Construction of Type-II Heterojunctions in Crystalline Carbon Nitride for Efficient Photocatalytic H2 Evolution
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Synthesis of Crystalline C3N4 (CCN)
2.3. Material Characterizations
2.4. Photocatalytic Measurements
2.5. Density Functional Theory (DFT) Calculations
2.6. Calculation of Built-In Electric Field (BIEF)
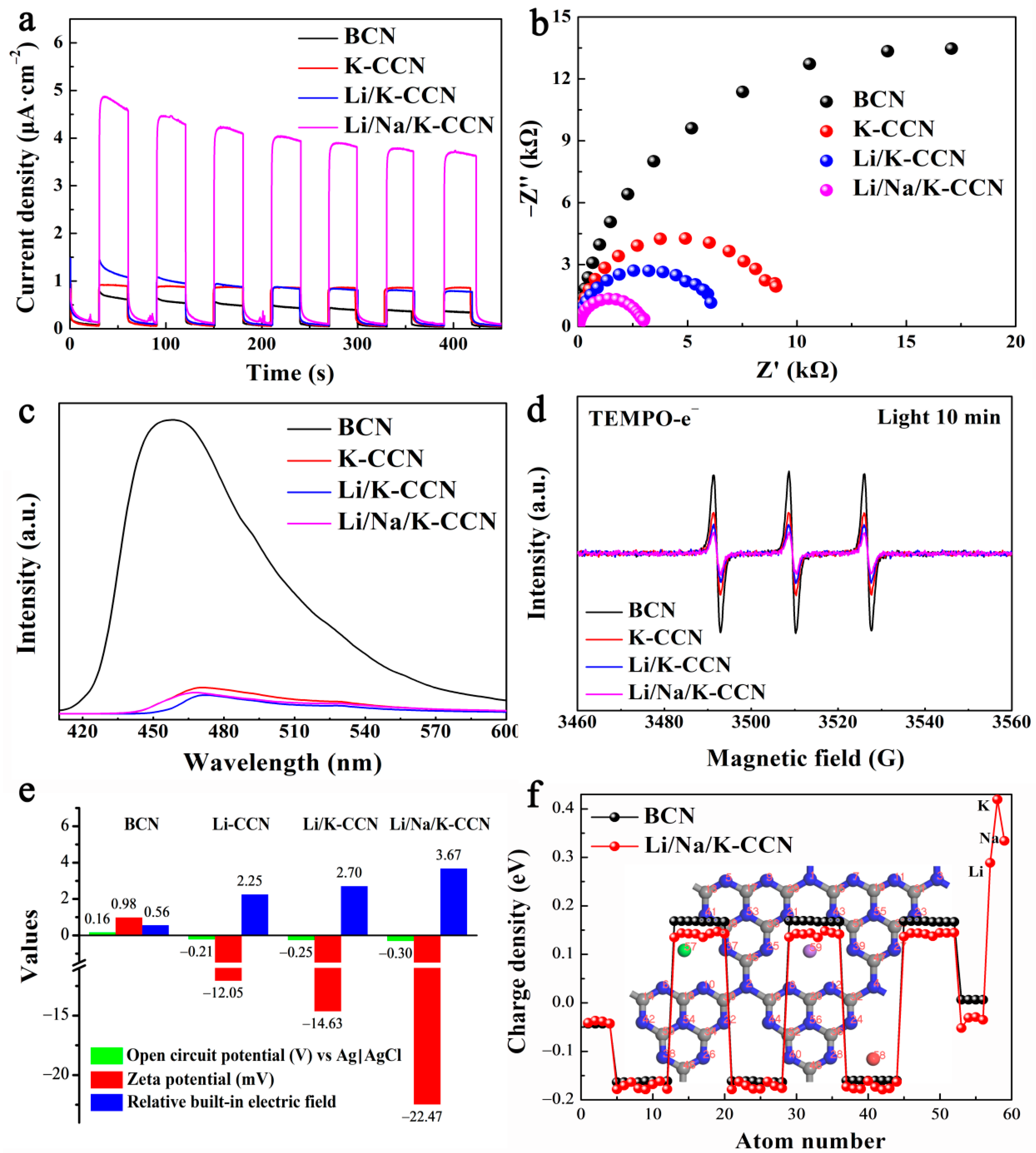
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, S.; Tang, R.; Gong, D.; Deng, Y.; Zheng, J.; Li, L.; Zhou, Z.; Yang, L.; Su, L. Environmentally-friendly carbon nanomaterials for photocatalytic hydrogen production. Chin. J. Catal. 2022, 43, 1719–1748. [Google Scholar] [CrossRef]
- Lan, K.; Wang, R.; Zhang, W.; Zhao, Z.; Elzatahry, A.; Zhang, X.; Liu, Y.; Al-Dhayan, D.; Xia, Y.; Zhao, D. Mesoporous TiO2 Microspheres with Precisely Controlled Crystallites and Architectures. Chem 2018, 4, 2436–2450. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Liu, S.; Yuan, Y.; Guo, S.; Ren, Z. Octahedral Shaped PbTiO3-TiO2 Nanocomposites for High-Efficiency Photocatalytic Hydrogen Production. Nanomaterials 2021, 11, 2295. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Han, Q.; Cheng, Z.; Gao, J.; Zhao, Y.; Zhang, Z.; Dai, L.; Qu, L. Mesh-on-Mesh Graphitic-C3N4@Graphene for Highly Efficient Hydrogen Evolution. Adv. Funct. Mater. 2017, 27, 1606352. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Ma, Y.; Zhang, Y.; Wang, L.; Bi, Y. Ultrathin graphitic C3N4 nanosheets as highly efficient metal-free cocatalyst for water oxidation. Appl. Catal. B-Environ. 2017, 205, 19–23. