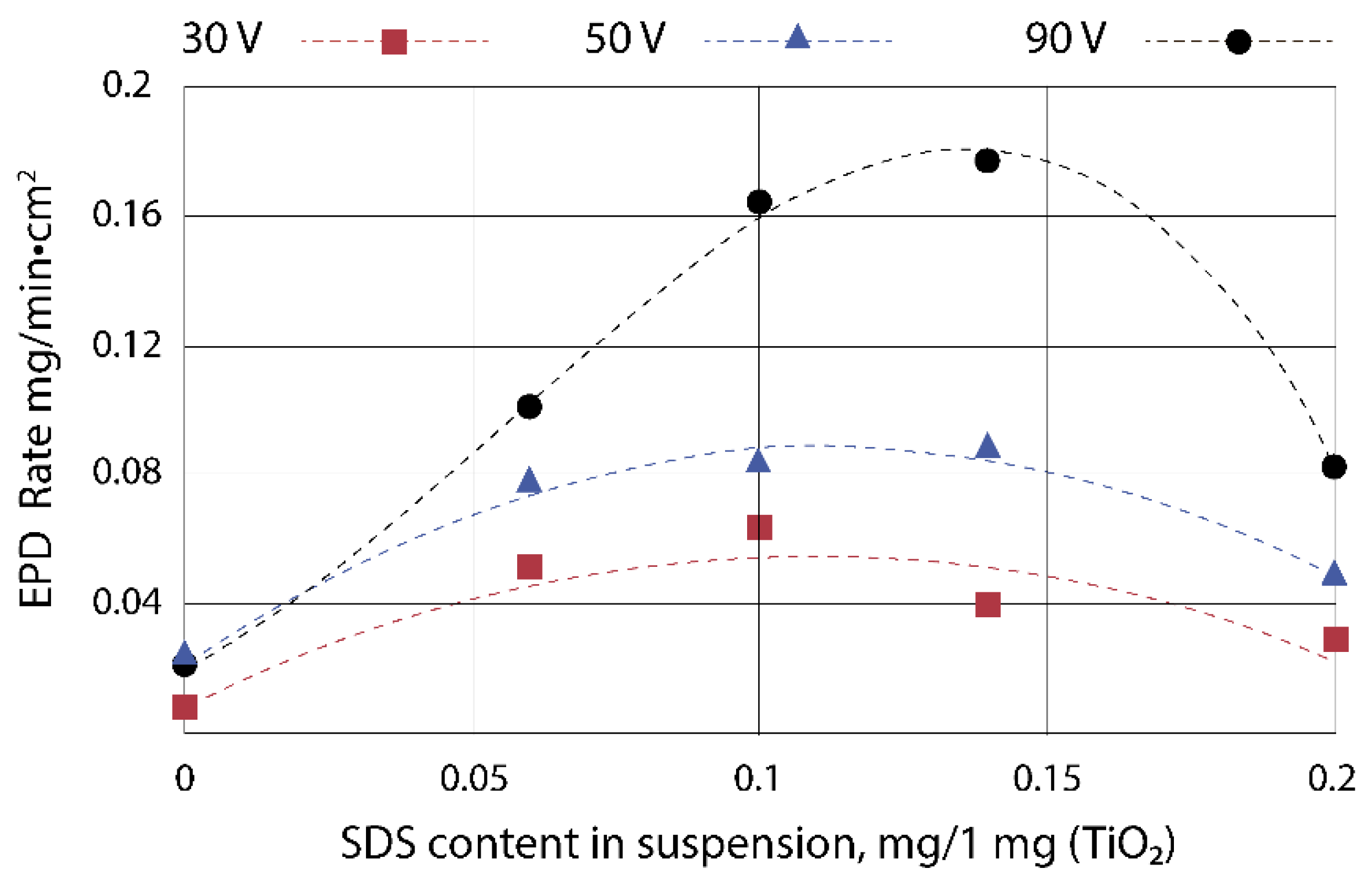
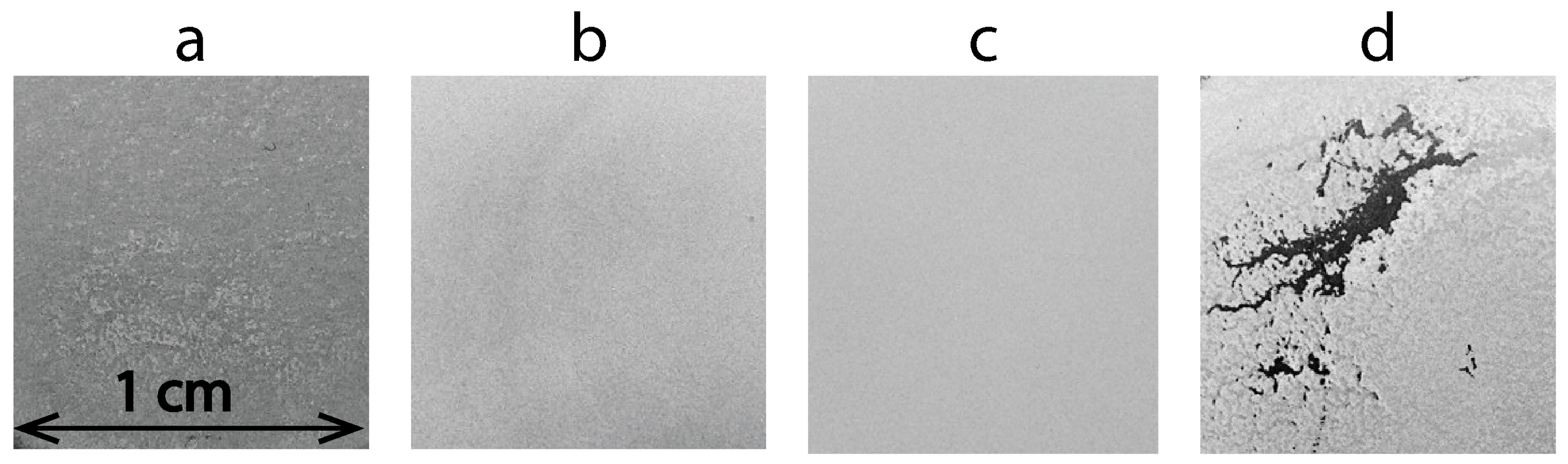
3.1. Electrophoretic Deposition of TiO2 Layers
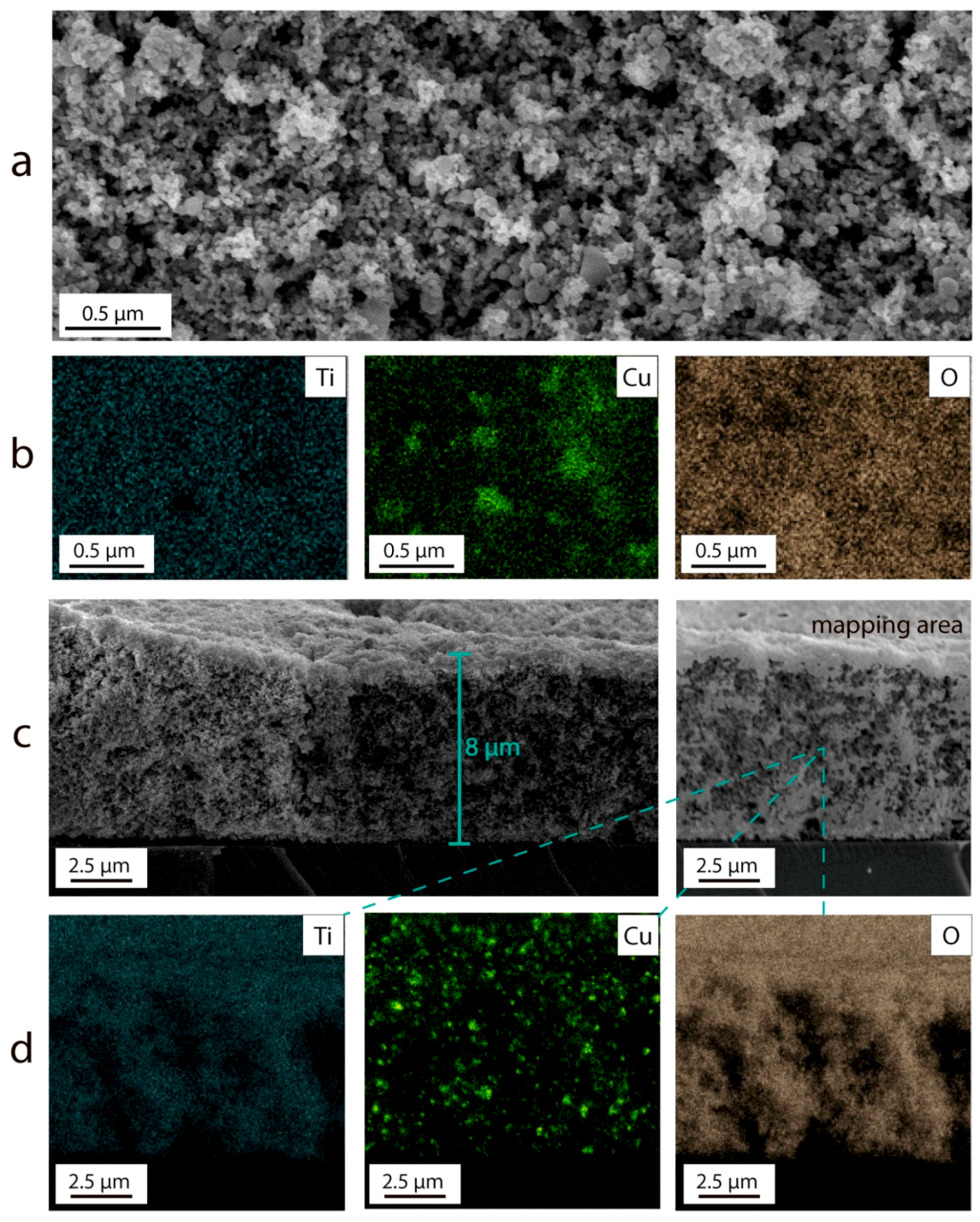
3.2. Electrophoretic Deposition of TiO2–CuOx Layers
3.3. Optical Band Gap
3.4. Open-Circuit Potential
3.5. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luévano-Hipólito, E.; Garay-Rodríguez, L.F.; Torres-Martínez, L.M. Photocatalytic Heterostructured Materials for Air Decontamination and Solar Fuels Production. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 593–636. ISBN 9780128224465. [Google Scholar]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A Heterojunction Strategy to Improve the Visible Light Sensitive Water Splitting Performance of Photocatalytic Materials. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 21696–21718. [Google Scholar] [CrossRef]
- Heterostructured Photocatalysts for Solar Energy Conversion; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128200728.
- Shawky, A.; Mohamed, R.M.; Alahmadi, N.; Zaki, Z.I. Enhanced Photocatalytic Reduction of Hexavalent Chromium Ions over S-Scheme Based 2D MoS2-Supported TiO2 Heterojunctions under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128564. [Google Scholar] [CrossRef]
- Cosma, D.; Urda, A.; Radu, T.; Rosu, M.C.; Mihet, M.; Socaci, C. Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules 2022, 27, 5803. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, B.; Zhang, W.; Xiao, Y.; Xu, H.; Liu, Y.; Liu, Z.; Zhang, J.; Jiang, Y. Visible-Light-Driven Double-Shell SnIn4S8/TiO2 Heterostructure with Enhanced Photocatalytic Activity for MO Removal and Cr(VI) Cleanup. Appl. Surf. Sci. 2022, 587, 152867. [Google Scholar] [CrossRef]
- Matias, M.L.; Reis-Machado, A.S.; Rodrigues, J.; Calmeiro, T.; Deuermeier, J.; Pimentel, A.; Fortunato, E.; Martins, R.; Nunes, D. Microwave Synthesis of Visible-Light-Activated g-C3N4/TiO2 Photocatalysts. Nanomaterials 2023, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, J.; Wang, X.; Wang, Z.; Zhang, M.; He, G.; Sun, Z. A Unique Cu2O/TiO2 Nanocomposite with Enhanced Photocatalytic Performance under Visible Light Irradiation. Ceram. Int. 2017, 43, 4866–4872. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Zhao, X.; Wu, Q.; Qi, X.; Chen, X.; Hu, T.; Cao, Y. Photocatalytic Oxidation of Gas-Phase Hg0 by CuO/TiO2. Appl. Catal. B 2015, 176–177, 559–569. [Google Scholar] [CrossRef]
- Lu, D.; Zelekew, O.A.; Abay, A.K.; Huang, Q.; Chen, X.; Zheng, Y. Synthesis and Photocatalytic Activities of a CuO/TiO2 Composite Catalyst Using Aquatic Plants with Accumulated Copper as a Template. RSC Adv. 2019, 9, 2018–2025. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Ismail, A.A. Uniform Dispersion of CuO Nanoparticles on Mesoporous TiO2 Networks Promotes Visible Light Photocatalysis. Ceram. Int. 2020, 46, 8819–8826. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Colbeau-Justin, C.; Nitta, A.; Kowalska, E. P25 and Its Components—Electronic Properties and Photocatalytic Activities. Surf. Interfaces 2022, 31, 102057. [Google Scholar] [CrossRef]
