Nanocrystal Array Engineering and Optoelectronic Applications of Organic Small-Molecule Semiconductors
Abstract
:1. Introduction
2. Methods
2.1. Spin Coating
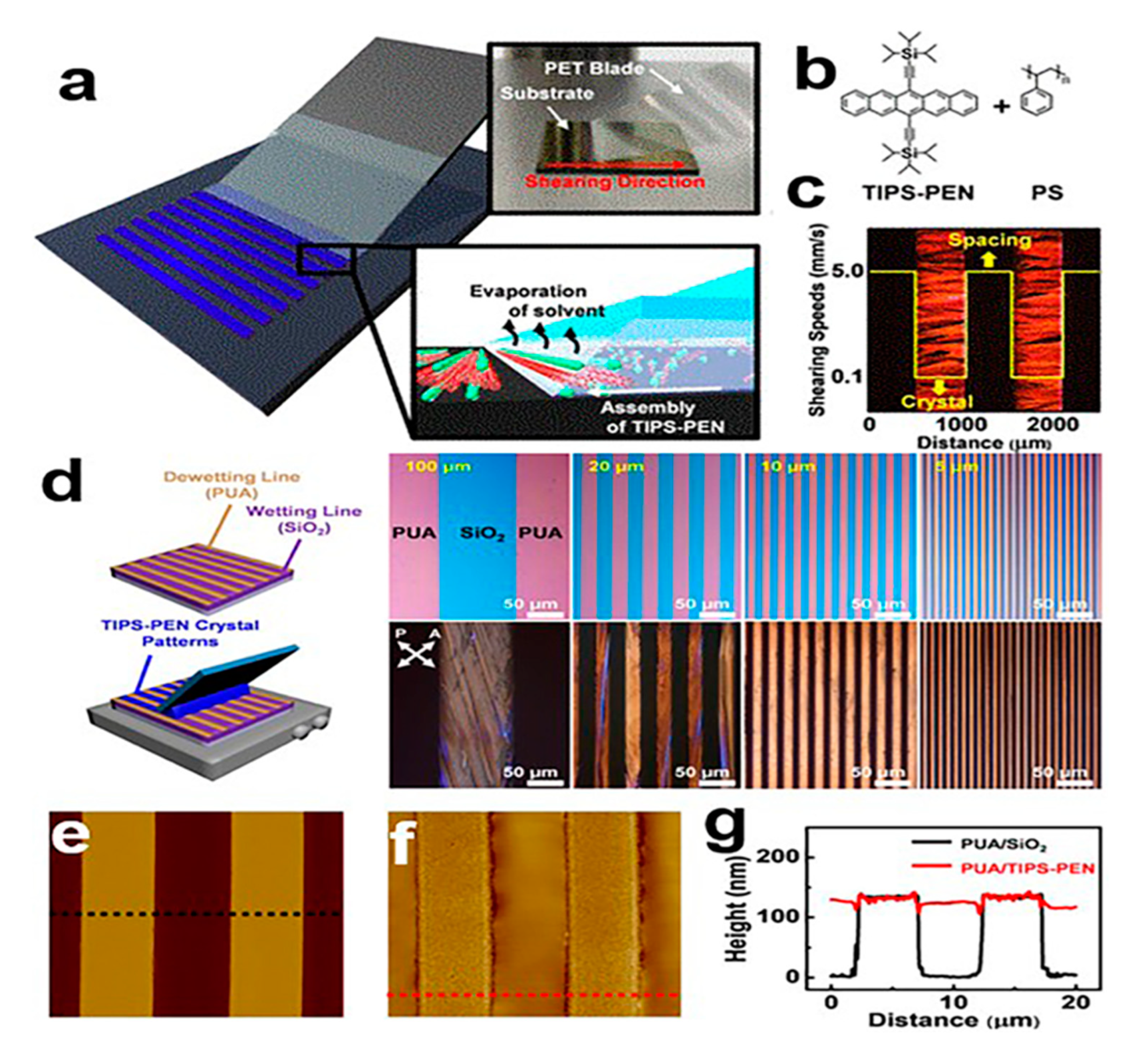
2.2. Blade Coating
2.3. Microstructural Template Patterning Method
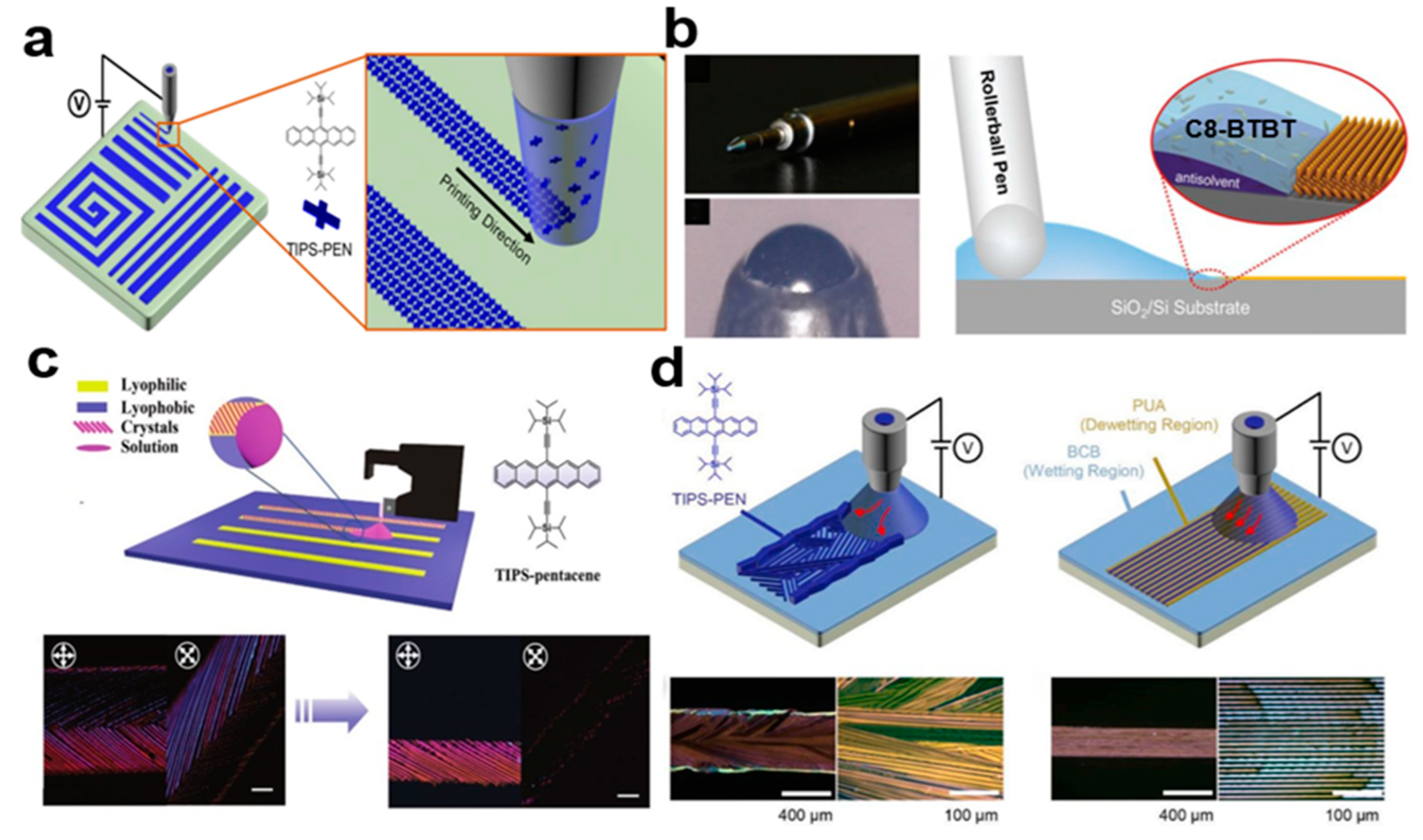
2.3.1. Selective Contact Evaporation Printing
2.3.2. Template-Assisted Self-Assembly (TASA)
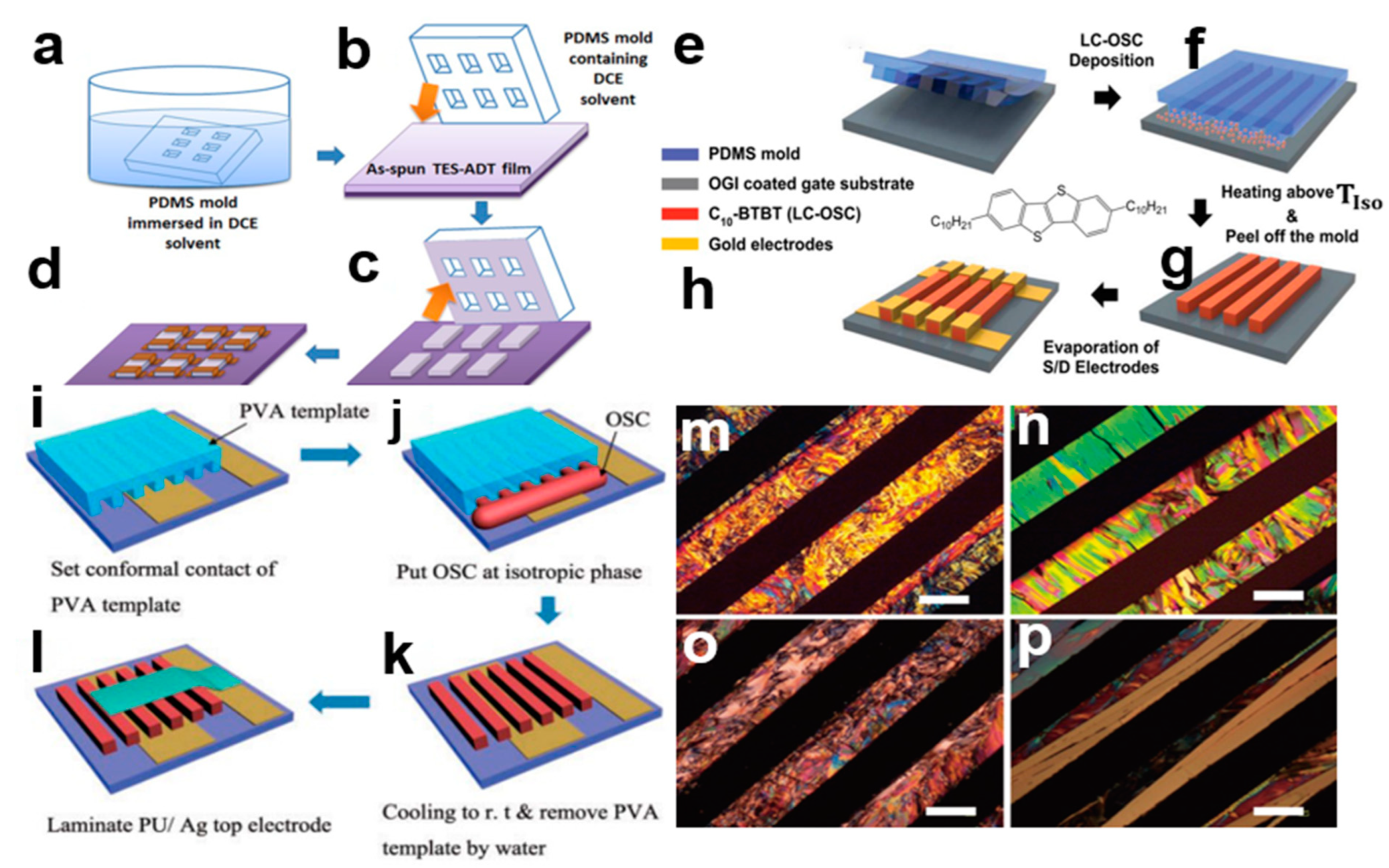
2.3.3. Soft Photolithography
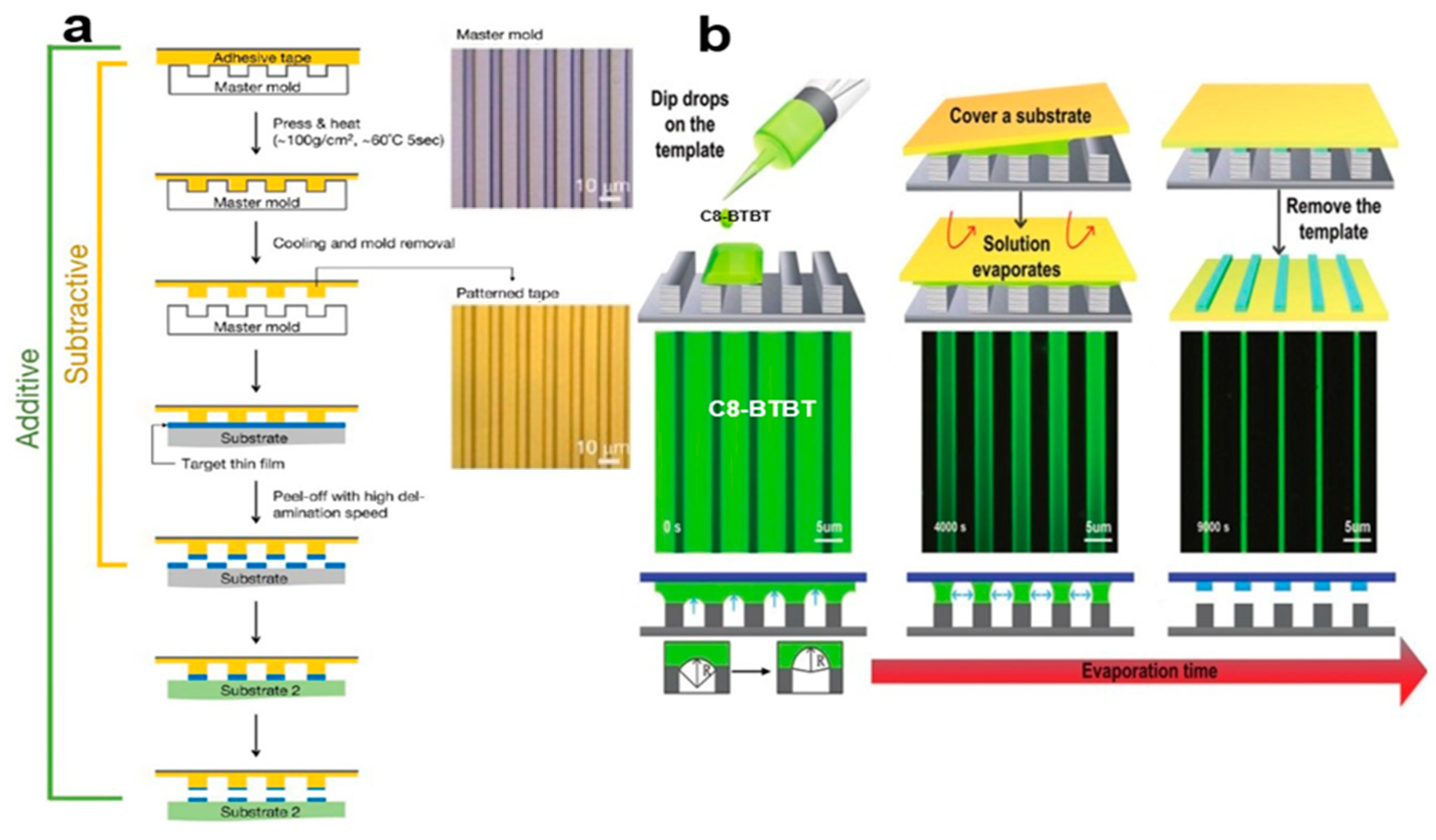
2.3.4. Evaporative Assembly Method
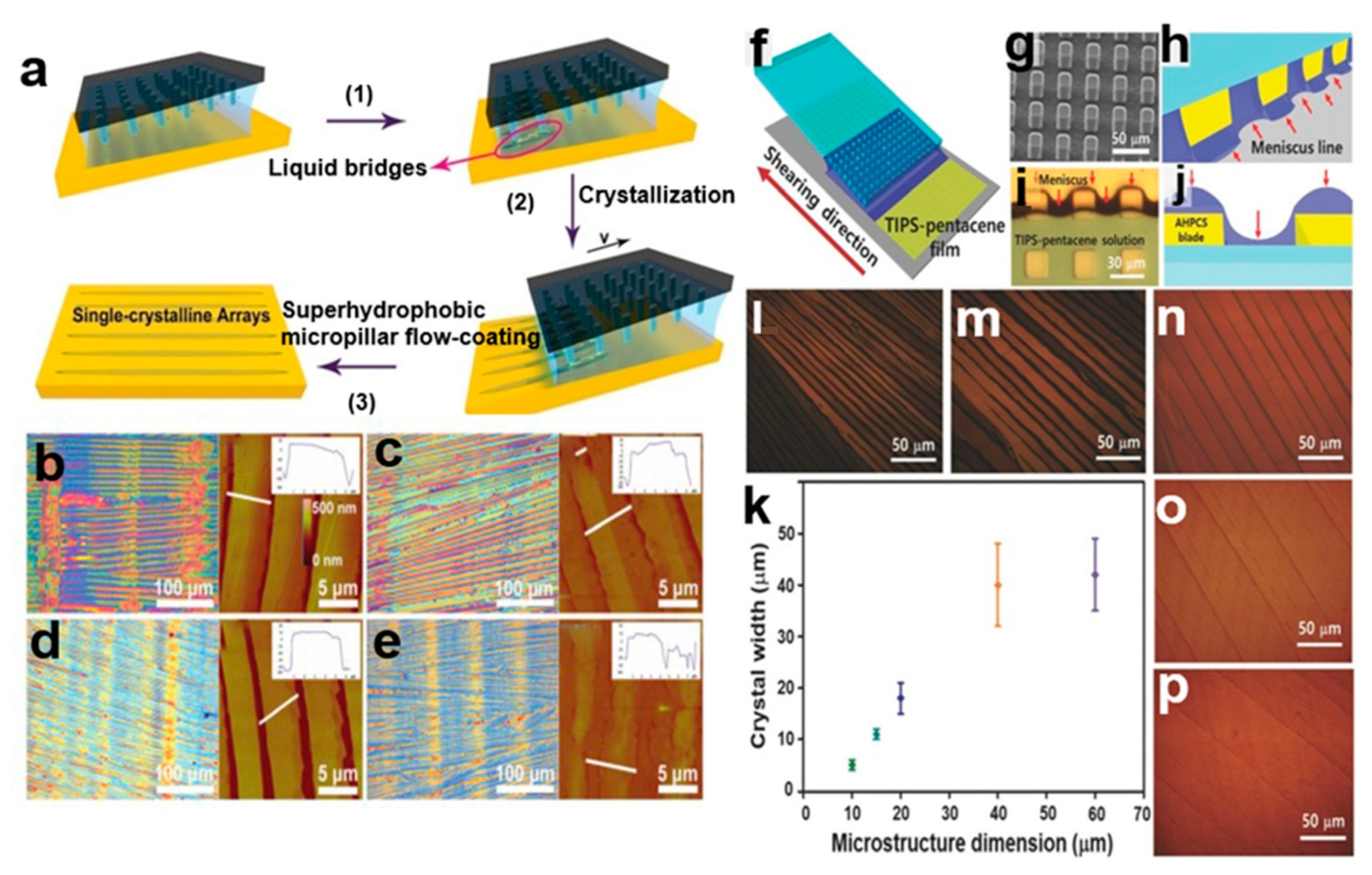
2.3.5. Superhydrophobic Microcolumn Flow Coating Method
2.3.6. Electro-Fluid (EHD) Jet Printing
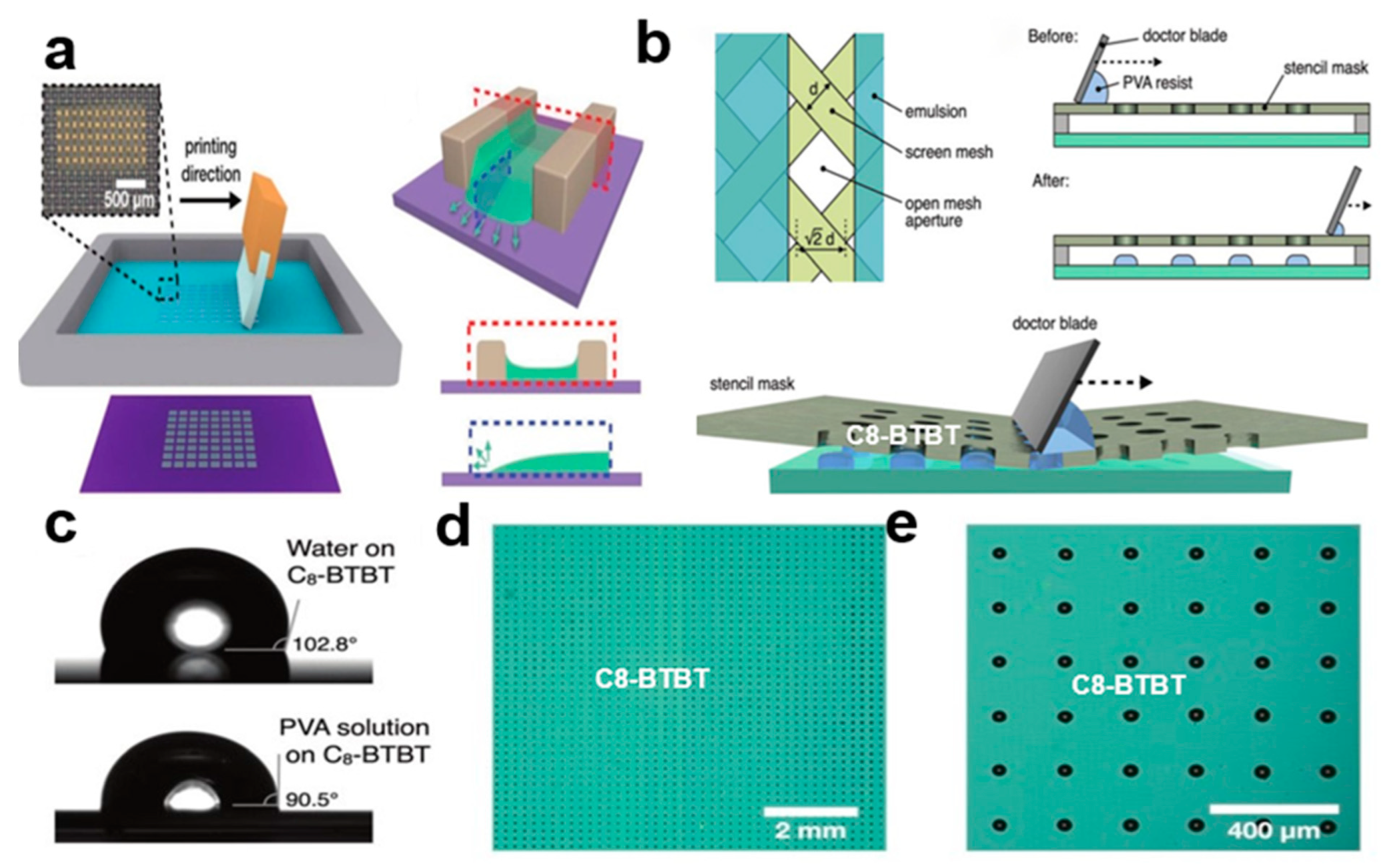
2.3.7. Programmed Scraper Method
2.3.8. Screen Printing
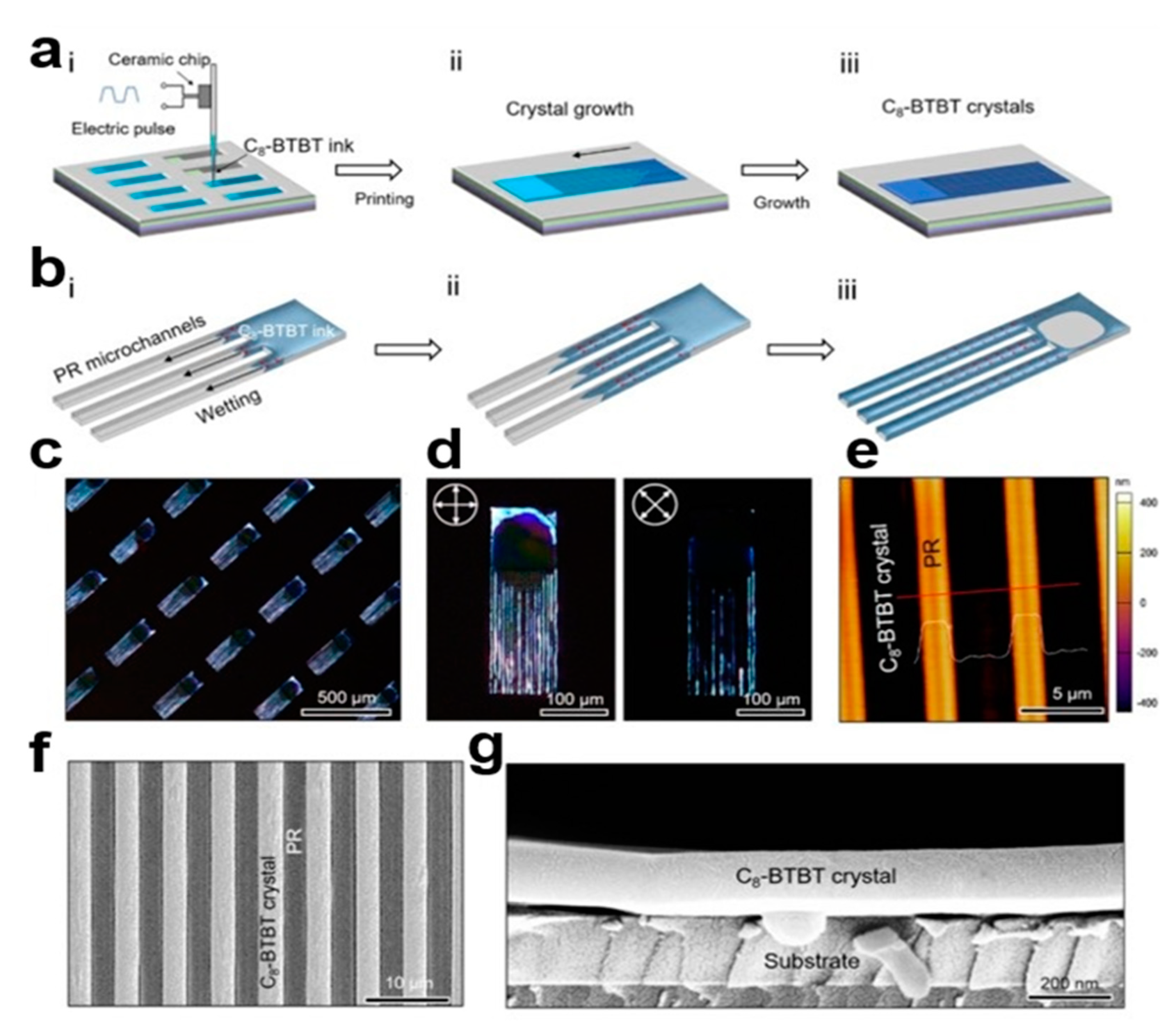
2.3.9. Microchannel-Assisted Inkjet Printing (MA-IJP) Method
2.3.10. Double Blade Coating Printing Technology
2.4. Bottom-Up Method
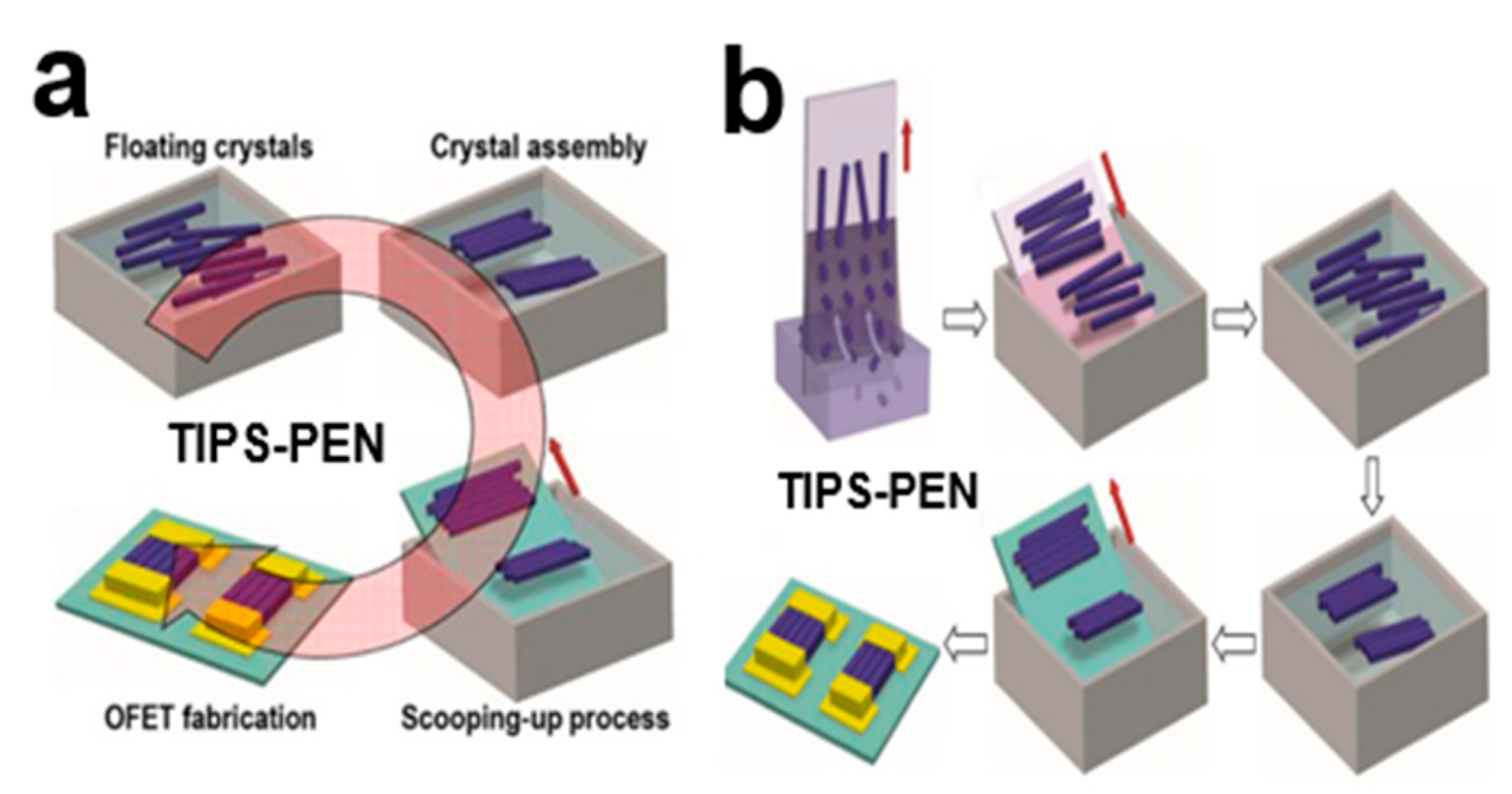
2.4.1. Straightforward Scooping-Up
2.4.2. Patterned Microchannel Dip Coating (PMDC) Method
2.4.3. Meniscus Coating Method
2.5. Crystallization Dynamic Control
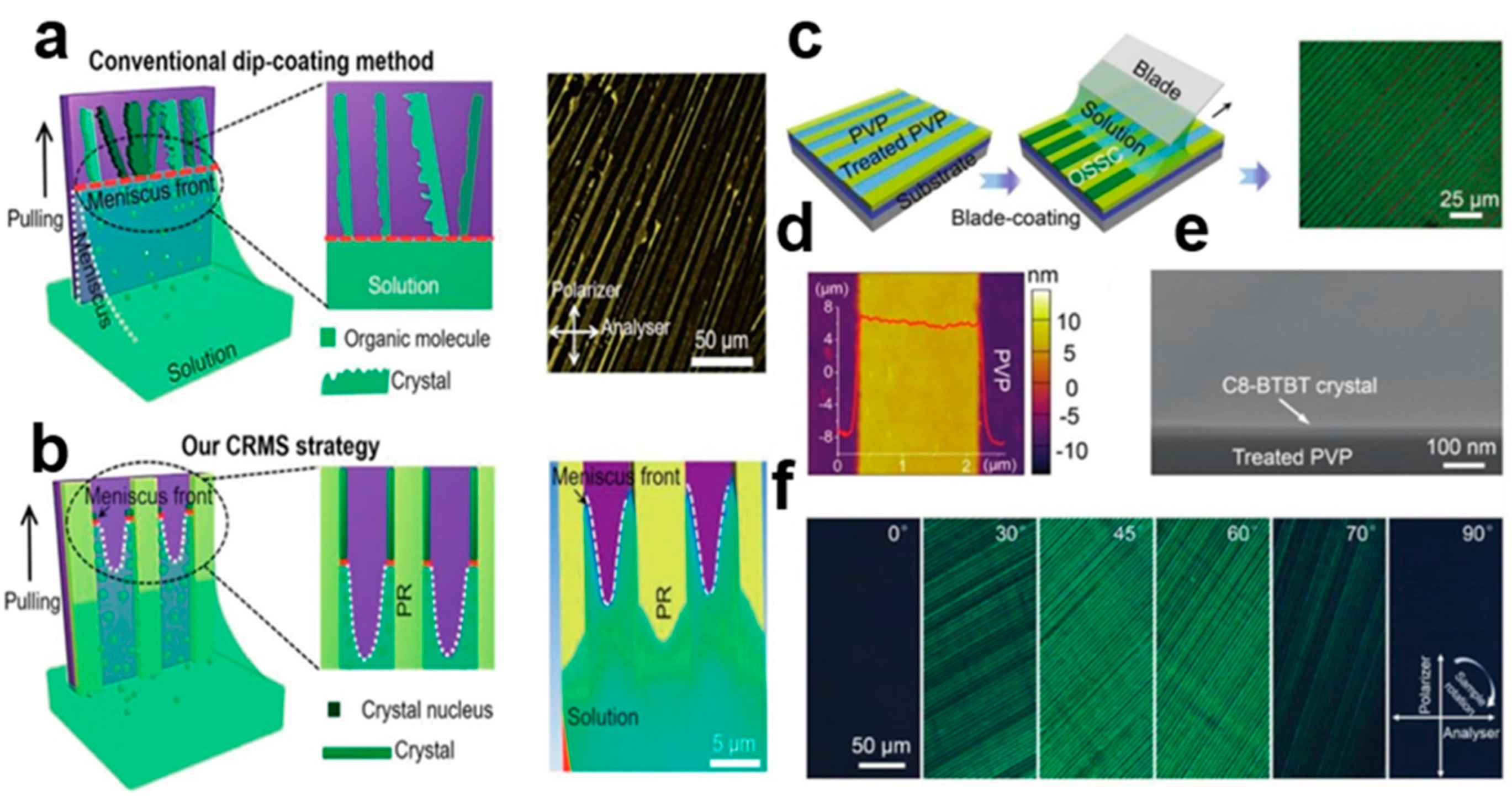
2.5.1. Solvent Crystallization Resistance Technology
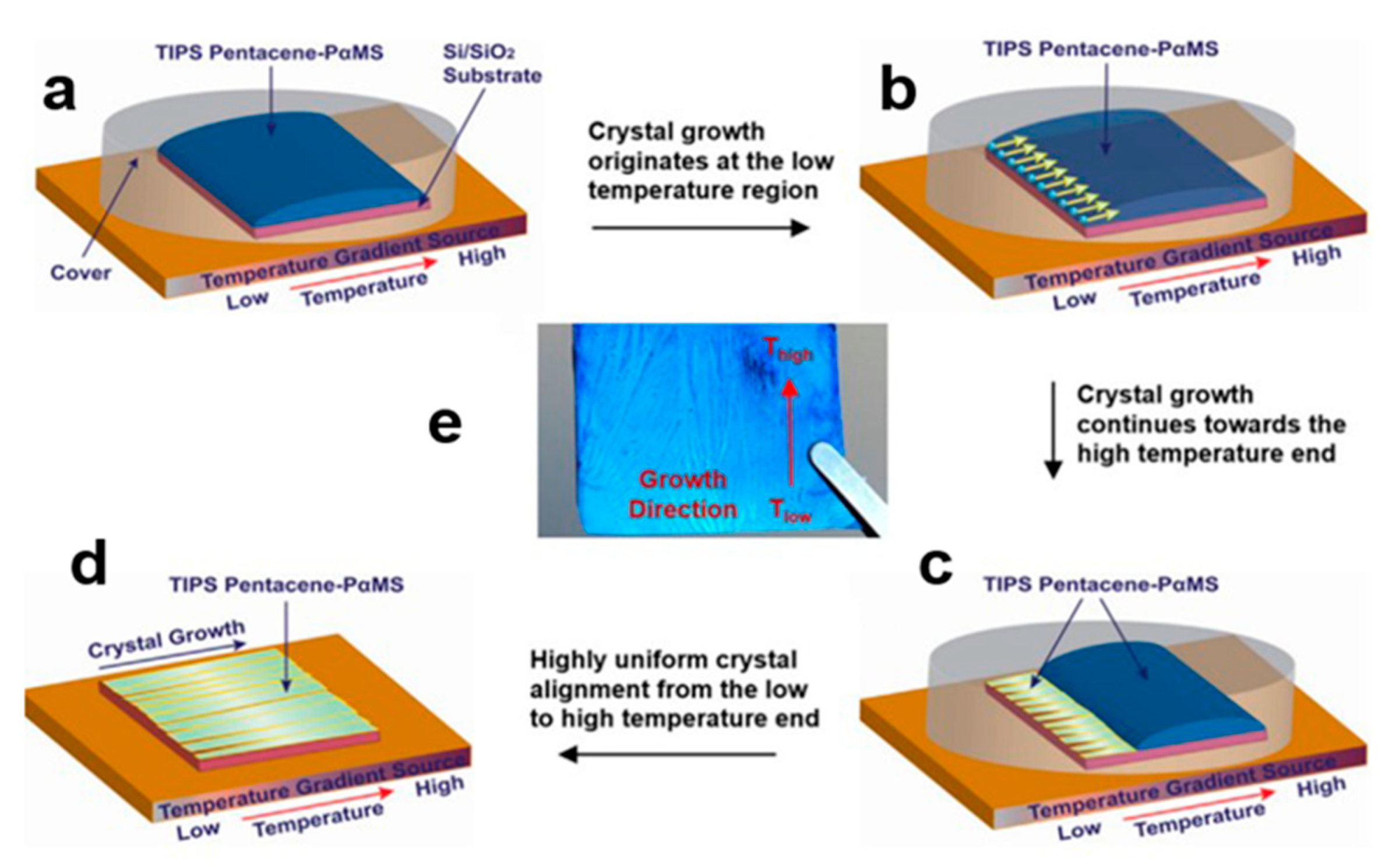
2.5.2. Temperature Gradient Technique
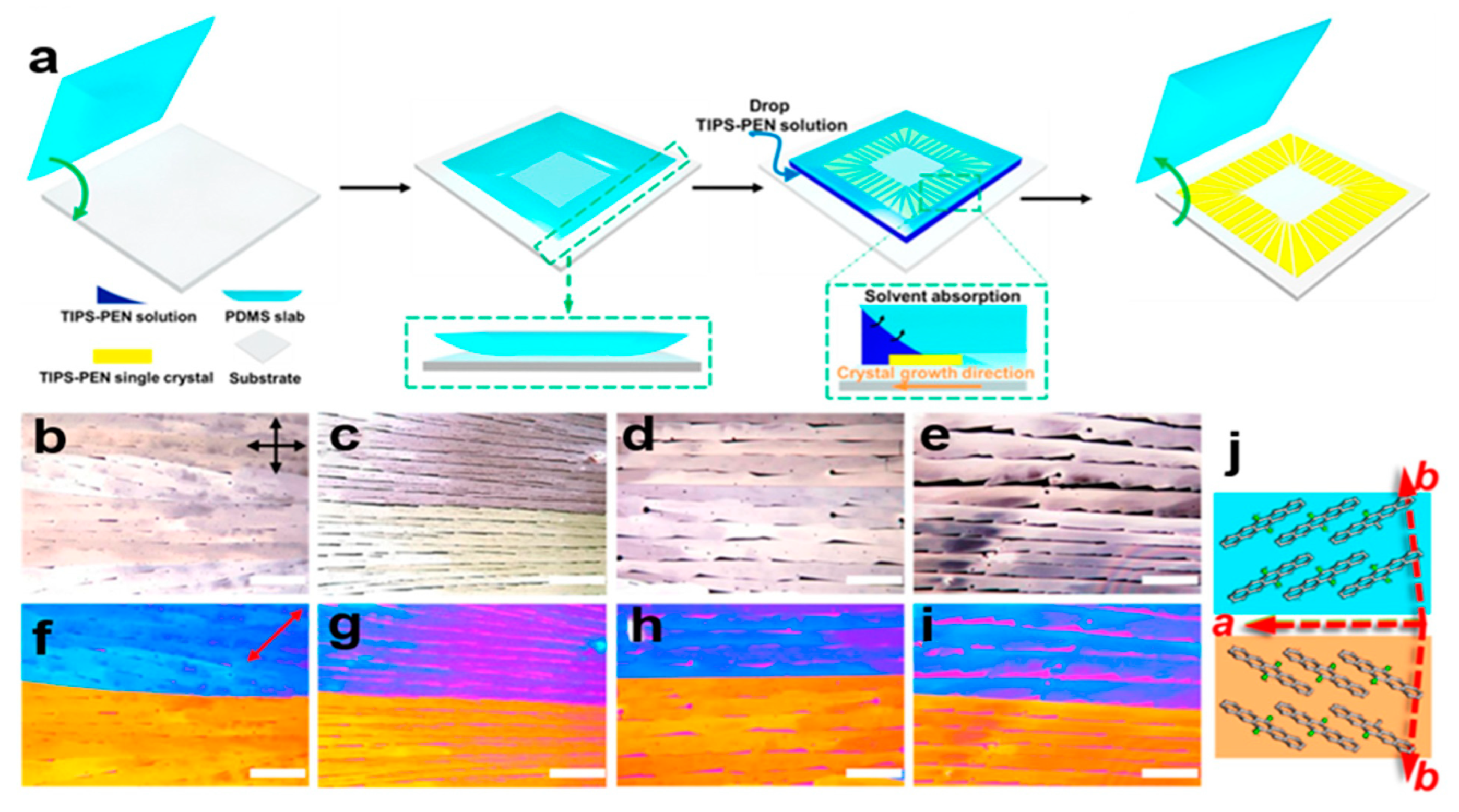
2.5.3. PDMS-Assisted Crystallization Method
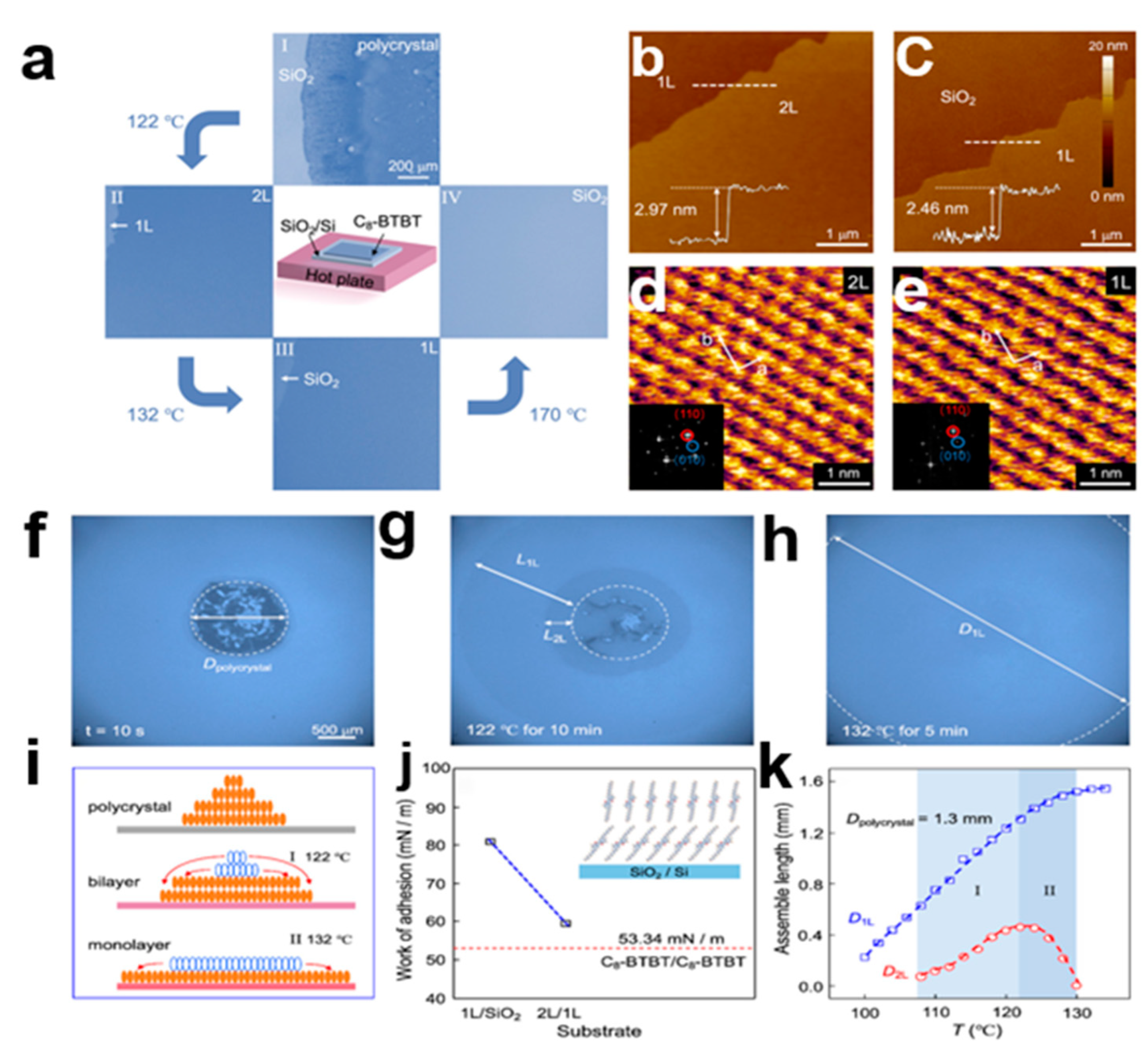
2.5.4. Heat-Induced Self-Assembly Method
2.5.5. Two-Step Crystallization Process
2.5.6. Micro-Spacing Air Sublimation Method
2.5.7. Vapor-Induced Coating Method
2.6. Self-Assembly Method
2.6.1. Van Der Waals Force Epitaxial Growth
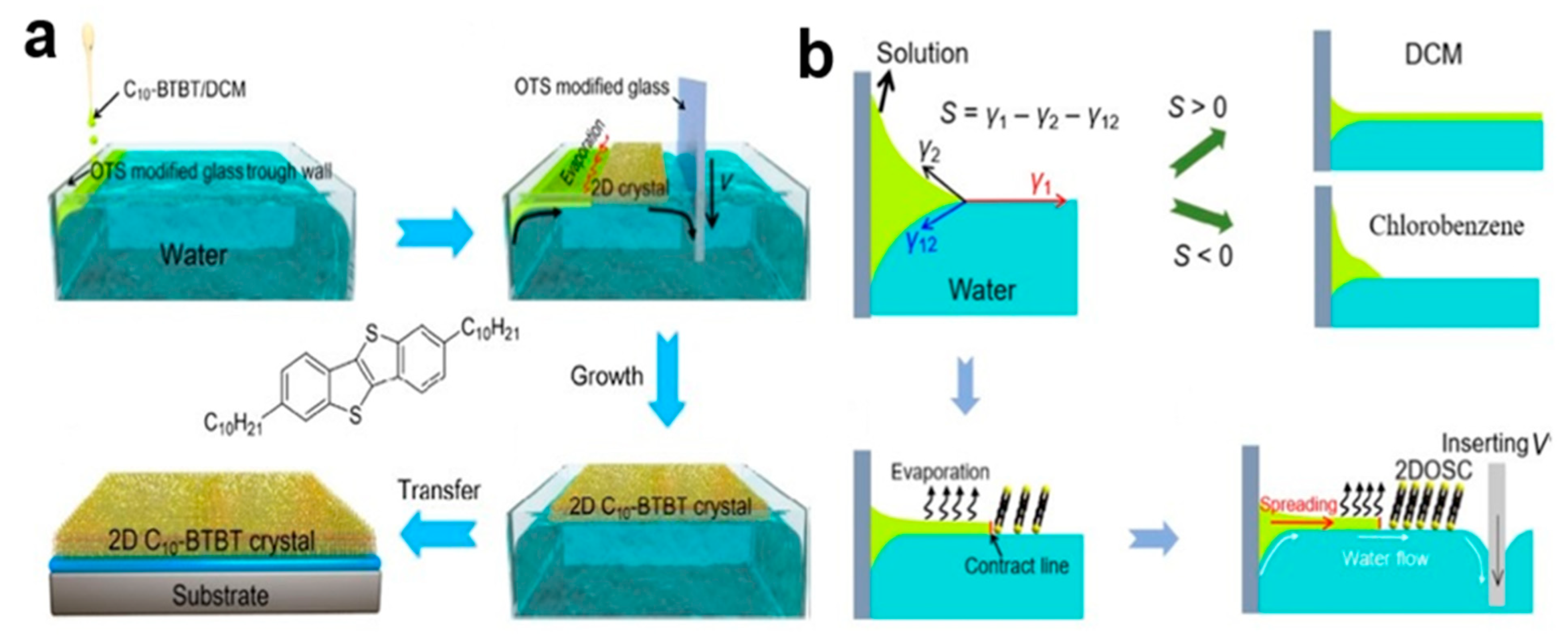
2.6.2. External Force Driven Solution Epitaxy (EFDSE) Method
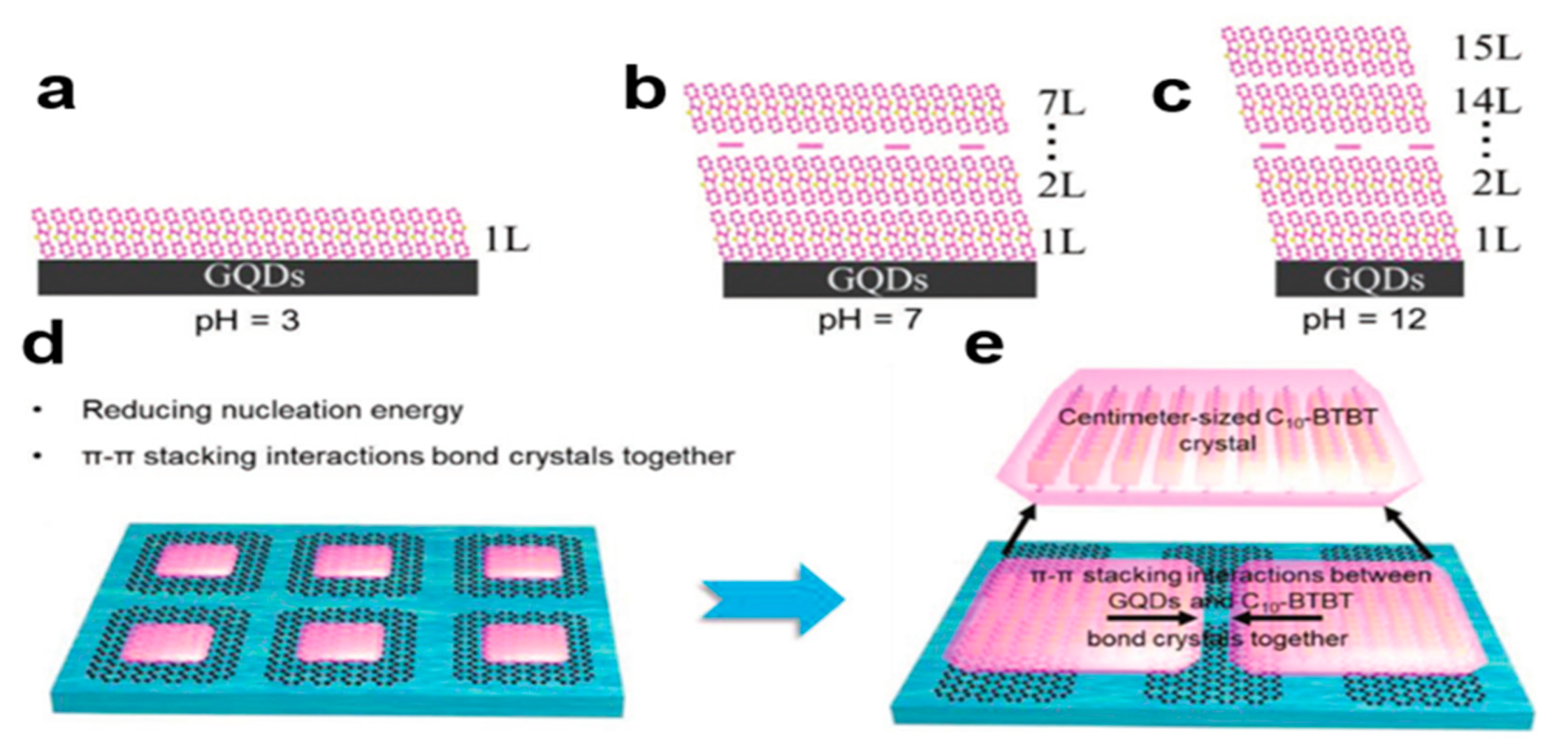
2.6.3. Self-Assembly Induced by Graphene Quantum Dots (GQDs)
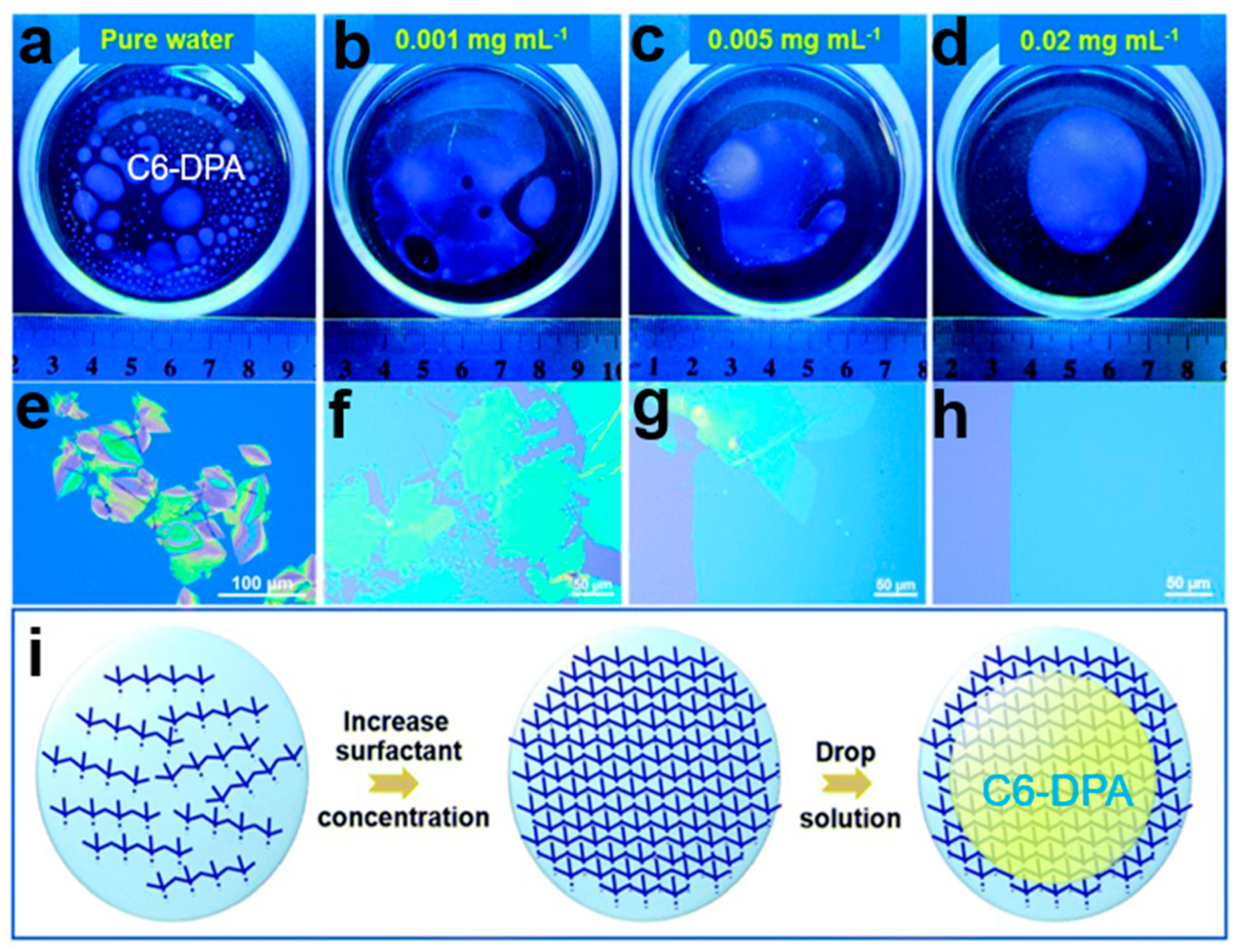
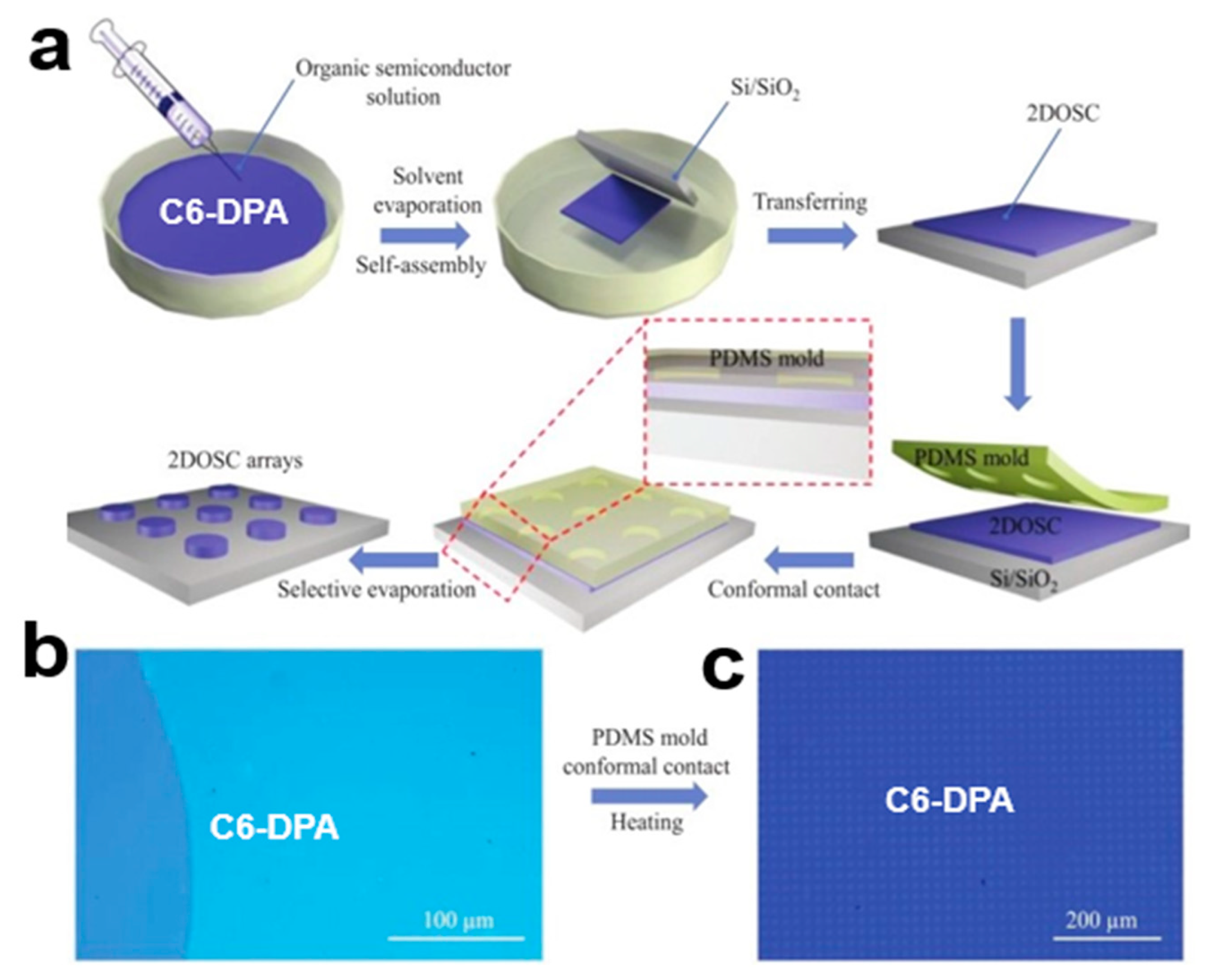
2.6.4. Soft Template Assisted Self-Assembly
2.6.5. DOSC Two-Step Strategy
3. Photoelectric Application
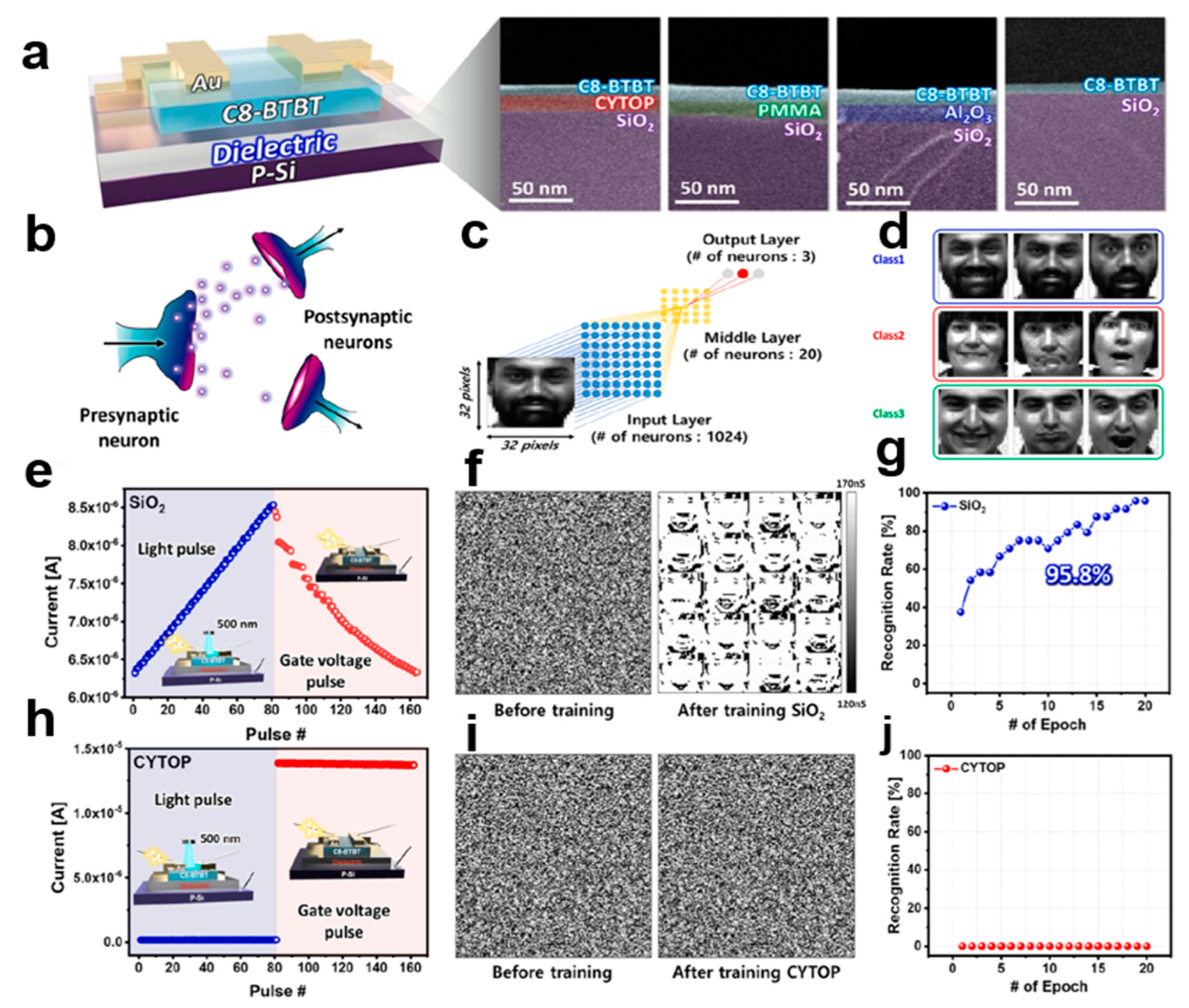
3.1. Artificial Neural Network Synapses
3.2. Organic Photodetector
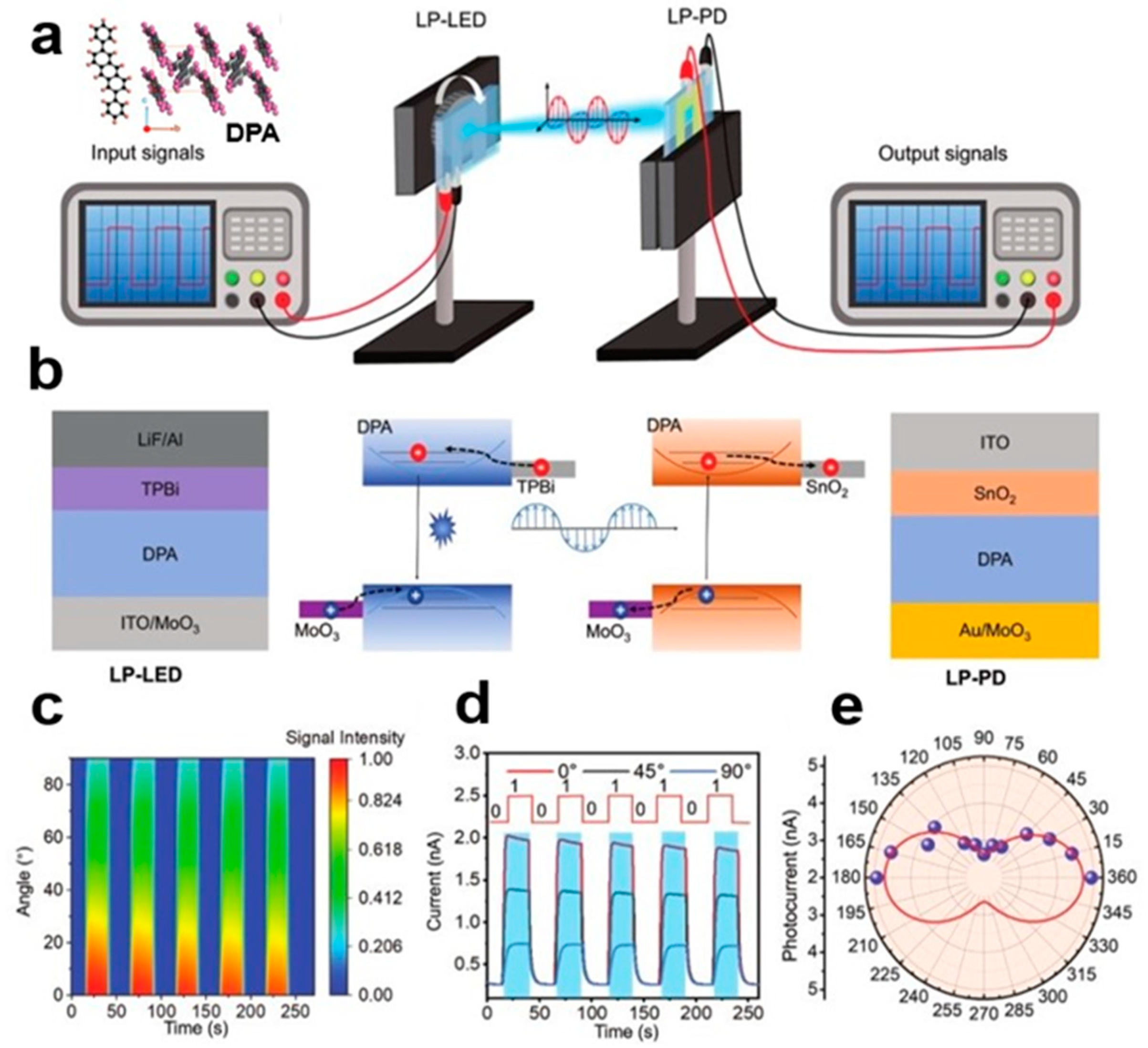
3.3. DPA SC Polarized Light Communication System
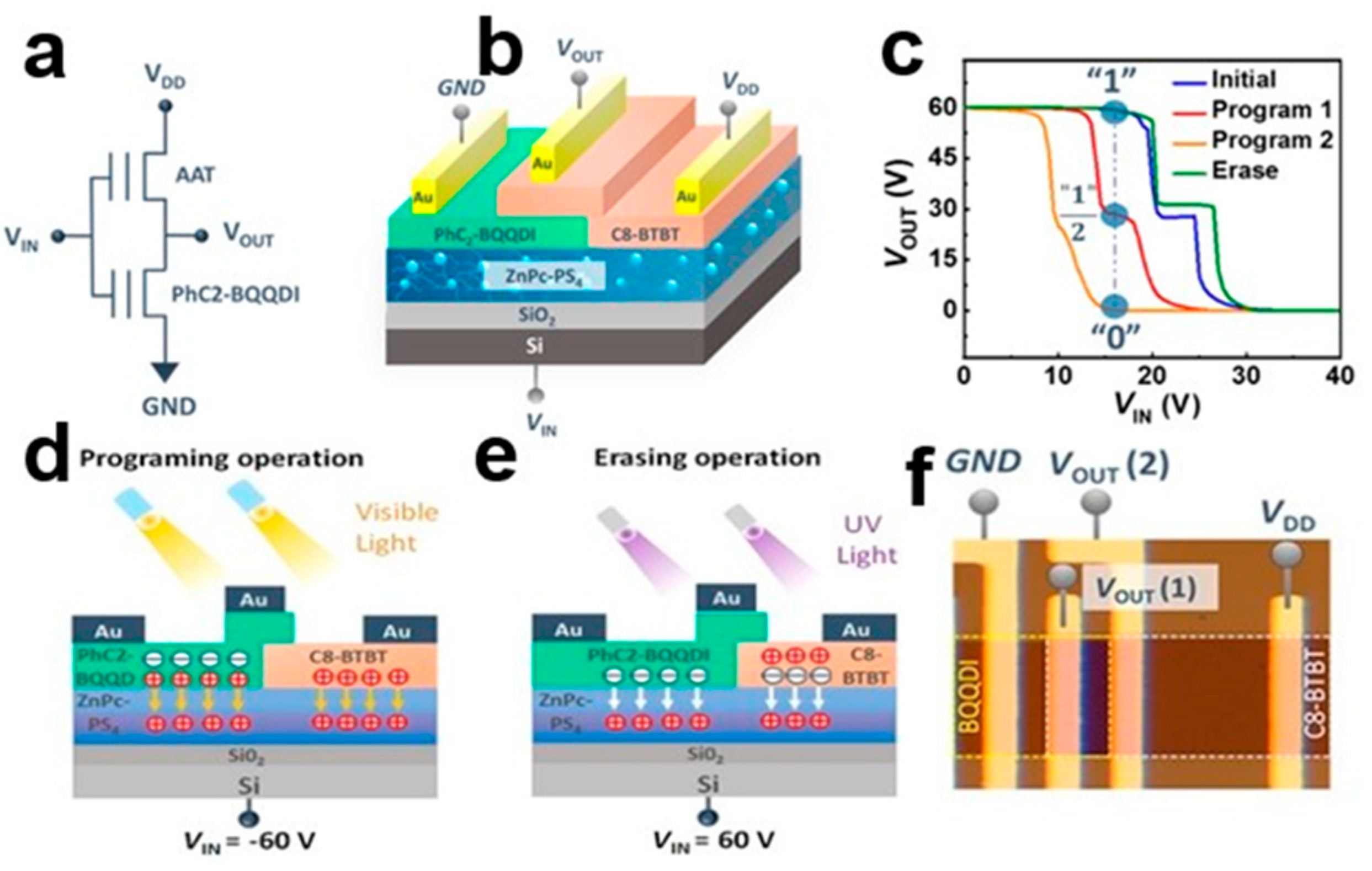
3.4. Photoelectric Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chai, Z.; Abbasi, S.A.; Busnaina, A.A. Scalable Directed Assembly of Highly Crystalline 2,7-Dioctyl 1 benzothieno 3,2-b 1 benzothiophene (C8-BTBT) Films. ACS Appl. Mater. Interfaces 2018, 10, 18123–18130. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Abbasi, S.A.; Busnaina, A.A. Solution-processed organic field-effect transistors using directed assembled carbon nanotubes and 2,7-dioctyl 1 benzothieno 3,2-b 1 benzothiophene (C8-BTBT). Nanotechnology 2019, 30, 485203. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Childress, A.; Busnaina, A.A. Directed Assembly of Nanomaterials for Making Nanoscale Devices and Structures: Mechanisms and Applications. ACS Nano 2022, 16, 17641–17686. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Cui, N.; Tang, Q.; Tong, Y.; Zhao, X.; Liu, Y. High-Performance, Ultrathin, Ultraflexible Organic Thin-Film Transistor Array Via Solution Process. Small 2018, 14, 1801020. [Google Scholar] [CrossRef]