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B-Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Ma, Z.; Dong, L.; Jia, X.; Zhang, J. Enhancing Photocatalytic Hydrogen Production of g-C3N4 by Selective Deposition of Pt Cocatalyst. Nanomaterials 2021, 11, 3266. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.-T.F.; et al. High Efficiency Photocatalytic Water Splitting Using 2D α-Fe2O3/g-C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Hu, Y.; Qu, Y.; Zhou, Y.; Wang, Z.; Wang, H.; Yang, B.; Yu, Z.; Wu, Y. Single Pt atom-anchored C3N4: A bridging Pt–N bond boosted electron transfer for highly efficient photocatalytic H2 generation. Chem. Eng. J. 2021, 412, 128749. [Google Scholar] [CrossRef]
- Yu, H.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.I.N.; Zhao, Y.; Zhou, C.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Nitrogen-Doped Porous Carbon Nanosheets Templated from g-C3N4 as Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Wang, Z.; Wu, H.; Zhou, X.; Ma, H.; Zhong, H.; Xing, Z.; Cai, G.; Jiang, C.; et al. C/N Vacancy Co-Enhanced Visible-Light-Driven Hydrogen Evolution of g-C3N4 Nanosheets Through Controlled He+ Ion Irradiation. Sol. RRL 2019, 3, 1800298. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Shi, T.; Cui, W.; Zhang, X.; Dong, F.; Cheng, J.; Fan, J.; Lv, K. Carbon vacancy in C3N4 nanotube: Electronic structure, photocatalysis mechanism and highly enhanced activity. Appl. Catal. B-Environ. 2020, 262, 118281. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, B.; Liu, Y.; Ren, Y.; Ma, J.; Zhou, X.; Cheng, X.; Deng, Y. Controllable Interface-Induced Co-Assembly toward Highly Ordered Mesoporous Pt@TiO2/g-C3N4 Heterojunctions with Enhanced Photocatalytic Performance. Adv. Funct. Mater. 