- Xu, N.; Huang, H.; Ouyang, H.; Wang, H. Preparation of the Heterojunction Catalyst N-Doping Carbon Quantum Dots/P25 and Its Visible Light Photocatalytic Activity. Sci. Rep. 2019, 9, 9971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, Z.; Xing, Z.; Song, Z.; Ye, B.; Wang, Z.; Wu, Q. UV-LED/P25-Based Photocatalysis for Effective Degradation of Isothiazolone Biocide. Front. Environ. Sci. Eng. 2021, 15, 85. [Google Scholar] [CrossRef]
- Zouzelka, R.; Remzova, M.; Brabec, L.; Rathousky, J. Photocatalytic Performance of Porous TiO2 Layers Prepared by Quantitative Electrophoretic Deposition from Organic Solvents. Appl. Catal. B 2018, 227, 70–78. [Google Scholar] [CrossRef]
- Dhiflaoui, H.; Khlifi, K.; Barhoumi, N.; Ben Cheikh Larbi, A. The Tribological and Corrosion Behavior of TiO2 Coatings Deposited by the Electrophoretic Deposition Process. Proc. Inst. Mech. Eng. Part C 2020, 234, 1231–1238. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Song, S. Electrophoretic Mobility Study for Heterocoagulation of Montmorillonite with Fluorite in Aqueous Solutions. Powder Technol. 2017, 309, 61–67. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Zhitomirsky, I. Electrophoretic Deposition of TiO2 and Composite TiO2-MnO2 Films Using Benzoic Acid and Phenolic Molecules as Charging Additives. J. Colloid Interface Sci. 2010, 352, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Zhitomirsky, I. Dispersing Agents for Electrophoretic Deposition of TiO2 and TiO2–Carbon Nanotube Composites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 131–138. [Google Scholar] [CrossRef]
- Parsi Benehkohal, N.; Demopoulos, G.P. Electrophoretically Self-Assembled Mixed Metal Oxide-TiO2 Nano-Composite Film Structures for Photoelectrochemical Energy Conversion: Probing of Charge Recombination and Electron Transport Resistances. J. Power Sources 2013, 240, 667–675. [Google Scholar] [CrossRef]
- Kubelka, P. Ein Beitrag Zur Optik Der Farbanstriche (Contribution to the Optic of Paint). Z. Fur Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Simmons, E.L. Diffuse Reflectance Spectroscopy: A Comparison of the Theories. Appl. Opt. 1975, 14, 1380. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-Gap Energy Estimation from Diffuse Reflectance Measurements on Sol–Gel and Commercial TiO2: A Comparative Study. J. Solgel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Kotarba, S.; Sulka, G.D.; Syrek, K. Synthesis and Spectroelectrochemical Investigation of Anodic Black TiOx Nanotubes. Nanomaterials 2023, 13, 931. [Google Scholar] [CrossRef]
- Landi, S., Jr.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and Misuse of the Kubelka-Munk Function to Obtain the Band Gap Energy from Diffuse Reflectance Measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Yeredla, R.R.; Xu, H. An Investigation of Nanostructured Rutile and Anatase Plates for Improving the Photosplitting of Water. Nanotechnology 2008, 19, 055706. [Google Scholar] [CrossRef] [PubMed]