- Lyu, L.; Niu, D.; Xie, H.; Cao, N.; Zhang, H.; Zhang, Y.; Liu, P.; Gao, Y. Orientation-dependent energy level alignment and film growth of 2,7-diocty[1]benzothieno[3,2-b]benzothiophene (C8-BTBT) on HOPG. J. Chem. Phys. 2016, 144, 034701. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Han, Y.; Li, J.; Wang, E. Beyond Conventional Patterns: New Electrochemical Lithography with High Precision for Patterned Film Materials and Wearable Sensors. Anal. Chem. 2017, 89, 2569–2574. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, C.; Cong, H.; Yang, Q.; Wu, Y.; Su, B.; Zhao, Y.; Wang, J.; Jiang, L. Hybrid Top-Down/Bottom-Up Strategy Using Superwettability for the Fabrication of Patterned Colloidal Assembly. ACS Appl. Mater. Interfaces 2016, 8, 4985–4993. [Google Scholar] [CrossRef]
- Molnár, G.; Cobo, S.; Real, J.A.; Carcenac, F.; Daran, E.; Vieu, C.; Bousseksou, A. A Combined Top-Down/Bottom-Up Approach for the Nanoscale Patterning of Spin-Crossover Coordination Polymers. Adv. Mater. 2007, 19, 2163–2167. [Google Scholar] [CrossRef]
- Kollipara, P.S.; Li, J.; Zheng, Y. Optical Patterning of Two-Dimensional Materials. Research 2020, 2020, 6581250. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, J.; Shao, J.; Ouyang, D.; Zhang, W.; Liu, S.; Li, Y.; Zhai, T. Nanopatterning Technologies of 2D Materials for Integrated Electronic and Optoelectronic Devices. Adv. Mater. 2022, 34, e2200734. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Qiu, Y.; Feng, J.; Li, S.; Wang, H.; Zhao, Y.; Wei, X.; Jiang, X.; Su, Y.; Wu, Y.; et al. Nano-confined crystallization of organic ultrathin nanostructure arrays with programmable geometries. Nat. Commun. 2019, 10, 3912. [Google Scholar] [CrossRef] [Green Version]
- Chou, K.W.; Khan, H.U.; Niazi, M.R.; Yan, B.; Li, R.; Payne, M.M.; Anthony, J.E.; Smilgies, D.-M.; Amassian, A. Late stage crystallization and healing during spin-coating enhance carrier transport in small-molecule organic semiconductors. J. Mater. Chem. C 2014, 2, 5681–5689. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, J.H.; Lee, W.H.; Kim, H.H.; Park, Y.D. Tailoring the crystallinity of solution-processed 6,13-bis(triisopropylsilylethynyl)pentacene via controlled solidification. Soft Matter 2019, 15, 7369–7373. [Google Scholar] [CrossRef]
- Shen, T.; Zhou, H.; Xin, J.; Fan, Q.; Yang, Z.; Wang, J.; Mei, T.; Wang, X.; Wang, N.; Li, J. Controllable microstructure of polymer-small molecule blend thin films for high-performance organic field-effect transistors. Appl. Surf. Sci. 2019, 498, 143822. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, W.; Lu, B.; Fang, X.; Zhang, X.; Jie, J. Fast deposition of an ultrathin, highly crystalline organic semiconductor film for high-performance transistors. Nanoscale Horiz. 2020, 5, 1096–1105. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, X.; Zhou, L.; Zhang, C.; Chang, J.; Wu, J.; Zhang, J. Solution-processed high performance organic thin film transistors enabled by roll-to-roll slot die coating technique. Org. Electron. 2018, 54, 80–88. [Google Scholar] [CrossRef]
- Paulus, F.; Engelhart, J.U.; Hopkinson, P.E.; Schimpf, C.; Leineweber, A.; Sirringhaus, H.; Vaynzof, Y.; Bunz, U.H.F. The effect of tuning the microstructure of TIPS-tetraazapentacene on the performance of solution processed thin film transistors. J. Mater. Chem. C 2016, 4, 1194–1200. [Google Scholar] [CrossRef]
- Wan, J.; Li, Y.; Benson, J.; Miller, R.; Zhernenkov, M.; Freychet, G.; Headrick, R.L. Dynamic processes in transient phases during self-assembly of organic semiconductor thin films. Mol. Syst. Des. Eng. 2022, 7, 34–43. [Google Scholar] [CrossRef]
- Bae, I.; Kang, S.J.; Shin, Y.J.; Park, Y.J.; Kim, R.H.; Mathevet, F.; Park, C. Tailored Single Crystals of Triisopropylsilylethynyl Pentacene by Selective Contact Evaporation Printing. Adv. Mater. 2011, 23, 3398–3402. [Google Scholar] [CrossRef]
- Park, Y.; Park, J.; Cho, S.; Sung, M.M. Large-area single-crystal organic patterned thin films by vertically confined lateral crystal growth via capillary force lithography. Appl. Surf. Sci. 2019, 494, 1023–1029. [Google Scholar] [CrossRef]