2018, 28, 1806214. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, S.; Ye, H.; Kong, K.; Gong, X.; Hua, J.; Tian, H. Molecular Engineering of Donor–Acceptor Conjugated Polymer/g-C3N4 Heterostructures for Significantly Enhanced Hydrogen Evolution Under Visible-Light Irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, A.; Hou, J.; Liu, Q.; Li, H.; Guo, X. Ag–Au Core–Shell Triangular Nanoprisms for Improving p-g-C3N4 Photocatalytic Hydrogen Production. Nanomaterials 2021, 11, 3347. [Google Scholar] [CrossRef]
- Sun, L.; Yang, M.; Huang, J.; Yu, D.; Hong, W.; Chen, X. Freestanding Graphitic Carbon Nitride Photonic Crystals for Enhanced Photocatalysis. Adv. Funct. Mater. 2016, 26, 4943–4950. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Yuan, Y.; Li, L.; Zhang, M.; Zhou, C.; Lin, Z. A Rapid Microwave-Assisted Thermolysis Route to Highly Crystalline Carbon Nitrides for Efficient Hydrogen Generation. Angew. Chem. Int. Ed. 2016, 55, 14693–14697. [Google Scholar] [CrossRef]
- Zhao, G.; Pang, H.; Liu, G.; Li, P.; Liu, H.; Zhang, H.; Shi, L.; Ye, J. Co-porphyrin/carbon nitride hybrids for improved photocatalytic CO2 reduction under visible light. Appl. Catal. B-Environ. 2017, 200, 141–149. [Google Scholar] [CrossRef]
- Zhai, B.; Li, H.; Gao, G.; Wang, Y.; Niu, P.; Wang, S.; Li, L. A Crystalline Carbon Nitride Based Near-Infrared Active Photocatalyst. Adv. Funct. Mater. 2022, 32, 2207375. [Google Scholar] [CrossRef]