- Boukhoubza, I.; Matei, E.; Jorio, A.; Enculescu, M.; Enculescu, I. Electrochemical Deposition of ZnO Nanowires on CVD-Graphene/Copper Substrates. Nanomaterials 2022, 12, 2858. [Google Scholar] [CrossRef] [PubMed]
- Landi, S., Jr. Comment on “Photocatalytic Degradation of RhB from an Aqueous Solution Using Ag3PO4/N-TiO2 Heterostructure” and “Evaluation of the Effect of Dose Change of Fe3O4 Nanoparticles on Electrochemical Biosensor Compatibility Using Hydrogels as an Experimental Living Organism Model. ” J. Mol. Liq. 2021, 338, 116635. [Google Scholar] [CrossRef]
- Landi, S., Jr.; Segundo, I.R.; Afonso, C.; Lima, O., Jr.; Costa, M.F.M.; Freitas, E.; Carneiro, J. Evaluation of Band Gap Energy of TiO2 Precipitated from Titanium Sulphate. Phys. B Condens. Matter 2022, 639, 414008. [Google Scholar] [CrossRef]
- Patel, M.; Chavda, A.; Mukhopadhyay, I.; Kim, J.; Ray, A. Nanostructured SnS with Inherent Anisotropic Optical Properties for High Photoactivity. Nanoscale 2016, 8, 2293–2303. [Google Scholar] [CrossRef]
- Emeline, A.V.; Rudakova, A.V.; Mikhaylov, R.V.; Bulanin, K.M.; Bahnemann, D.W. Photoactive Heterostructures: How They Are Made and Explored. Catalysts 2021, 11, 294. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, A. Novel CuO/TiO2 Nanocomposite Films with a Graded Band Gap for Visible Light Irradiation. Mater. Express 2012, 2, 238–244. [Google Scholar] [CrossRef]