- Deng, W.; Lv, Y.; Zhang, X.; Fang, X.; Lu, B.; Lu, Z.; Jie, J. High-resolution patterning of organic semiconductor single crystal arrays for high-integration organic field-effect transistors. Mater. Today 2020, 40, 82–90. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Iino, H.; Hanna, J.-I. Planar-orientation polycrystalline thin film of liquid-crystalline organic semiconductor by template-directed self-assembly. Appl. Phys. Express 2017, 10, 101601. [Google Scholar] [CrossRef]
- Nguyen, K.V.; Lee, J.H.; Lee, S.C.; Ku, G.M.; Lee, W.H. Simultaneous control of molecular orientation and patterning of small-molecule organic semiconductors for organic transistors. Org. Electron. 2017, 41, 107–113. [Google Scholar] [CrossRef]
- Kim, A.; Jang, K.-S.; Kim, J.; Won, J.C.; Yi, M.H.; Kim, H.; Yoon, D.K.; Shin, T.J.; Lee, M.-H.; Ka, J.-W.; et al. Solvent-Free Directed Patterning of a Highly Ordered Liquid Crystalline Organic Semiconductor via Template-Assisted Self-Assembly for Organic Transistors. Adv. Mater. 2013, 25, 6219–6225. [Google Scholar] [CrossRef]
- Oh, S.; Park, S.K.; Kim, J.H.; Cho, I.; Kim, H.-J.; Park, S.Y. Patterned Taping: A High-Efficiency Soft Lithographic Method for Universal Thin Film Patterning. ACS Nano 2016, 10, 3478–3485. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, X.; Feng, J.; Wang, X.; Wu, Y.; Su, B.; Jiang, L. Regulated Dewetting for Patterning Organic Single Crystals with Pure Crystallographic Orientation toward High Performance Field-Effect Transistors. Adv. Funct. Mater. 2018, 28, 1800470. [Google Scholar] [CrossRef]
- Fesenko, P.; Flauraud, V.; Xie, S.; Brugger, J.; Genoe, J.; Heremans, P.; Rolin, C. Arrays of Pentacene Single Crystals by Stencil Evaporation. Cryst. Growth Des. 2016, 16, 4694–4700. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, J.S.; Choi, Y.J.; Lee, W.H.; Lee, D.Y.; Cho, J.H. Gate- and Light-Tunable pn Heterojunction Microwire Arrays Fabricated via Evaporative Assembly. ACS Appl. Mater. Interfaces 2017, 9, 3857–3864. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Kan, X.; Liu, C.; Jiang, W.; Zhao, G.; Zhao, Q.; Zhang, L.; Hu, W.; Wang, Z.; Jiang, L. Controlled formation of large-area single-crystalline TIPS-pentacene arrays through superhydrophobic micropillar flow-coating. J. Mater. Chem. C 2017, 5, 2702–2707. [Google Scholar] [CrossRef]
- Kim, J.-O.; Lee, J.-C.; Kim, M.-J.; Noh, H.; Yeom, H.-I.; Ko, J.B.; Lee, T.H.; Ko Park, S.-H.; Kim, D.-P.; Park, S. Inorganic Polymer Micropillar-Based Solution Shearing of Large-Area Organic Semiconductor Thin Films with Pillar-Size-Dependent Crystal Size. Adv. Mater. 2018, 30, 1800647. [Google Scholar] [CrossRef]
- Kim, K.; Oh, S.M.; Hong, J.; Jung, C.; Seo, J.; Jeong, Y.J.; Lee, H.S.; Kim, S.H. Electrohydrodynamic jet printing of small-molecule semiconductor crystals on chemically patterned surface for high-performance organic field-effect transistors. Mater. Chem. Phys. 2022, 285, 126165. [Google Scholar] [CrossRef]
- Kim, K.; Bae, J.; Noh, S.H.; Jang, J.; Kim, S.H.; Park, C.E. Direct Writing and Aligning of Small-Molecule Organic Semiconductor Crystals via “Dragging Mode” Electrohydrodynamic Jet Printing for Flexible Organic Field-Effect Transistor Arrays. J. Phys. Chem. Lett. 2017, 8, 5492–5500. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-j.; Tang, X.; Kim, S.; Li, Z.; Wang, R.; Park, B.H.; Kim, C.; Kim, S.; Hong, J.; Ryu, K.Y.; et al. Molecular Engineering of Printed Semiconducting Blends to Develop Organic Integrated Circuits: Crystallization, Charge Transport, and Device Application Analyses. ACS Appl. Mater. Interfaces 2022, 14, 23678–23691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, Y.; Song, L.; Qian, J.; Jiang, S.; Wang, Q.; Wang, X.; Shi, Y.; Wang, X.; Li, Y. Directly writing 2D organic semiconducting crystals for high-performance field-effect transistors. J. Mater. Chem. C 2017, 5, 11246–11251. [Google Scholar] [CrossRef]