- Sun, K.; Wang, Y.; Chang, C.; Yang, S.; Di, S.; Niu, P.; Wang, S.; Li, L. Molten-salt synthesis of crystalline C3N4/C nanosheet with high sodium storage capability. Chem. Eng. J. 2021, 425, 131591. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, K.; Ding, X.; Wang, S.; Zhang, C.; Liu, J.; Zhan, Z.; Cheng, G.; Li, B.; Chen, H.; et al. Controlling Monomer Feeding Rate to Achieve Highly Crystalline Covalent Triazine Frameworks. Adv. Mater. 2019, 31, 1807865. [Google Scholar] [CrossRef] [PubMed]
- Brazovskii, S.; Kirova, N. Physical theory of excitons in conducting polymers. Chem. Soc. Rev. 2010, 39, 2453–2465. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Chen, S.; Zhang, X.; Shao, W.; Sun, X.; Zhao, Z.; Zhang, Q.; Luo, Y.; Xie, Y. Insights into the excitonic processes in polymeric photocatalysts. Chem. Sci. 2017, 8, 4087–4092. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, M.; Fan, F.; Li, G.; Duan, J.; Li, Y.; Jiang, G.; Yao, W. Enhanced full-spectrum photocatalytic activity of 3D carbon-coated C3N4 nanowires via giant interfacial electric field. Appl. Catal. B-Environ. 2022, 318, 121829. [Google Scholar] [CrossRef]
- Tan, T.; Wang, X.; Zhou, X.; Ma, H.; Fang, R.; Geng, Q.; Dong, F. Highly active Cs2SnCl6/C3N4 heterojunction photocatalysts operating via interfacial charge transfer mechanism. J. Hazard. Mater. 2022, 439, 129694. [Google Scholar] [CrossRef]
- Ran, J.; Guo, W.; Wang, H.; Zhu, B.; Yu, J.; Qiao, S.-Z. Metal-Free 2D/2D Phosphorene/g-C3N4 Van der Waals Heterojunction for Highly Enhanced Visible-Light Photocatalytic H2 Production. Adv. Mater. 2018, 30, 1800128. [Google Scholar] [CrossRef]
