Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal
Abstract
:1. Introduction
2. Advantages of LDH/g-C3N4 Heterojunctions
3. Synthetic Strategy of LDH/g-C3N4 Heterojunctions
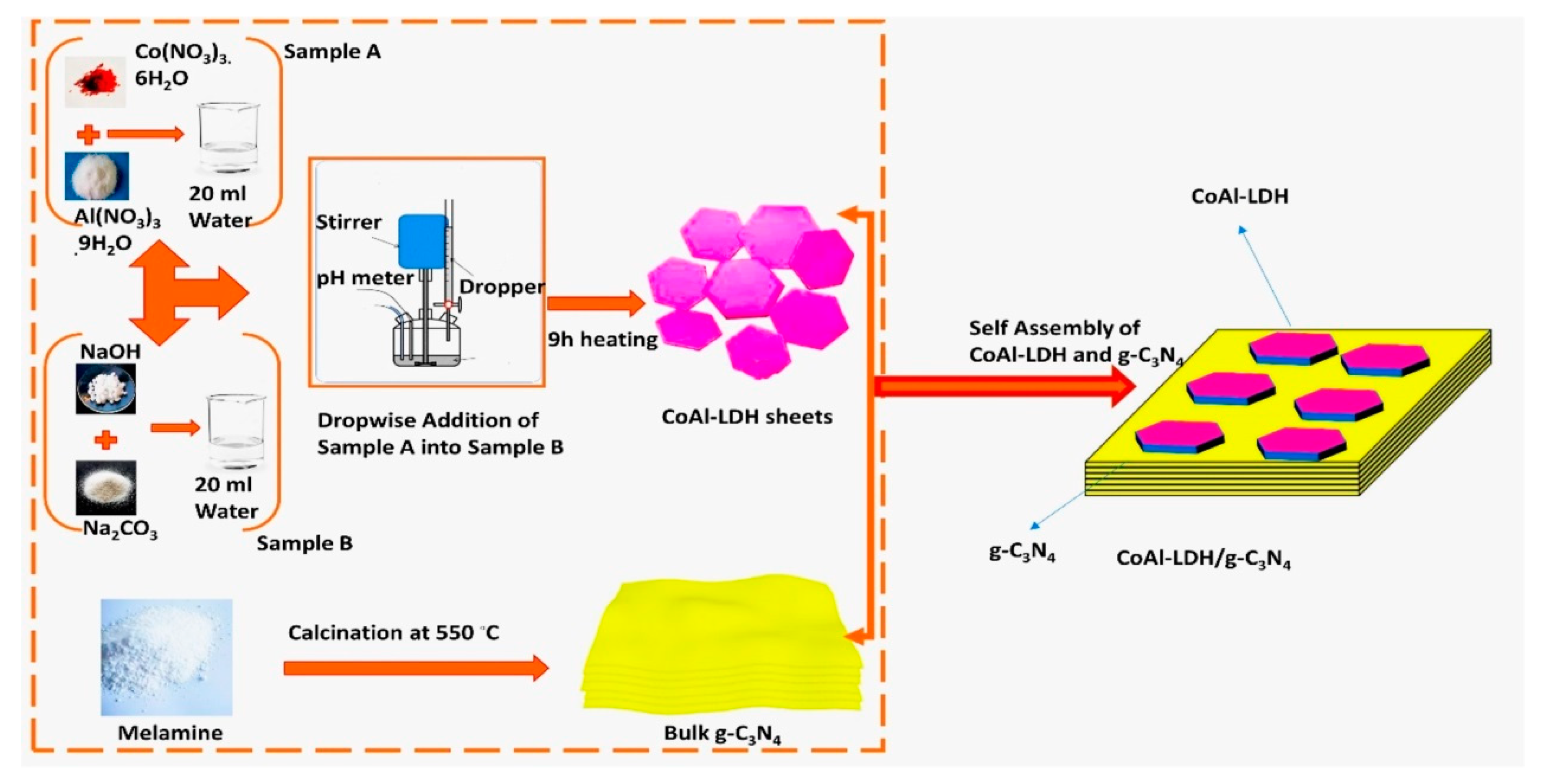
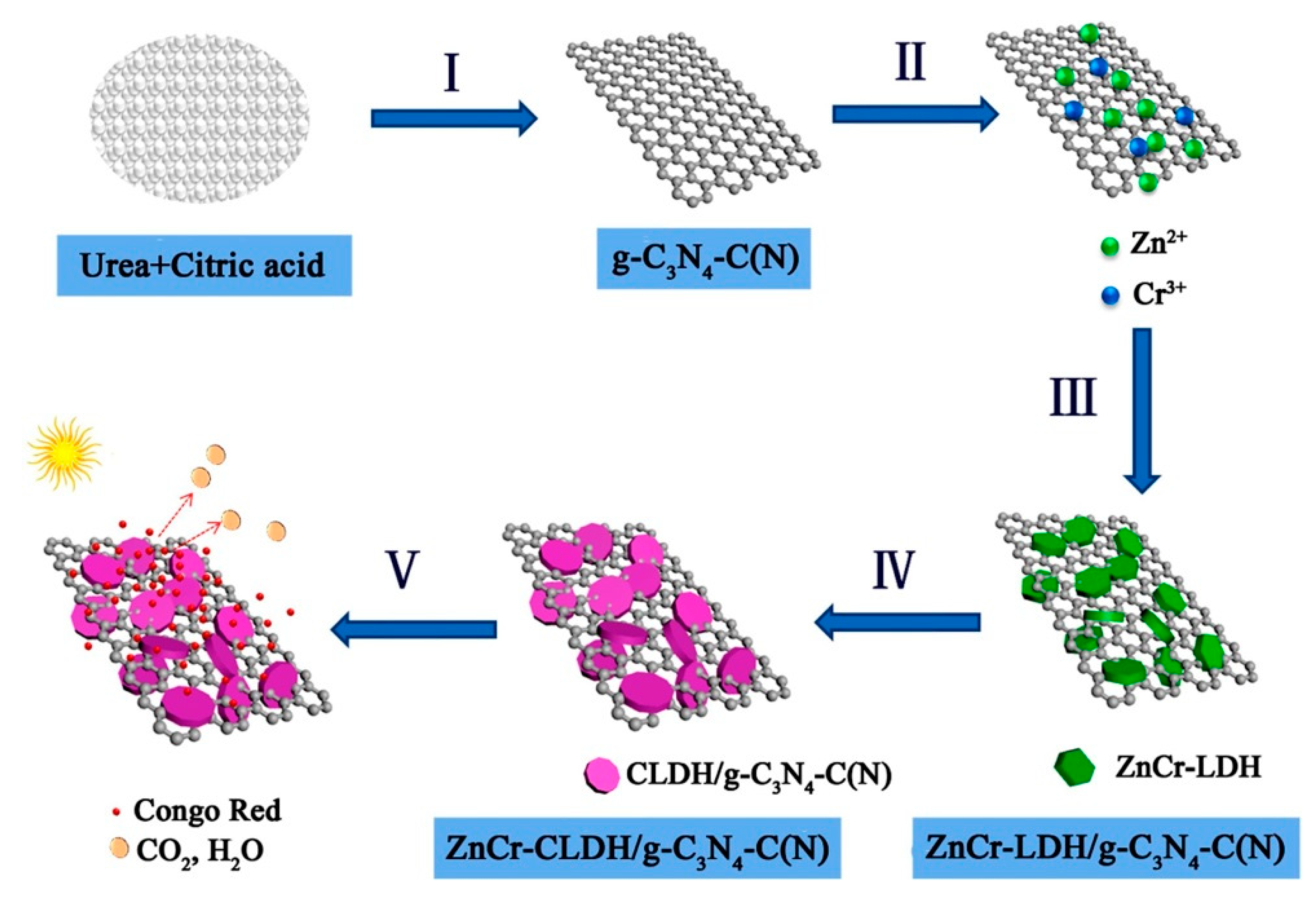
3.1. Electrostatic Self-Assembly Method
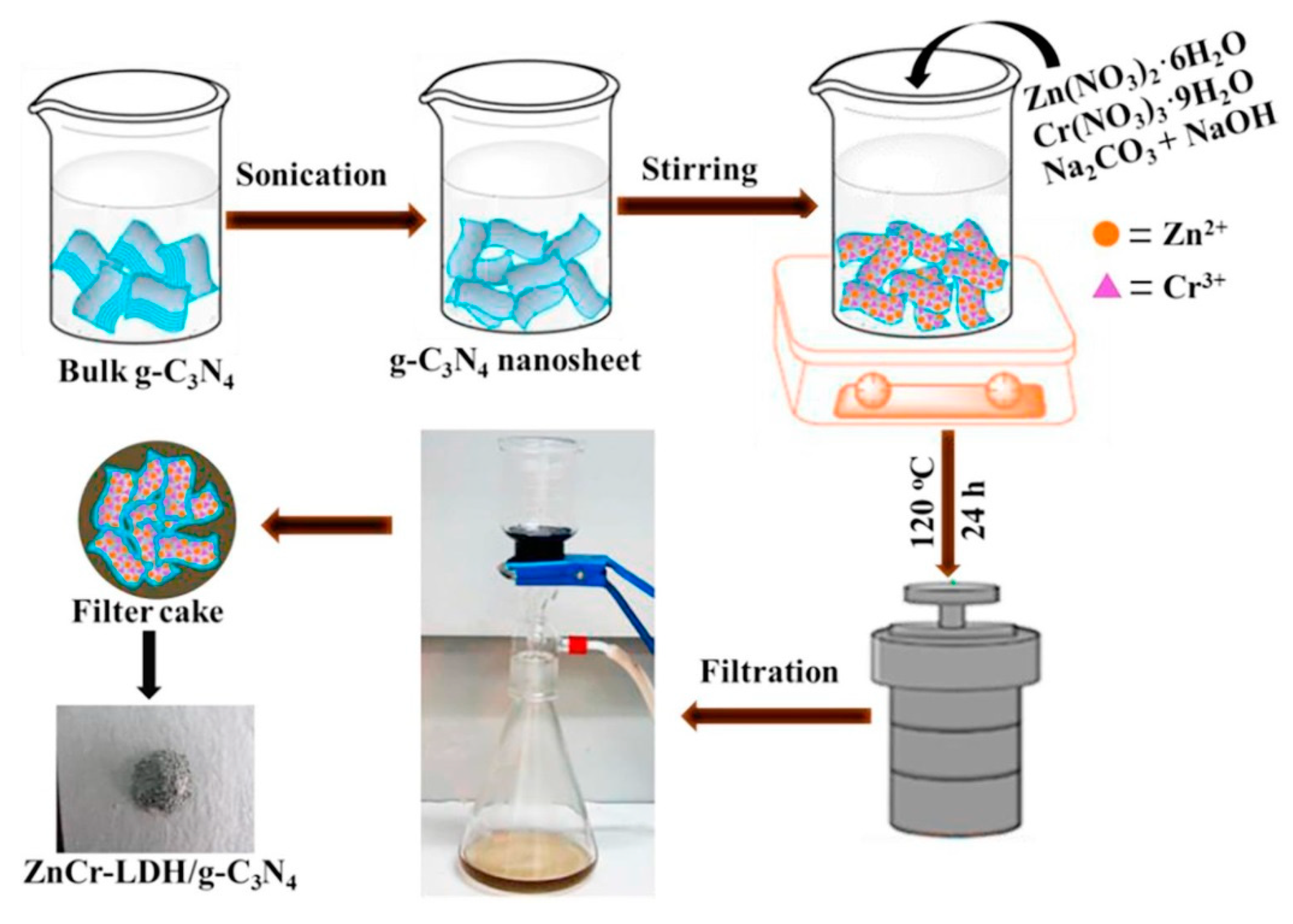
3.2. In Situ Coprecipitation Strategy
3.3. Hydrothermal Strategy
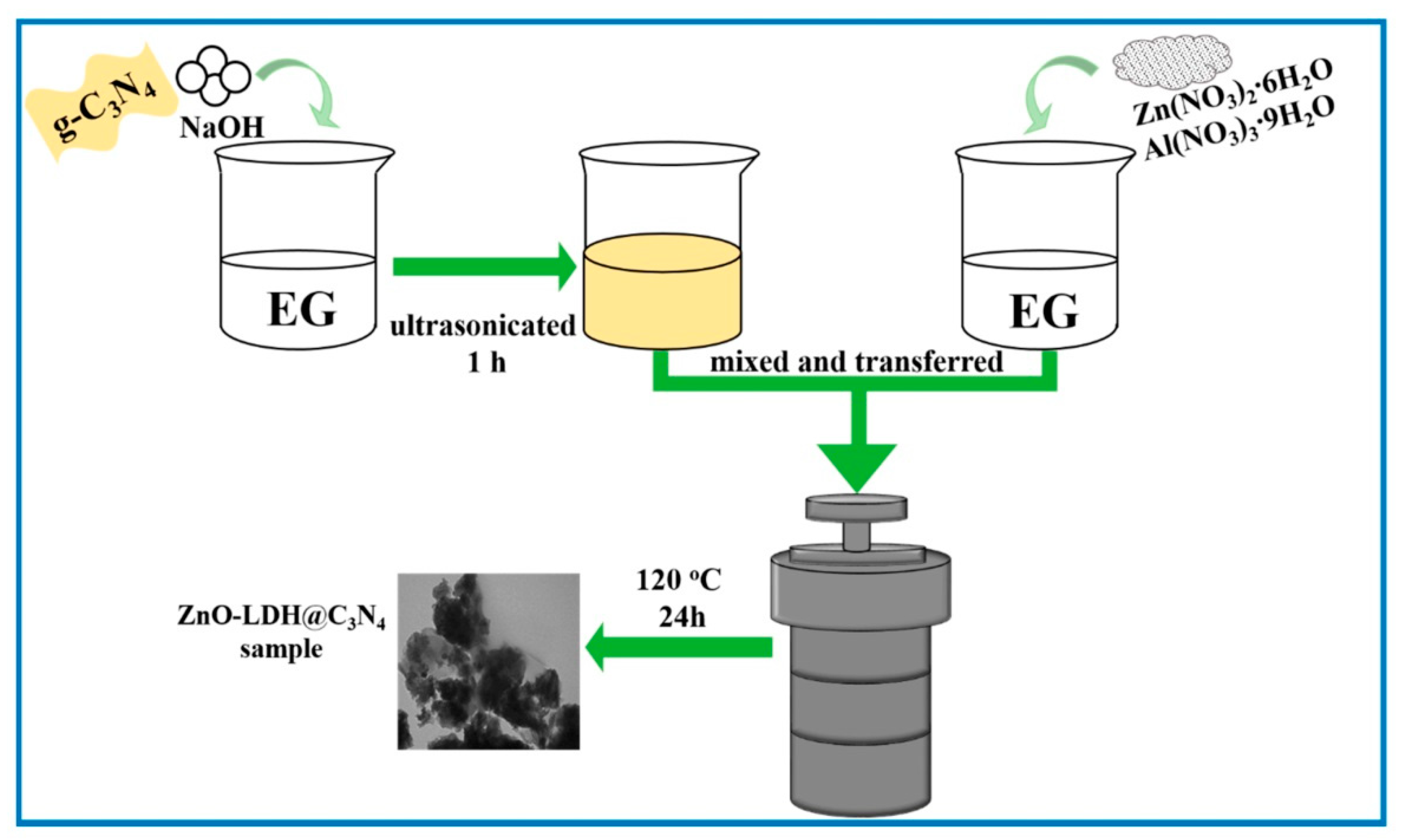
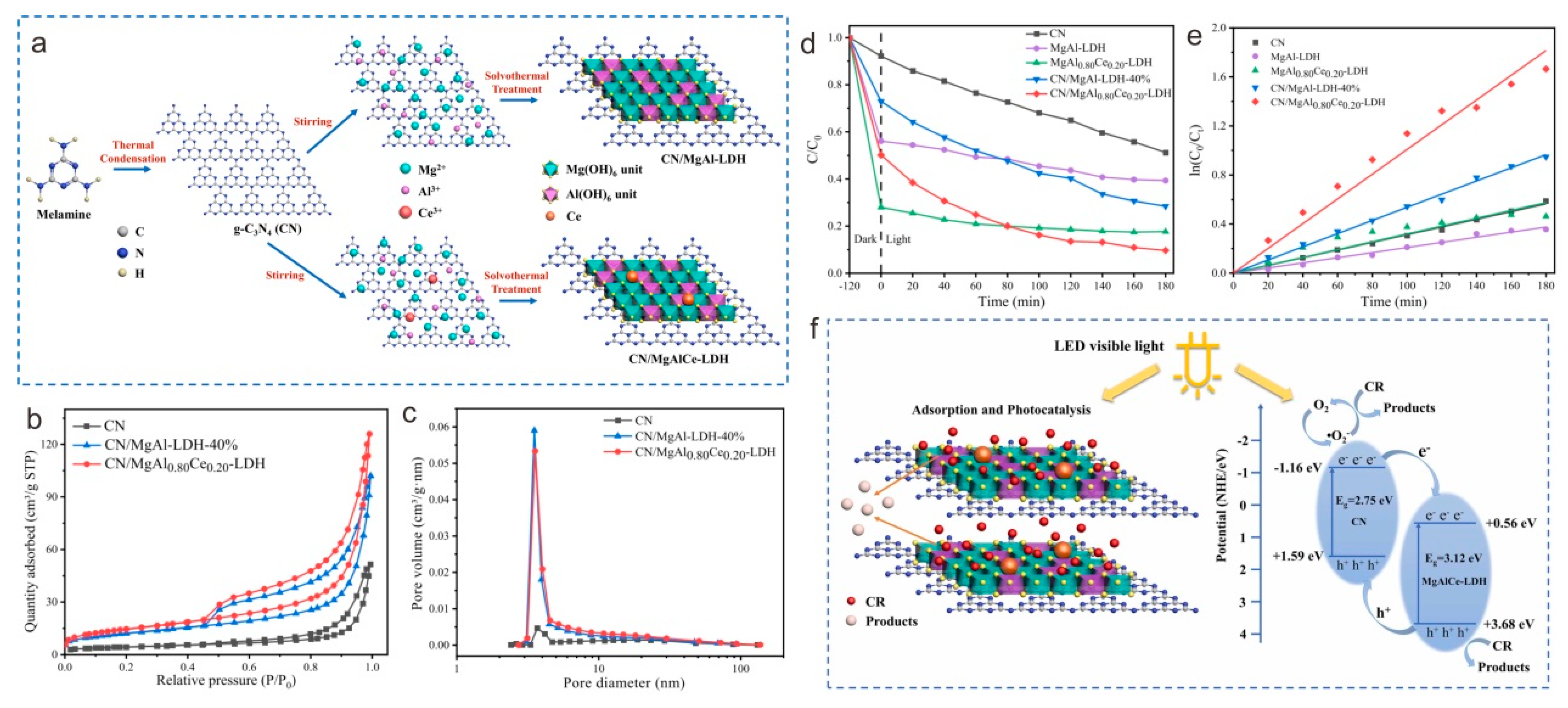
3.4. Solvothermal Method
3.5. Calcination Method