- Koohestani, H.; Sadrnezhaad, S.K. Photocatalytic Degradation of Methyl Orange and Cyanide by Using TiO2/CuO Composite. Desalination Water Treat. 2016, 57, 22029–22038. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobedo-Morales, A.; Ruiz-López, I.I.; Ruiz-Peralta, M.D.; Tepech-Carrillo, L.; Sánchez-Cantú, M.; Moreno-Orea, J.E. Automated Method for the Determination of the Band Gap Energy of Pure and Mixed Powder Samples Using Diffuse Reflectance Spectroscopy. Heliyon 2019, 5, e01505. [Google Scholar] [CrossRef] [Green Version]
- Balık, M.; Bulut, V.; Erdogan, I.Y. Optical, Structural and Phase Transition Properties of Cu2O, CuO and Cu2O/CuO: Their Photoelectrochemical Sensor Applications. Int. J. Hydrogen Energy 2019, 44, 18744–18755. [Google Scholar] [CrossRef]
- van de Krol, R.; Grätzel, M. (Eds.) Photoelectrochemical Hydrogen Production; Springer: Boston, MA, USA, 2012; ISBN 9781461413790. [Google Scholar]
- Beranek, R. (Photo)Electrochemical Methods for the Determination of the Band Edge Positions of TiO2-Based Nanomaterials. Adv. Phys. Chem. 2011, 2011, 786759. [Google Scholar] [CrossRef] [Green Version]
- Zare, M.; Solaymani, S.; Shafiekhani, A.; Kulesza, S.; Ţălu, Ş.; Bramowicz, M. Evolution of Rough-Surface Geometry and Crystalline Structures of Aligned TiO2 Nanotubes for Photoelectrochemical Water Splitting. Sci. Rep. 2018, 8, 10870. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Van Le, Q. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Hamad, H.; Elsenety, M.M.; Sadik, W.; El-Demerdash, A.-G.; Nashed, A.; Mostafa, A.; Elyamny, S. The Superior Photocatalytic Performance and DFT Insights of S-Scheme CuO@TiO2 Heterojunction Composites for Simultaneous Degradation of Organics. Sci. Rep. 2022, 12, 2217. [Google Scholar] [CrossRef]
- Park, S.-M.; Razzaq, A.; Park, Y.H.; Sorcar, S.; Park, Y.; Grimes, C.A.; In, S.-I. Hybrid CuxO-TiO2 Heterostructured Composites for Photocatalytic CO2 Reduction into Methane Using Solar Irradiation: Sunlight into Fuel. ACS Omega 2016, 1, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Konstantinova, E.; Savchuk, T.; Pinchuk, O.; Kytina, E.; Ivanova, E.; Volkova, L.; Zaitsev, V.; Pavlikov, A.; Elizarova, E. Photoelectron Properties and Organic Molecules Photodegradation Activity of Titania Nanotubes with CuxO Nanoparticles Heat Treated in Air and Argon. Molecules 2022, 27, 8080. [Google Scholar] [CrossRef] [PubMed]
- Savchuk, T.P.; Kytina, E.V.; Konstantinova, E.A.; Kytin, V.G.; Pinchuk, O.; Tarhanov, A.K.; Zaitsev, V.B.; Maniecki, T. Photocatalytic CO2 Conversion Using Anodic TiO2 Nanotube-CuxO Composites. Catalysts 2022, 12, 1011. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 Heterostructures for CO2 Reduction through a Direct Z-Scheme: Protecting Cu2O from Photocorrosion. Appl. Catal. B 2017, 217, 485–493. [Google Scholar] [CrossRef]




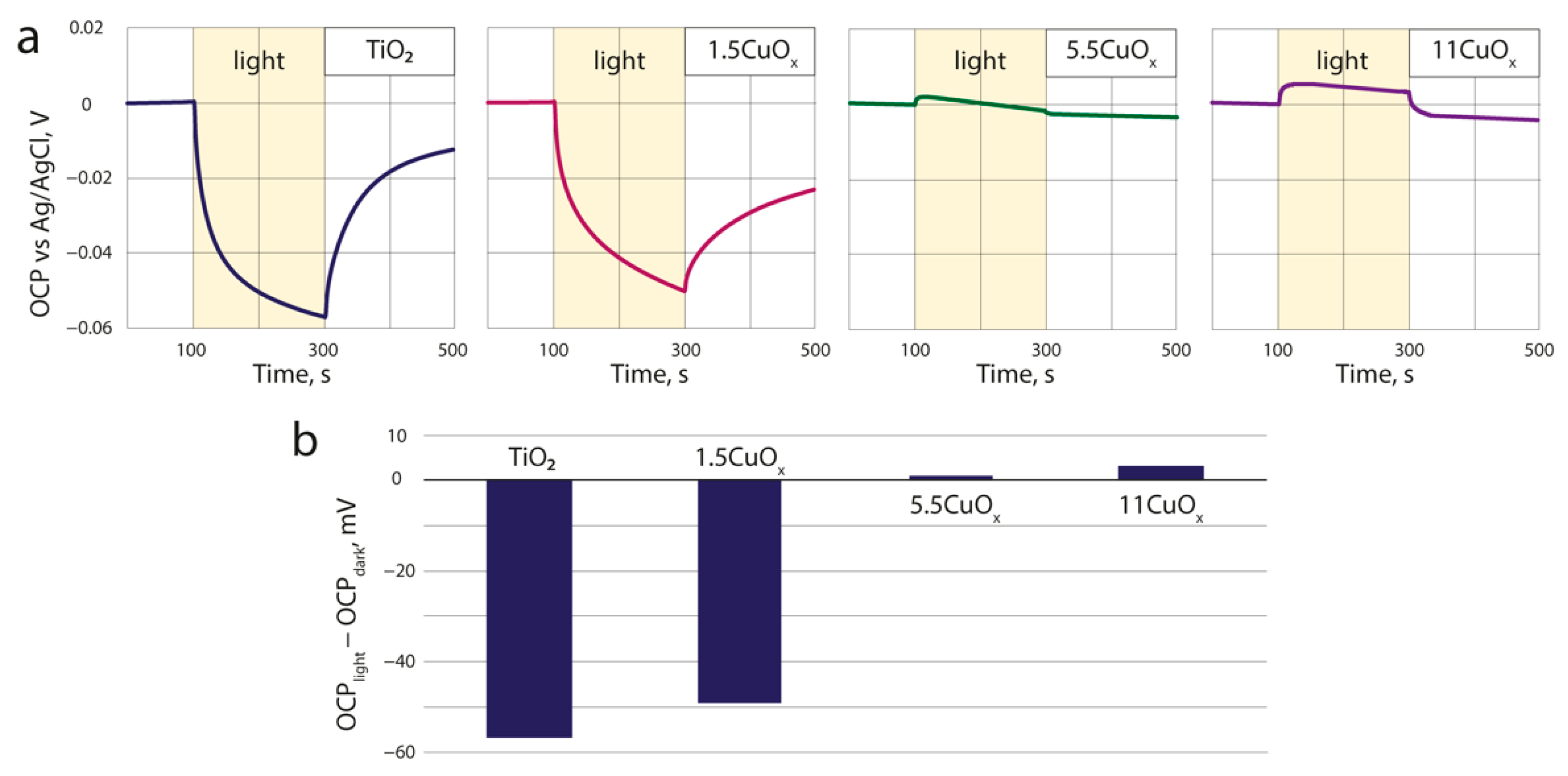