- Shan, H.; Qin, J.; Ding, Y.; Sari, H.M.K.; Song, X.; Liu, W.; Hao, Y.; Wang, J.; Xie, C.; Zhang, J.; et al. Controllable Heterojunctions with a Semicoherent Phase Boundary Boosting the Potassium Storage of CoSe2/FeSe2. Adv. Mater. 2021, 33, 2102471. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, H.; Quan, X.; Chen, S.; Zhang, S. Structuring phase junction between tri-s-triazine and triazine crystalline C3N4 for efficient photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2018, 227, 153–160. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, W.; Ke, X.; Cai, X.; Chen, X.; Wang, S.; Lin, W.; Wang, X. A heptazine-based polymer photocatalyst with donor-acceptor configuration to promote exciton dissociation and charge separation. Appl. Catal. B-Environ. 2023, 325, 122312. [Google Scholar] [CrossRef]
- Liu, B.; Xu, B.; Li, S.; Du, J.; Liu, Z.; Zhong, W. Heptazine-based porous graphitic carbon nitride: A visible-light driven photocatalyst for water splitting. J. Mater. Chem. A 2019, 7, 20799–20805. [Google Scholar] [CrossRef]
- Ou, H.; Lin, L.; Zheng, Y.; Yang, P.; Fang, Y.; Wang, X. Tri-s-triazine-Based Crystalline Carbon Nitride Nanosheets for an Improved Hydrogen Evolution. Adv. Mater. 2017, 29, 1700008. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule Self-Assembly Synthesis of Porous Few-Layer Carbon Nitride for Highly Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.; Li, K.; Yang, G.; Wang, K.; Xu, R.; Xie, X. One-step synthesis of novel K+ and cyano groups decorated triazine-/heptazine-based g-C3N4 tubular homojunctions for boosting photocatalytic H2 evolution. Appl. Catal. B-Environ. 2020, 262, 118252. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef]