- Chen, S.; Ma, X.; Cai, Z.; Long, H.; Wang, X.; Li, Z.; Qu, Z.; Zhang, F.; Qiao, Y.; Song, Y. A Direct Writing Approach for Organic Semiconductor Single-Crystal Patterns with Unique Orientation. Adv. Mater. 2022, 34, 2200928. [Google Scholar] [CrossRef]
- Kim, K.; Nam, K.; Li, X.; Lee, D.Y.; Kim, S.H. Programmed Design of Highly Crystalline Organic Semiconductor Patterns with Uniaxial Alignment via Blade Coating for High-Performance Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2019, 11, 42403–42411. [Google Scholar] [CrossRef]
- Kim, K.; Hong, J.; Hahm, S.G.; Rho, Y.; An, T.K.; Kim, S.H.; Park, C.E. Facile and Microcontrolled Blade Coating of Organic Semiconductor Blends for Uniaxial Crystal Alignment and Reliable Flexible Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2019, 11, 13481–13490. [Google Scholar] [CrossRef]
- Duan, S.; Gao, X.; Wang, Y.; Yang, F.; Chen, M.; Zhang, X.; Ren, X.; Hu, W. Scalable Fabrication of Highly Crystalline Organic Semiconductor Thin Film by Channel-Restricted Screen Printing toward the Low-Cost Fabrication of High-Performance Transistor Arrays. Adv. Mater. 2019, 31, 1807975. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, T.; Mu, Q.; Huang, C.; Duan, S.; Ren, X.; Hu, W. Stencil mask defined doctor blade printing of organic single crystal arrays for high-performance organic field-effect transistors. Mater. Chem. Front. 2021, 5, 3236–3245. [Google Scholar] [CrossRef]
- Duan, S.; Wang, T.; Geng, B.; Gao, X.; Li, C.; Zhang, J.; Xi, Y.; Zhang, X.; Ren, X.; Hu, W. Solution-Processed Centimeter-Scale Highly Aligned Organic Crystalline Arrays for High-Performance Organic Field-Effect Transistors. Adv. Mater. 2020, 32, 1908388. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Tan, Y.; Deng, W.; Ren, X.; Liu, X.; Shi, Y.; Zhang, X. Patterning of organic semiconductor crystal arrays via microchannel-assisted inkjet printing for organic field-effect transistors. J. Phys. Mater. 2022, 5, 035001. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, W.; Hong, J.; Ren, X.; Zhang, X.; Shi, J.; Sheng, F.; Zhang, X.; Jie, J. Water-Surface-Mediated Precise Patterning of Organic Single-Crystalline Films via Double-Blade Coating for High-Performance Organic Transistors. Adv. Funct. Mater. 2023, 33, 2213788. [Google Scholar] [CrossRef]
- Arai, S.; Inoue, S.; Hamai, T.; Kumai, R.; Hasegawa, T. Semiconductive Single Molecular Bilayers Realized Using Geometrical Frustration. Adv. Mater. 2018, 30, e1707256. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Han, S.; Baek, S.; Kim, S.H.; Lee, H.S. Dense Assembly of Soluble Acene Crystal Ribbons and Its Application to Organic Transistors. ACS Appl. Mater. Interfaces 2016, 8, 24753–24760. [Google Scholar] [CrossRef]
- Nam, S.; Jeong, Y.J.; Jung, J.; Kim, S.H.; Ahn, J.; Shin, K.; Jang, J. Direct printing of soluble acene crystal stripes by a programmed dip-coating process for organic field-effect transistor applications. J. Mater. Chem. C 2018, 6, 799–807. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Z.; Deng, W.; Zhao, W.; Lu, B.; Xiao, J.; Zhang, X.; Jie, J.; Zhang, X. Precise patterning of single crystal arrays of organic semiconductors by a patterned microchannel dip-coating method for organic field-effect transistors. J. Mater. Chem. C 2021, 9, 5174–5181. [Google Scholar] [CrossRef]
- Wu, H.; Iino, H.; Hanna, J.-I. Scalable Ultrahigh-Speed Fabrication of Uniform Polycrystalline Thin Films for Organic Transistors. ACS Appl. Mater. Interfaces 2020, 12, 29497–29504. [Google Scholar] [CrossRef]