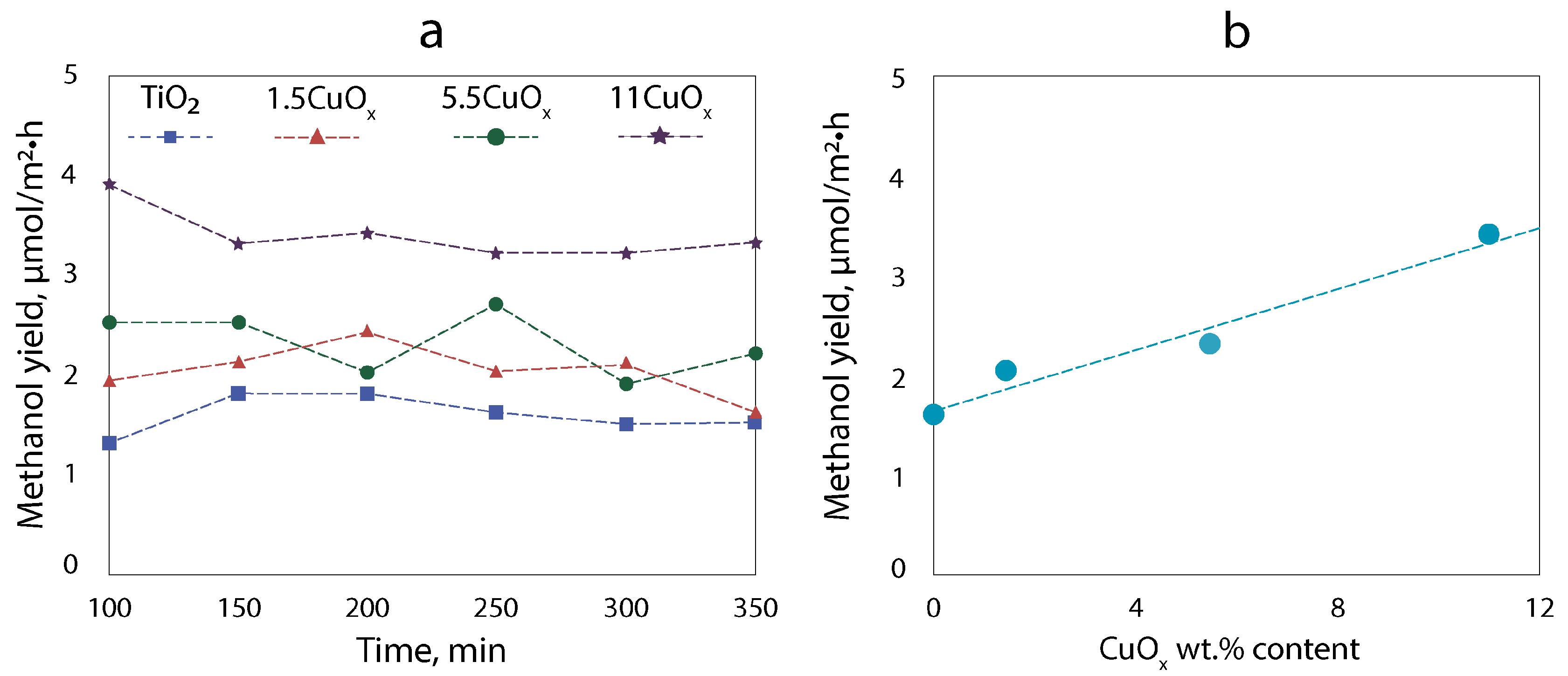
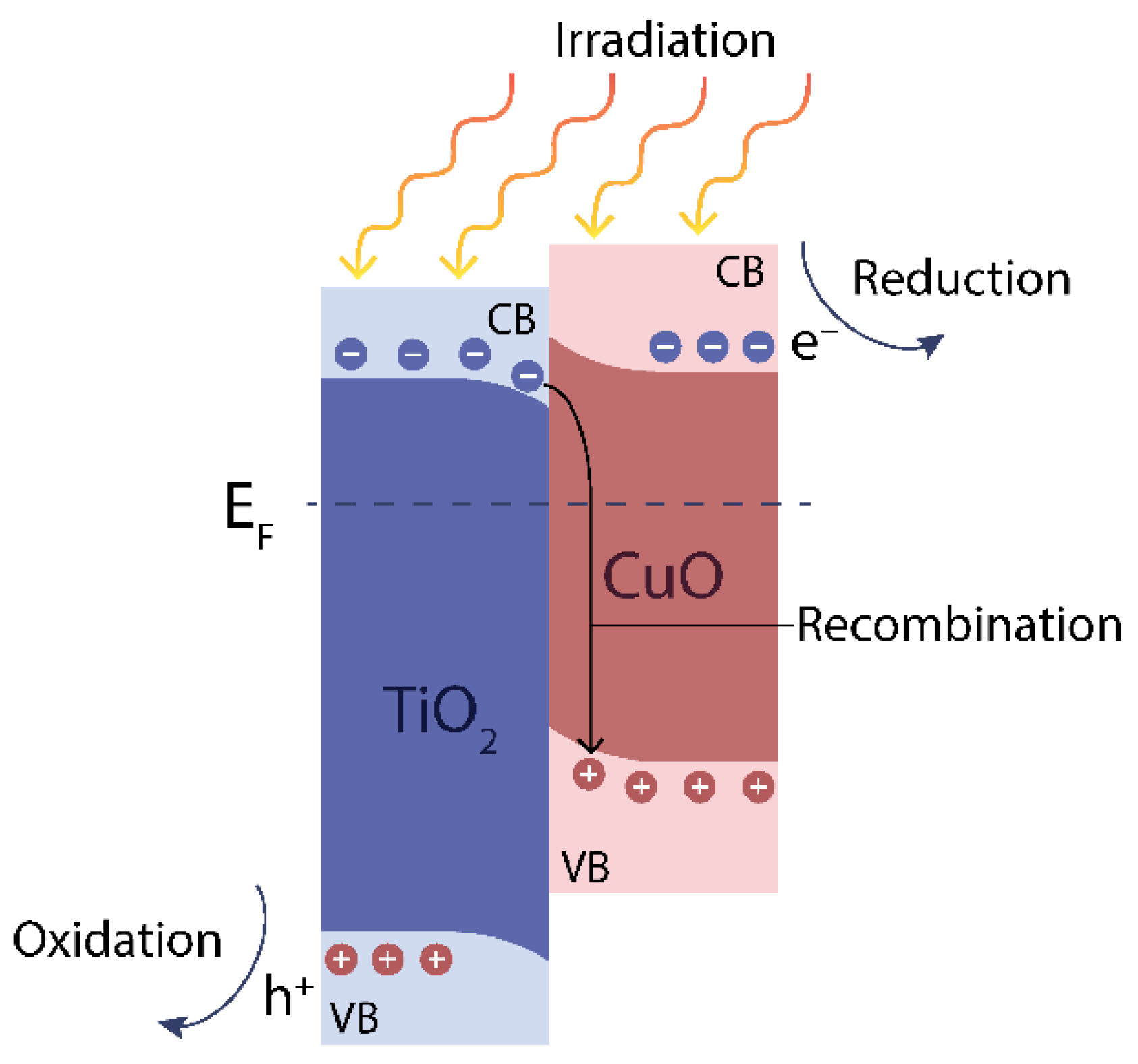
| CuOx wt.% in the Suspension | CuOx wt.% in the Deposited Layer |
|---|---|
| 3 | 1.5 |
| 10 | 5.5 |
| 20 | 11 |
| CuOx Content, wt.% | Eg1opt, eV | Eg21opt, eV | Eg22opt, eV |
|---|---|---|---|
| 0 | - | 3.08 | 3.08 |
| 1.5 | 1.41 | 2.89 | 3.13 |
| 5.5 | 1.44 | 2.55 | 3.25 |
| 11 | 1.44 | 2.03 | 3.36 |
| 100 | 1.36 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokina, L.I.; Tarasov, A.M.; Pepelyaeva, A.I.; Lazarenko, P.I.; Trifonov, A.Y.; Savchuk, T.P.; Kuzmin, A.V.; Tregubov, A.V.; Shabaeva, E.N.; Zhurina, E.S.; et al. The Composite TiO2–CuOx Layers Formed by Electrophoretic Method for CO2 Gas Photoreduction. Nanomaterials 2023, 13, 2030. https://doi.org/10.3390/nano13142030
Sorokina LI, Tarasov AM, Pepelyaeva AI, Lazarenko PI, Trifonov AY, Savchuk TP, Kuzmin AV, Tregubov AV, Shabaeva EN, Zhurina ES, et al. The Composite TiO2–CuOx Layers Formed by Electrophoretic Method for CO2 Gas Photoreduction. Nanomaterials. 2023; 13(14):2030. https://doi.org/10.3390/nano13142030
Chicago/Turabian StyleSorokina, Larisa I., Andrey M. Tarasov, Anastasiya I. Pepelyaeva, Petr I. Lazarenko, Alexey Yu. Trifonov, Timofey P. Savchuk, Artem V. Kuzmin, Aleksey V. Tregubov, Elena N. Shabaeva, Ekaterina S. Zhurina, and et al. 2023. "The Composite TiO2–CuOx Layers Formed by Electrophoretic Method for CO2 Gas Photoreduction" Nanomaterials 13, no. 14: 2030. https://doi.org/10.3390/nano13142030