- Wang, K.; Fu, J.; Zheng, Y. Insights into photocatalytic CO2 reduction on C3N4: Strategy of simultaneous B, K co-doping and enhancement by N vacancies. Appl. Catal. B-Environ. 2019, 254, 270–282. [Google Scholar] [CrossRef]
- Wei, J.; Xin, C.; Shang, W.; Hu, J.; Guo, J.; Cheng, X.; Liu, W.; Shi, Y. Enhanced CO2-to-methane photoconversion over carbon nitride via interfacial charge kinetics steering. Mater. Des. 2022, 219, 110756. [Google Scholar] [CrossRef]
- Shi, L.; Yang, L.; Zhou, W.; Liu, Y.; Yin, L.; Hai, X.; Song, H.; Ye, J. Photoassisted Construction of Holey Defective g-C3N4 Photocatalysts for Efficient Visible-Light-Driven H2O2 Production. Small 2018, 14, 1703142. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.; Zhang, X.; Liu, Y.; Ren, M.; Yang, Y.; Guo, Y. Controllable Approach to Carbon-Deficient and Oxygen-Doped Graphitic Carbon Nitride: Robust Photocatalyst Against Recalcitrant Organic Pollutants and the Mechanism Insight. Adv. Funct. Mater. 2021, 31, 2010763. [Google Scholar] [CrossRef]
- Zhao, Z.; Long, Y.; Luo, S.; Luo, Y.; Chen, M.; Ma, J. Metal-Free C3N4 with plentiful nitrogen vacancy and increased specific surface area for electrocatalytic nitrogen reduction. J. Energy Chem. 2021, 60, 546–555. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, J.; Yu, X.; Yang, X.; Liu, W.; Yang, J.; Tang, H.; Xu, H.; Li, H.; Li, Y.; et al. Unveiling the origin of boosted photocatalytic hydrogen evolution in simultaneously (S, P, O)-Codoped and exfoliated ultrathin g-C3N4 nanosheets. Appl. Catal. B-Environ. 2019, 248, 84–94. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Xing, Y.; Liu, X.; Yang, Y.; Wang, X.; Song, S. Preparation of Carbon-Rich g-C3N4 Nanosheets with Enhanced Visible Light Utilization for Efficient Photocatalytic Hydrogen Production. Small 2017, 13, 1701552. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liu, X.; Zhao, X.; Zhang, J.; Dong, P.; Zhang, Y.; Zhao, C.; Sun, H.; Duan, X.; Wang, S.; et al. Manipulation of n → π* electronic transitions via implanting thiophene rings into two-dimensional carbon nitride nanosheets for efficient photocatalytic water purification. J. Mater. Chem. A 2022, 10, 20559–20570. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Li, H.; Cao, L.; Jiang, Z.; Chai, Z.; Wang, X. Molecular Self-Assembly of Oxygen Deep-Doped Ultrathin C3N4 with a Built-In Electric Field for Efficient Photocatalytic H2 Evolution. Inorg. Chem. 2021, 60, 15782–15796. [Google Scholar] [CrossRef]
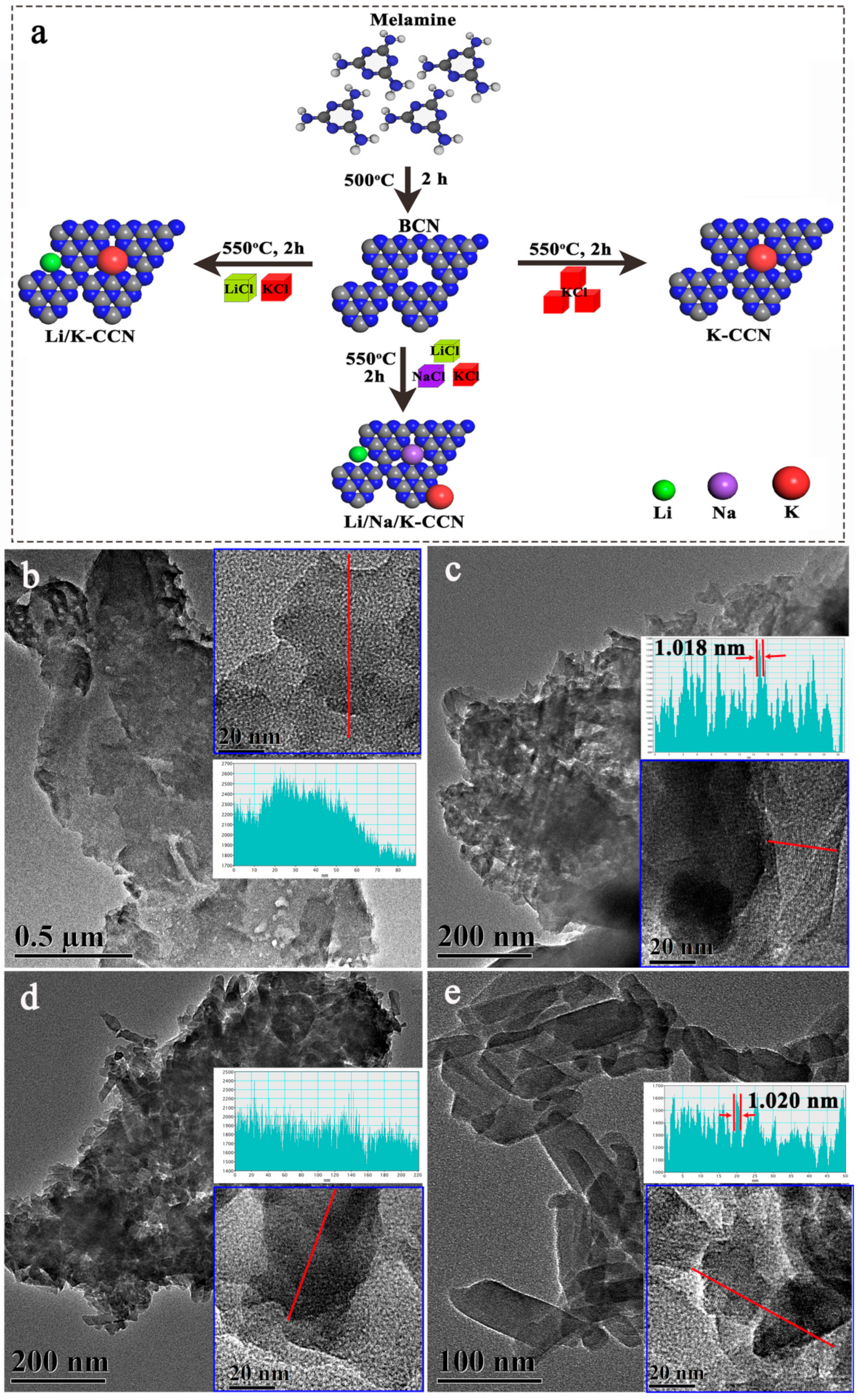
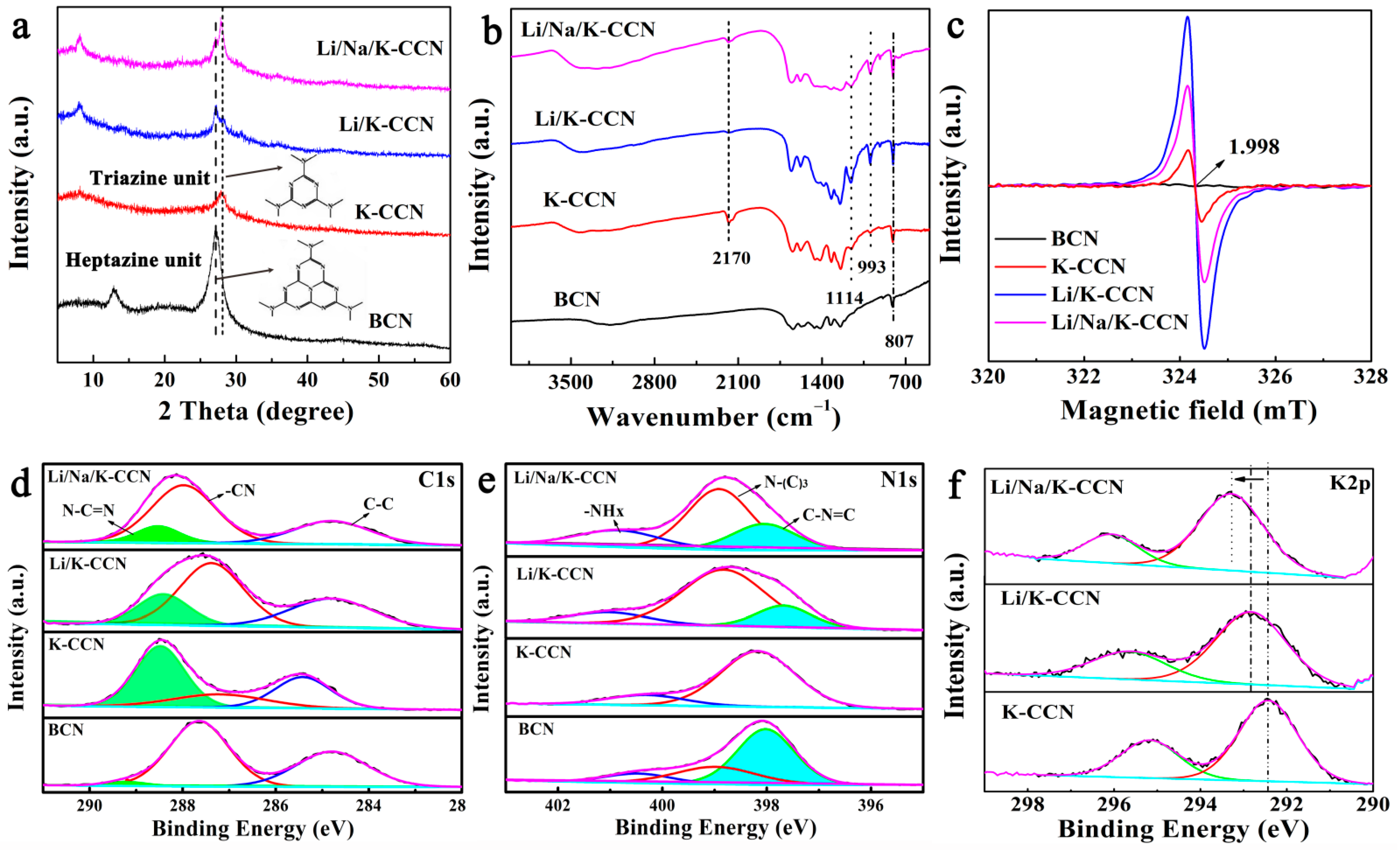
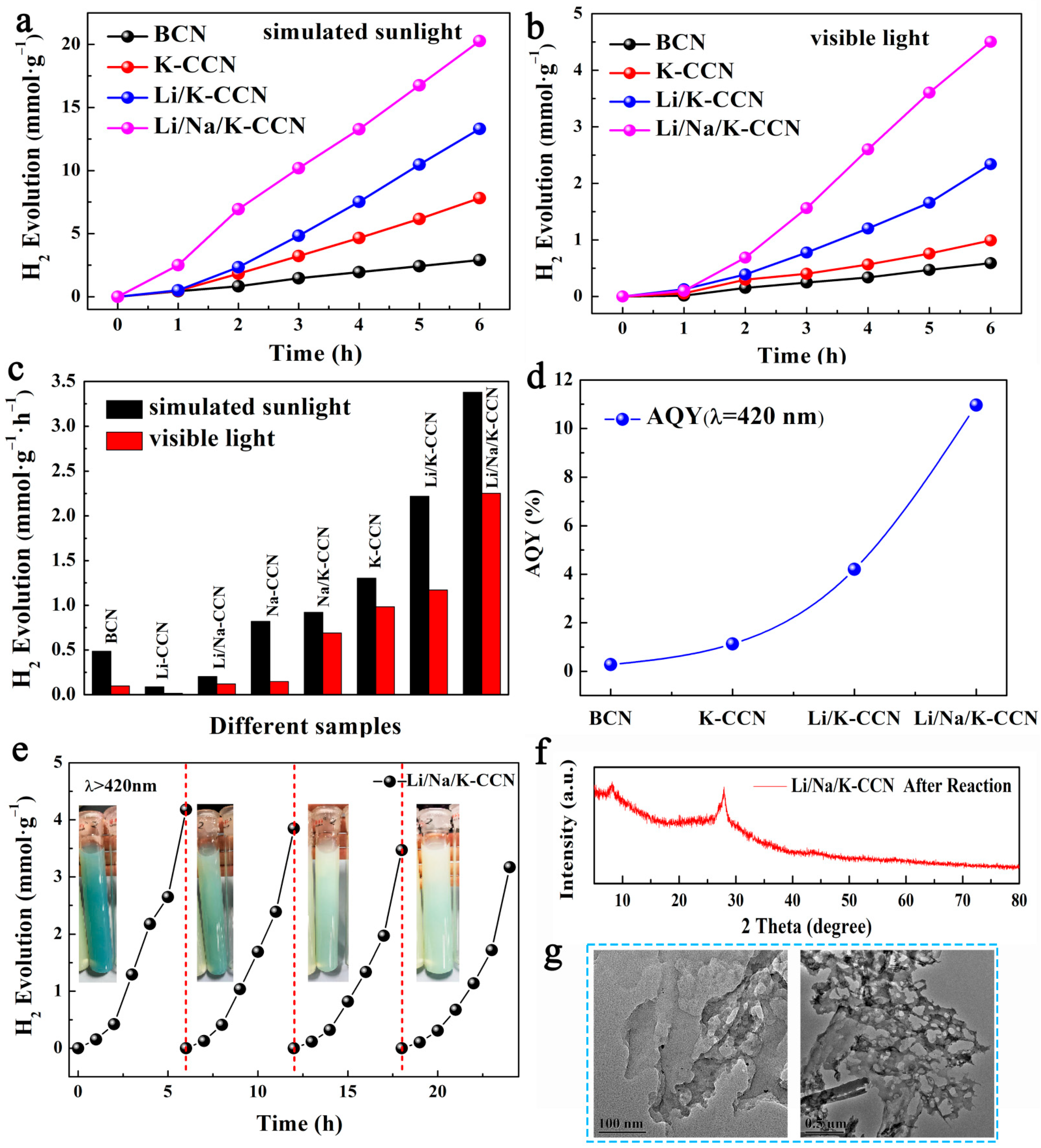
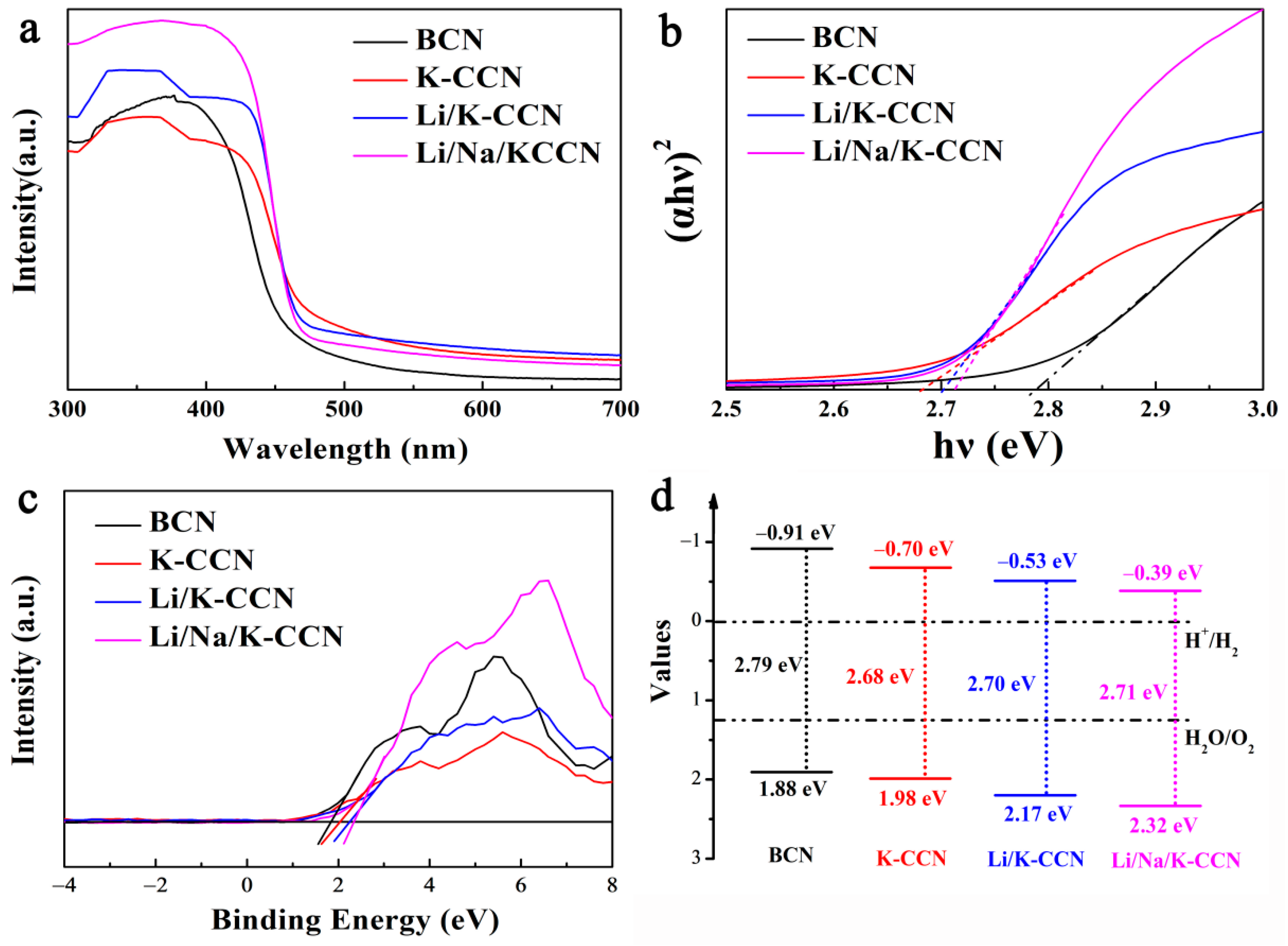

| Technique | Elemental Contents (at.%) | ||
|---|---|---|---|
| Li | Na | K | |
| XPS | 1.21 | 0.88 | 3.92 |
| ICP-MS | 0.1843 | 0.0499 | 0.1377 |
| Photocatalyst | Amt. (mg) | Experimental Conditions | Light Source | H2 Evolution (μmol·g−1·h−1) | Reference |
|---|---|---|---|---|---|
| MC-CN | 100 | 10% TEOA solution, 3% Pt | 300 W Xe lamp | 1125 | [21] |
| tri-/tri-s-tri-C3N4-90 | 50 | 10% TEOA solution, 3% Pt | 300 W Xe lamp (>420 nm) | 36 | [30] |
| P3/CN | 50 | 17% TEOA solution | 300 W Xe lamp | 13,000 | [16] |