- Salzillo, T.; Montes, N.; Pfattner, R.; Mas-Torrent, M. Selection of the two enantiotropic polymorphs of diF-TES-ADT in solution sheared thin film transistors. J. Mater. Chem. C 2020, 8, 15361–15367. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, X.; Dong, H.; Jie, J.; Xu, X.; Liu, J.; He, L.; Xu, L.; Hu, W.; Zhang, X. Channel-restricted meniscus self-assembly for uniformly aligned growth of single-crystal arrays of organic semiconductors. Mater. Today 2019, 24, 17–25. [Google Scholar] [CrossRef]
- Kim, D.-K.; Vincent, P.; Jang, J.; Kang, I.M.; Kim, H.; Lang, P.; Choi, M.; Bae, J.-H. Contact line curvature-induced molecular misorientation of a surface energy patterned organic semiconductor in meniscus-guided coating. Appl. Surf. Sci. 2020, 504, 144362. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Marszalek, T.; Borkowski, M.; Fytas, G.; Blom, P.W.M.; Pisula, W. Key role of the meniscus shape in crystallization of organic semiconductors during meniscus-guided coating. Mater. Horiz. 2020, 7, 1631–1640. [Google Scholar] [CrossRef]
- Yildiz, O.; Wang, Z.; Borkowski, M.; Fytas, G.; Blom, P.W.M.; Michels, J.J.; Pisula, W.; Marszalek, T. Optimized Charge Transport in Molecular Semiconductors by Control of Fluid Dynamics and Crystallization in Meniscus-Guided Coating. Adv. Funct. Mater. 2022, 32, 2107976. [Google Scholar] [CrossRef]
- Kitahara, G.; Inoue, S.; Higashino, T.; Ikawa, M.; Hayashi, T.; Matsuoka, S.; Arai, S.; Hasegawa, T. Meniscus-controlled printing of single-crystal interfaces showing extremely sharp switching transistor operation. Sci. Adv. 2020, 6, eabc8847. [Google Scholar] [CrossRef]
- Zhao, W.; Jie, J.; Wei, Q.; Lu, Z.; Jia, R.; Deng, W.; Zhang, X.; Zhang, X. A Facile Method for the Growth of Organic Semiconductor Single Crystal Arrays on Polymer Dielectric toward Flexible Field-Effect Transistors. Adv. Funct. Mater. 2019, 29, 1902494. [Google Scholar] [CrossRef]
- Minemawari, H.; Yamada, T.; Matsui, H.; Tsutsumi, J.Y.; Haas, S.; Chiba, R.; Kumai, R.; Hasegawa, T. Inkjet printing of single-crystal films. Nature 2011, 475, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Rigas, G.P.; Payne, M.M.; Anthony, J.E.; Horton, P.N.; Castro, F.A.; Shkunov, M. Spray printing of organic semiconducting single crystals. Nat. Commun. 2016, 7, 13531. [Google Scholar] [CrossRef] [Green Version]
- Minemawari, H.; Yamada, T.; Hasegawa, T. Crystalline film growth of TIPS-pentacene by double-shot inkjet printing technique. Jpn. J. Appl. Phys. 2014, 53, 05HC10. [Google Scholar] [CrossRef]
- Fang, X.; Shi, J.; Zhang, X.; Ren, X.; Lu, B.; Deng, W.; Jie, J.; Zhang, X. Patterning Liquid Crystalline Organic Semiconductors via Inkjet Printing for High-Performance Transistor Arrays and Circuits. Adv. Funct. Mater. 2021, 31, 2100237. [Google Scholar] [CrossRef]
- Pipan, G.; Bogar, M.; Ciavatti, A.; Basiricò, L.; Cramer, T.; Fraboni, B.; Fraleoni-Morgera, A. Direct Inkjet Printing of TIPS-Pentacene Single Crystals onto Interdigitated Electrodes by Chemical Confinement. Adv. Mater. Interfaces 2018, 5, 1700925. [Google Scholar] [CrossRef]
- Schweicher, G.; Liu, G.; Fastre, P.; Resel, R.; Abbas, M.; Wantz, G.; Geerts, Y.H. Directional crystallization of C8-BTBT-C8 thin films in a temperature gradient. Mater. Chem. Front. 2021, 5, 249–258. [Google Scholar] [CrossRef]