4. LDH/g-C3N4 Heterojunctions for Organic Pollutant Removal
5. Conclusions and Outlook
- (i)
- Matching the redox potential of a particular photocatalytic process with the CB (or VB) potential of LDH/g-C3N4 photocatalysts is crucial. This is likely to modify the CB (or VB) position of LDHs/g-C3N4 to offer high redox potential for a variety of photocatalytic processes since the band structure of the compound is customizable. The precise adjustment technique, however, requires more research.
- (ii)
- Gaining an in-depth understanding of the transfer mechanism of charge is also crucial. The precise charge transfer pathway and photocatalytic process can be further provided using theoretical calculations and characterization methods [61,105,106] including in situ FTIR, in situ XPS, photo-KPFM, and synchrotron radiation. The search for additional LDH can combine with g-C3N4 to improve photocatalytic activity, which is made easier with a greater knowledge of the mechanism.
- (iii)
- Constructing ultrathin 2D/2D structures is highly worth considering. The ultrathin LDHs and g-C3N4 have been suggested as photocatalysts in certain investigations. This is also likely to construct ultrathin LDH/g-C3N4 heterojunctions that will enable more rapid photocarrier migration because of the further shortened migration distance and decreased photocarrier recombination.
- (iv)
- Enhancing the capacity to capture visible or even near-infrared light, which accounts for over half of solar radiation, is strongly advised. Even while LDH/g-C3N4 photocatalysts can work with visible light, surface sensitization, doping, band gap correction, and other techniques can help them gather sunlight more effectively.
- (v)
- Expanding the applications of LDH/g-C3N4 photocatalysts in light of their special advantages is essential to creating a sustainable society.
- (vi)
- Some effective enhancement strategies, such as morphological modulation, loading co-catalysts, interface engineering, doping, and exposing more reactive facets, should be seriously studied to achieve an actual application of LDH/g-C3N4 systems.