| MS-550 | 100 | 10% TEOA solution, 3% Pt | 300 W Xe lamp (>420 nm) | 661 | [35] |
| PbTiO3-TiO2 | 30 | 20% methanol solution | 500 W high-pressure mercury lamp (<420 nm) | 21,017 | [3] |
| CN2 | 10 | 10% TEOA solution, 3% Pt | 300 W Xe lamp (>420 nm) | 1271 | [13] |
| 1.8PCN | 20 | 18% lactic acid aqueous solution | 300 W Xe lamp (≥400 nm) | 571 | [28] |
| CCNNSs | 50 | 10% methanol solution, 3% Pt | monochromatic LED lamps (>420 nm) | 1060 | [33] |
| CN-SPO | 10 | 20% TEOA solution, 3% Pt | 300 W Xe lamp (>420 nm) | 2479 | [42] |
| CN16 | 20 | 15% TEOA solution, 0.5% Pt | 300 W Xe lamp (>420 nm) | 2025 | [19] |
| CNSC | 10 | 12% methanol solution, 3% Pt | 300 W Xe lamp (>400 nm) | 3960 | [43] |
| Li/Na/K-CCN | 100 | 20% TEOA solution, 3% Pt | 300 W Xe lamp (>420 nm) | 2250 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, Z.; Li, J.; He, Y.; Tong, H.; Li, S.; Chai, Z.; Lan, K. Construction of Type-II Heterojunctions in Crystalline Carbon Nitride for Efficient Photocatalytic H2 Evolution. Nanomaterials 2023, 13, 2300. https://doi.org/10.3390/nano13162300
Zhang J, Li Z, Li J, He Y, Tong H, Li S, Chai Z, Lan K. Construction of Type-II Heterojunctions in Crystalline Carbon Nitride for Efficient Photocatalytic H2 Evolution. Nanomaterials. 2023; 13(16):2300. https://doi.org/10.3390/nano13162300
Chicago/Turabian StyleZhang, Jingyu, Zhongliang Li, Jialong Li, Yalin He, Haojie Tong, Shuang Li, Zhanli Chai, and Kun Lan. 2023. "Construction of Type-II Heterojunctions in Crystalline Carbon Nitride for Efficient Photocatalytic H2 Evolution" Nanomaterials 13, no. 16: 2300. https://doi.org/10.3390/nano13162300