- Asare-Yeboah, K.; Bi, S.; He, Z.; Li, D. Temperature gradient controlled crystal growth from TIPS pentacene-poly(alpha-methyl styrene) blends for improving performance of organic thin film transistors. Org. Electron. 2016, 32, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Tsujita, K.; Maruyama, S.; Shibata, Y.; Koganezawa, T.; Kaminaga, K.; Fujikake, H.; Matsumoto, Y. Directional lateral crystallization of vacuum-deposited C8-BTBT thin films via liquid crystal phase by a seeded horizontal temperature gradient cooling technique. CrystEngComm 2023, 25, 64–71. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Li, H.; Zhang, J.; Li, C.; Dong, Y.; Shi, X.; Sun, Y.; Liu, J.; Lei, M.; et al. Polymer assisted transferring of patterned electrodes for a weak anisotropic charge transport study in monolayer molecular crystals. J. Mater. Chem. C 2022, 10, 10799–10804. [Google Scholar] [CrossRef]
- Wu, K.-Y.; Wang, C.-L. Influences of out-of-plane lattice alignment on the OFET performance of TIPS-PEN crystal arrays. Cryst. Growth Des. 2016, 16, 6160–6166. [Google Scholar] [CrossRef]
- Jiang, S.; Qian, J.; Duan, Y.; Wang, H.; Guo, J.; Guo, Y.; Liu, X.; Wang, Q.; Shi, Y.; Li, Y. Millimeter-Sized Two-Dimensional Molecular Crystalline Semiconductors with Precisely Defined Molecular Layers via Interfacial-Interaction-Modulated Self-Assembly. J. Phys. Chem. Lett. 2018, 9, 6755–6760. [Google Scholar] [CrossRef]
- Lin, Q.; Ye, X.; Guo, Q.; Zheng, X.; Han, Q.; Zhang, L.; Cui, S.; Li, C.; Jiang, J.; Liu, Y.; et al. Fabrication of Wafer-Scale Organic Single-Crystal Films with Uniform In-Plane Orientation via Wetting-Assisted In-Air Sublimation for High-Performance Transistor Arrays. Chem. Mater. 2022, 34, 1030–1040. [Google Scholar] [CrossRef]
- Perez-Rodriguez, A.; Temino, I.; Ocal, C.; Mas-Torrent, M.; Barrena, E. Decoding the Vertical Phase Separation and Its Impact on C8-BTBT/PS Transistor Properties. ACS Appl. Mater. Interfaces 2018, 10, 7296–7303. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, T.; Liu, H.; Wang, S.; Li, X.; Zhang, Y.; Hou, X.; Liu, Z.; Shi, W.; Dennis, T.J.S. Organic Single-Crystalline p-n Heterojunctions for High-Performance Ambipolar Field-Effect Transistors and Broadband Photodetectors. ACS Appl. Mater. Interfaces 2018, 10, 42715–42722. [Google Scholar] [CrossRef]
- Zhang, L.; Pavlica, E.; Zhong, X.; Liscio, F.; Li, S.; Bratina, G.; Orgiu, E.; Samori, P. Fast-Response Photonic Device Based on Organic-Crystal Heterojunctions Assembled into a Vertical-Yet-Open Asymmetric Architecture. Adv. Mater. 2017, 29, 1605760. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, L.; Wang, S.; Li, Y.; Xie, H.; Niu, D.; Huang, H.; Gao, Y. Modification of an ultrathin C-60 interlayer on the electronic structure and molecular packing of C8-BTBT on HOPG. Phys. Chem. Chem. Phys. 2020, 22, 25264–25271. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Han, Q.; Ge, C.; Cui, S.; Zhang, L.; Zheng, X.; Liu, G.; Liu, J.; Liu, D.; et al. Microspacing In-Air Sublimation Growth of Organic Crystals. Chem. Mater. 2018, 30, 412–420. [Google Scholar] [CrossRef]
- Lin, Q.; Ye, X.; Guo, Q.; Zheng, X.; Han, Q.; Li, C.; Jiang, J.; Liu, Y.; Tao, X. Homogeneously Oriented Organic Single-Crystalline Patterns Grown by Microspacing In-Air Sublimation. Small Methods 2023, 7, 2201374. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, J.; Zhang, J.; Wang, S.; Huang, H.; Zhang, J.; Yan, D.; Gao, Y.; Yang, J. Controllable thin-film morphology and structure for 2,7-dioctyl 1 benzothieno 3,2-b 1 benzothiophene (C8BTBT) based organic field-effect transistors. Org. Electron. 2016, 36, 73–81. [Google Scholar] [CrossRef]
- Shao, L.-J.; Chen, S.-N.; Wang, Y.-M.; Li, Z.; Shi, X.-S.; Long, H.-R.; Jiang, L.; Yang, J.-H.; Qiao, Y.-L.; Song, Y.-L. Vapor-Induced Coating Method for Well-Aligned and Uniform Organic Semiconductor Single Crystals. Chin. J. Polym. Sci. 2023, 1–8. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Long, H.; Yang, J.; Li, Z.; Cai, Z.; Qu, Z.; Shao, L.; Shi, X.; Jiang, L.; et al. A General Vapor-Induced Coating Approach for Layer—Controlled Organic Single Crystals. Adv. Funct. Mater. 2023, 33, 2212158. [Google Scholar] [CrossRef]