- (vii)
- Improving the ability to harness both visible light and near-infrared light, which collectively represent more than 50% of solar irradiation, is paramount. LDH/g-C3N4 photocatalysts show potential for efficient operation under visible light, and there are also opportunities to further enhance sunlight harvesting capacity through techniques such as surface sensitization, doping elements, adjusting the band gap, and more.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Liu, C.; Chen, Z.; Huang, H.; Liu, Y.; Xue, L.; Sun, J.; Wang, X.; Xiong, P.; Zhu, J. Recent advances in two-dimensional materials for hydrovoltaic energy technology. Exploration 2023, 3, 20220061. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; He, C.; Li, Q.; Liu, S.-Y.; Liao, G. Renewable plant-derived lignin for electrochemical energy systems. Trends Biotechnol. 2022, 40, 1425–1438. [Google Scholar] [CrossRef]
- Li, C.; Jia, R.; Yang, Y.; Liao, G. A Hierarchical Helical Carbon Nanotube Fiber Artificial Ligament. Adv. Fiber Mater. 2023, 5, 1549–1551. [Google Scholar] [CrossRef]
- Lan, Q.; Jin, S.-J.; Wang, Z.; Li, X.-Y.; Xiong, Y.; Wang, Z.-C.; Liu, S.-S.; Zhang, Z.-M.; Zhao, Q. Design and synthesis of polyoxovanadate-based framework for efficient dye degradation. Tungsten 2023. [Google Scholar] [CrossRef]
- Khan, A.; Nilam, B.; Rukhsar, C.; Sayali, G.; Mandlekar, B.; Kadam, A. A review article based on composite graphene @tungsten oxide thin films for various applications. Tungsten 2023, 5, 391–418. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Yu, Y.; Wang, J.; Liu, J.; Ihara, H.; Qiu, H. Composite materials based on covalent organic frameworks for multiple advanced applications. Exploration 2023, 3, 20220144. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, G.-K. Enhanced photocatalytic performance of tungsten-based photocatalysts for degradation of volatile organic compounds: A review. Tungsten 2020, 2, 240–250. [Google Scholar] [CrossRef]
- Lin, J.-H.; Yan, Y.-T.; Qi, J.-L.; Zha, C.-Y. Atomic tailoring-induced deficiency in tungsten oxides for high-performance energy-related devices. Tungsten 2023. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, C.; Jian, J.; Zhou, T.; Xue, X.; Shi, J.; Che, G.; Liao, G. Efficient removal of organic pollution via photocatalytic degradation over a TiO2@HKUST-1 yolk-shell nanoreactor. J. Mol. Liq. 2023, 385, 122383. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-decorated BixTiyOz/TiO2 flexible carbon nanofibers and their applications on degradating of organic pollutants under solar radiation. J. Mater. Sci. Technol. 2023, 150, 114–123. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Fang, B. Donor-acceptor organic semiconductor heterojunction nanoparticles for efficient photocatalytic H2 evolution. Matter 2022, 5, 1635–1637. [Google Scholar] [CrossRef]
- Liao, G.; Fang, B.; Li, C. A high-pressure-tolerant vapor-fed photocatalytic system for efficient water splitting. Chem Catal. 2023, 3, 100671. [Google Scholar] [CrossRef]
- Ghugal, S.G.; Kumar Tadi, K.; Rani, K.; Mendhe, A.C.; Jeong, Y.; Yoon, J. Improved photocatalytic performance of CdS/α-Fe2O3 heterostructure for anionic dye mineralization and charge storage applications. J. Mol. Liq. 2023, 392, 123384. [Google Scholar] [CrossRef]
- Duta, A.; Andronic, L.; Enesca, A. The influence of low irradiance and electrolytes on the mineralization efficiency of organic pollutants using the Vis-active photocatalytic tandem CuInS2/TiO2/SnO2. Catal. Today 2018, 300, 18–27. [Google Scholar] [CrossRef]
- Tao, X.; Ren, J.; Wang, D.; Huang, H.; Li, Y.; Guo, D.; Shan, B.; Liu, Y.; Wang, J.; Zhen, Y.; et al. Refining the d-band center of S-scheme CdIn2S4/MnZnFe2O4 heterostructures by interfacial electric field with boosting photocatalytic performance. Chem. Eng. J. 2023, 478, 147347. [Google Scholar] [CrossRef]
- Palani, G.; Apsari, R.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Kannan, K.; Shivanna, A.T.; Idris, A.M.; Yadav, C.H. Metal-Doped Graphitic Carbon Nitride Nanomaterials for Photocatalytic Environmental Applications-A Review. Nanomaterials 2022, 12, 1754. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Liao, G.; Tao, X.; Fang, B. An innovative synthesis strategy for high-efficiency and defects-switchable-hydrogenated TiO2 photocatalysts. Matter 2022, 5, 377–379. [Google Scholar] [CrossRef]