- Shtein, M.; Peumans, P.; Benziger, J.B.; Forrest, S.R. Micropatterning of small molecular weight organic semiconductor thin films using organic vapor phase deposition. J. Appl. Phys. 2003, 93, 4005–4016. [Google Scholar] [CrossRef]
- He, D.; Zhang, Y.; Wu, Q.; Xu, R.; Nan, H.; Liu, J.; Yao, J.; Wang, Z.; Yuan, S.; Li, Y.; et al. Two-dimensional quasi-freestanding molecular crystals for high-performance organic field-effect transistors. Nat. Commun. 2014, 5, 5162. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Jiang, T.; Wang, X.; Chen, H.; Li, S.; Wei, F.; Ren, Z.; Yan, S.; Guo, X.; Tu, H. Preparation of highly oriented single crystal arrays of C8-BTBT by epitaxial growth on oriented isotactic polypropylene. J. Mater. Chem. C 2020, 8, 2155–2159. [Google Scholar] [CrossRef]
- Wu, B.; Zhao, Y.; Nan, H.; Yang, Z.; Zhang, Y.; Zhao, H.; He, D.; Jiang, Z.; Liu, X.; Li, Y.; et al. Precise, Self-Limited Epitaxy of Ultrathin Organic Semiconductors and Heterojunctions Tailored by van der Waals Interactions. Nano Lett. 2016, 16, 3754–3759. [Google Scholar] [CrossRef]
- Deng, W.; Xiao, Y.; Lu, B.; Zhang, L.; Xia, Y.; Zhu, C.; Zhang, X.; Guo, J.; Zhang, X.; Jie, J. Water-Surface Drag Coating: A New Route Toward High-Quality Conjugated Small-Molecule Thin Films with Enhanced Charge Transport Properties. Adv. Mater. 2021, 33, 2005915. [Google Scholar] [CrossRef]
- Wang, J.W.; Deng, W.; Wang, W.; Jia, R.F.; Xu, X.Z.; Xiao, Y.L.; Zhang, X.J.; Jie, J.S.; Zhang, X.H. External-force-driven solution epitaxy of large-area 2D organic single crystals for high-performance field-effect transistors. Nano Res. 2019, 12, 2796–2801. [Google Scholar] [CrossRef]
- Wang, C.; Fu, B.; Zhang, X.; Li, R.; Dong, H.; Hu, W. Solution-Processed, Large-Area, Two-Dimensional Crystals of Organic Semiconductors for Field-Effect Transistors and Phototransistors. ACS Cent. Sci. 2020, 6, 636–652. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Pan, J.; Feng, T.; Wu, D.; Zhang, X.; Yang, B.; Zhang, X.; Jie, J. Graphene-Quantum-Dots-Induced Centimeter-Sized Growth of Monolayer Organic Crystals for High-Performance Transistors. Adv. Mater. 2020, 32, 2003315. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, B.; Zheng, R.; Wu, S.; Zhao, D.; Qiu, M. Production and Patterning of Fluorescent Quantum Dots by Cryogenic Electron-Beam Writing. ACS Appl. Mater. Interfaces 2023, 15, 12154–12160. [Google Scholar] [CrossRef]
- Tian, X.; Yao, J.; Guo, S.; Wang, Z.; Xiao, Y.; Zhang, H.; Feng, Y.; Feng, W.; Jie, J.; Yang, F.; et al. Soft template-assisted self-assembly: A general strategy toward two-dimensional molecular crystals for high-performance organic field-effect transistors. J. Mater. Chem. C 2022, 10, 2575–2580. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, S.; Zhang, X.; Geng, B.; Xiao, Y.; Jie, J.; Dong, H.; Li, L.; Hu, W. Organic Semiconductor Crystal Engineering for High-Resolution Layer-Controlled 2D Crystal Arrays. Adv. Mater. 2022, 34, e2104166. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Rao, L.; Zhang, J.; Xiao, M.; Zhu, D.; Li, C.; Shi, X.; Liu, J.; Liu, J.; et al. Solution-processed top-contact electrodes strategy for organic crystalline field-effect transistor arrays. Nano Res. 2022, 15, 858–863. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Liu, J.; Li, T.; Li, C.; Zhang, J.; Hu, W.; Liu, Y.; Jiang, L. Capillary-Confinement Crystallization for Monolayer Molecular Crystal Arrays. Adv. Mater. 2022, 34, e2107574. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, S.; Jang, B.C.; Yoo, H. Dielectric surface-dependent photogating phenomenon in C8-BTBT leading to broad spectral ultraviolet to near-infrared photoresponse and linearly-weighted synaptic phototransistors. Dye. Pigment. 2022, 208, 110882. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, S.; Sun, L.; Liu, L.; Wei, H.; Xu, Z.; Xu, W.; Xu, W. A low-dimensional hybrid p-i-n heterojunction neuromorphic transistor with ultra-high UV sensitivity and immediate switchable plasticity. Appl. Mater. Today 2021, 25, 101223. [Google Scholar] [CrossRef]
- Li, Q.-X.; Wang, T.-Y.; Wang, X.-L.; Chen, L.; Zhu, H.; Wu, X.-H.; Sun, Q.-Q.; Zhang, D.W. Flexible organic field-effect transistor arrays for wearable neuromorphic device applications. Nanoscale 2020, 12, 23150–23158. [Google Scholar] [CrossRef]
- Li, Q.; Wang, T.; Hu, X.; Wu, X.; Zhu, H.; Ji, L.; Sun, Q.; Zhang, D.W.; Chen, L. Organic Optoelectronic Synaptic Devices for Energy-Efficient Neuromorphic Computing. IEEE Electron Device Lett. 2022, 43, 1089–1092. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, L.; Feng, J.; Liu, J.; Sun, L.; Xu, W. Flexible optoelectronic neural transistors with broadband spectrum sensing and instant electrical processing for multimodal neuromorphic computing. SmartMat 2022, 4, e1154. [Google Scholar] [CrossRef]
- Xie, P.; Chen, X.; Zeng, Z.; Wang, W.; Meng, Y.; Lai, Z.; Quan, Q.; Li, D.; Wang, W.; Bu, X.; et al. Artificial Visual Systems With Tunable Photoconductivity Based on Organic Molecule-Nanowire Heterojunctions. Adv. Funct. Mater. 2023, 33, 2209091. [Google Scholar] [CrossRef]
- Xu, X.; Deng, W.; Zhang, X.; Huang, L.; Wang, W.; Jia, R.; Wu, D.; Zhang, X.; Jie, J.; Lee, S.-T. Dual-Band, High-Performance Phototransistors from Hybrid Perovskite and Organic Crystal Array for Secure Communication Applications. ACS Nano 2019, 13, 5910–5919. [Google Scholar] [CrossRef]
- Gao, Y.; Yi, Y.; Wang, X.; Meng, H.; Lei, D.; Yu, X.-F.; Chu, P.K.; Li, J. A Novel Hybrid-Layered Organic Phototransistor Enables Efficient Intermolecular Charge Transfer and Carrier Transport for Ultrasensitive Photodetection. Adv. Mater. 2019, 31, 1900763. [Google Scholar] [CrossRef]
- Jia, R.; Li, J.; Zhang, H.; Zhang, X.; Cheng, S.; Pan, J.; Wang, C.; Pirzado, A.A.A.; Dong, H.; Hu, W.; et al. Highly Efficient Inherent Linearly Polarized Electroluminescence from Small-Molecule Organic Single Crystals. Adv. Mater. 2022, 35, 2208789. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, D.; Hayakawa, R.; Zhong, X.; Aimi, J.; Wakayama, Y. Optically Controllable Organic Logic-in-Memory: An Innovative Approach toward Ternary Data Processing and Storage. Nano Lett. 2022, 23, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kwon, J.; Lee, Y.; Baek, S.; Jung, S. Phase–Separated, Printed Organic Thin–Film Transistor–Based Nonvolatile Memory with Enhanced Data Retention. Adv. Mater. Technol. 2020, 5, 2000228. [Google Scholar] [CrossRef]
- Panigrahi, D.; Hayakawa, R.; Wakayama, Y. Antiambipolar Transistor with Double Negative Differential Transconductances for Organic Quaternary Logic Circuits. Adv. Funct. Mater. 2023, 33, 2213899. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, H.; Lin, J.; Sun, H. Nanocrystal Array Engineering and Optoelectronic Applications of Organic Small-Molecule Semiconductors. Nanomaterials 2023, 13, 2087. https://doi.org/10.3390/nano13142087
Gong H, Lin J, Sun H. Nanocrystal Array Engineering and Optoelectronic Applications of Organic Small-Molecule Semiconductors. Nanomaterials. 2023; 13(14):2087. https://doi.org/10.3390/nano13142087
Chicago/Turabian StyleGong, Haoyu, Jinyi Lin, and Huibin Sun. 2023. "Nanocrystal Array Engineering and Optoelectronic Applications of Organic Small-Molecule Semiconductors" Nanomaterials 13, no. 14: 2087. https://doi.org/10.3390/nano13142087