- Li, W.; Sohail, M.; Anwar, U.; Taha, T.A.; Al-Sehemi, A.G.; Muhammad, S.; Al-Ghamdi, A.A.; Amin, M.A.; Palamanit, A.; Ullah, S.; et al. Recent progress in g–C3N4–Based materials for remarkable photocatalytic sustainable energy. Int. J. Hydrog. Energy 2022, 47, 21067–21118. [Google Scholar] [CrossRef]
- Abu-Sari, S.M.; Daud, W.M.A.W.; Patah, M.F.A.; Ang, B.C. A review on synthesis, modification method, and challenges of light-driven H2 evolution using g-C3N4-based photocatalyst. Adv. Colloid Interface Sci. 2022, 307, 102722. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, G.; Yang, B.; Zhang, Y.; Ge, G.; Lipovka, A.; Liu, J.; Rodriguez, R.D.; Yang, X.; Jia, X. Structural reorganization of ultrathin g-C3N4 nanosheets for significantly boosting wide-spectrum-driven CO2 photoreduction. Appl. Surf. Sci. 2023, 638, 157989. [Google Scholar] [CrossRef]
- Tian, F.; Wu, X.; Chen, J.; Sun, X.; Yan, X.; Liao, G. One-step photodeposition of spatially separated CuOx and MnOx dual cocatalysts on g-C3N4 for enhanced CO2 photoreduction. Dalton Trans. 2023, 52, 11934–11940. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wu, X.; Liu, S.; Gu, Y.; Lin, Z.; Zhang, H.; Yan, X.; Liao, G. Boosting photocatalytic H2 evolution through interfacial manipulation on a lotus seedpod shaped Cu2O/g-C3N4 p-n heterojunction. Sustain. Energy Fuels 2023, 7, 786–796. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arabian J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Z.; Zhang, W.; Zhang, D. High-Efficiency g-C3N4 Based Photocatalysts for CO2 Reduction: Modification Methods. Adv. Fiber Mater. 2022, 4, 342–360. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.-Y.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2022, 4, 111–127. [Google Scholar] [CrossRef]
- Li, C.; Cheng, B.; Lu, H.; Ding, G.; Jiang, Z.; Liao, G. Kinetically and Thermodynamically Favorable Ni–Al Layered Double Hydroxide/Ni-Doped Zn0.5Cd0.5S S-Scheme Heterojunction Triggering Photocatalytic H2 Evolution. Inorg. Chem. 2023, 62, 6843–6850. [Google Scholar] [CrossRef]
- Ding, G.; Li, C.; Ni, Y.; Chen, L.; Shuai, L.; Liao, G. Layered double hydroxides and their composites as high-performance photocatalysts for CO2 reduction. EES Catal. 2023, 1, 369–391. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Xiao, R.; Ye, S.; Chen, M.; Lai, C.; Xu, P.; Tang, X. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications. Adv. Colloid Interface Sci. 2019, 272, 101999. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, H.; Ding, G.; Li, Q.; Liao, G. Recent advances on g-C3N4-based Z-scheme photocatalysts for organic pollutant removal. Catal. Sci. Technol. 2023, 13, 2877–2898. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Das, K.K.; Mansingh, S.; Sultana, S.; Parida, K. Recent progress in first row transition metal Layered double hydroxide (LDH) based electrocatalysts towards water splitting: A review with insights on synthesis. Coordin. Chem. Rev. 2022, 469, 214666. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coordin. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2D materials for photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Xue, J.; Xie, L.; Shen, J.; He, G.; Chen, H. Construction of magnetically separable NiAl LDH/Fe3O4–RGO nanocomposites with enhanced photocatalytic performance under visible light. Phys. Chem. Chem. Phys. 2018, 20, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D Hybrid Heterojunction for High-Performance Photocatalytic Reduction of CO2 into Renewable Fuels. ACS Appl. Mater. Interfaces 2018, 10, 2667–2678. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Sanati, S.; Rezvani, Z.; Hou, Z.; Dai, H. Ni–Ti Layered Double Hydroxide@Graphitic Carbon Nitride Nanosheet: A Novel Nanocomposite with High and Ultrafast Sonophotocatalytic Performance for Degradation of Antibiotics. Inorg. Chem. 2019, 58, 1834–1849. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-Y.; Lu, X.-L.; Tang, S.-F.; Yin, X.-P.; Wei, Z.-W.; Lu, T.-B. Metal-Free 2D/2D Heterojunction of Graphitic Carbon Nitride/Graphdiyne for Improving the Hole Mobility of Graphitic Carbon Nitride. Adv. Energy Mater. 2018, 8, 1702992. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-Linked g-C3N4/rGO Nanocomposites with Tunable Band Structure and Enhanced Visible Light Photocatalytic Activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Xue, C.; An, H.; Shao, G.; Yang, G. Accelerating directional charge separation via built-in interfacial electric fields originating from work-function differences. Chin. J. Catal. 2021, 42, 583–594. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.-Y.; Fang, B.; Yang, H. Z-scheme systems: From fundamental principles to characterization, synthesis, and photocatalytic fuel-conversion applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the layer-by-layer assembly of multilayered polymer composites: Strategy, structural control and applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. Ultrasonic assisted coating of multiwalled carbon nanotubes with NiFe-layered double hydroxide for improved electrocatalytic oxygen reduction. J. Electroanal. Chem. 2018, 823, 499–504. [Google Scholar] [CrossRef]
- Mao, N.; Zhou, C.H.; Tong, D.S.; Yu, W.H.; Cynthia Lin, C.X. Exfoliation of layered double hydroxide solids into functional nanosheets. Appl. Clay Sci. 2017, 144, 60–78. [Google Scholar] [CrossRef]
- Tahir, M.; Mansoor, R. Constructing Highly Stable CoAl-LDH-Coupled g-C3N4 2D/2D Heterojunctions for Solar Energy-Driven Conversion of Flared Gas to Syngas through Dry-/Bireforming of Methane. Energy Fuels 2023, 37, 5241–5256. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Qian, H.; Liu, J.; Liu, H.; Zhang, P.; Duan, E. Process and mechanism of toluene oxidation using Cu1-yMn2CeyOx/sepiolite prepared by the co-precipitation method. J. Hazard. Mater. 2018, 357, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—A review. Appl. Surf. Sci. 2016, 383, 200–213. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Bing, X.; Ng, D.H.L.; Cui, X.; Ji, F.; Kionga, D.D. ZnCr-LDH/N-doped graphitic carbon-incorporated g-C3N4 2D/2D nanosheet heterojunction with enhanced charge transfer for photocatalysis. Mater. Res. Bull. 2018, 102, 379–390. [Google Scholar] [CrossRef]
- Yuan, X.; Li, W. Graphitic-C3N4 modified ZnAl-layered double hydroxides for enhanced photocatalytic removal of organic dye. Appl. Clay Sci. 2017, 138, 107–113. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Merzari, F.; Goldfarb, J.; Andreottola, G.; Mimmo, T.; Volpe, M.; Fiori, L. Hydrothermal Carbonization as a Strategy for Sewage Sludge Management: Influence of Process Withdrawal Point on Hydrochar Properties. Energies 2020, 3, 2890. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Ding, G.; Huo, P.; Yan, Y.; Yan, Y.; Liao, G. Interior and Surface Synergistic Modifications Modulate the SnNb2O6/Ni-Doped ZnIn2S4 S-Scheme Heterojunction for Efficient Photocatalytic H2 Evolution. Inorg. Chem. 2022, 61, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Zhang, D. A novel strategy of hydrothermal in-situ grown bismuth based film on epoxy resin as recyclable photocatalyst for photodegrading antibiotics and sterilizing microorganism. Sep. Purif. Technol. 2022, 290, 120842. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Li, Y.; Liu, Y.; Ge, F. Nanostructure Design and Catalytic Performance of Mo/ZnAl-LDH in Cationic Orchid X-BL Removal. Materials 2018, 11, 2390. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, J.; Song, X.; Yang, H.; Li, X.; Dai, H.; Song, Y.; Liu, Y.; Hu, J.; Pan, X.; et al. Enhanced water dissociation performance of graphitic-C3N4 assembled with ZnCr-layered double hydroxide. Chem. Eng. J. 2018, 337, 560–566. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Sun, Y.; Xiao, T.; Tu, W.; Yuan, X.; Zeng, G.; Li, S.; Chew, J.W. Photogenerated charge transfer via interfacial internal electric field for significantly improved photocatalysis in direct Z-scheme oxygen-doped carbon nitrogen/CoAl-layered double hydroxide heterojunction. Appl. Catal. B Environ. 2018, 227, 530–540. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, R.; Patel, R.; Spreitzer, M.; Patel, N. A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. A Gen. 2023, 661, 119254. [Google Scholar] [CrossRef]
- Triolo, C.; Xu, W.; Petrovičovà, B.; Pinna, N.; Santangelo, S. Evaluation of Entropy-Stabilized (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O Oxides Produced via Solvothermal Method or Electrospinning as Anodes in Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2202892. [Google Scholar] [CrossRef]
- Lai, J.; Niu, W.; Luque, R.; Xu, G. Solvothermal synthesis of metal nanocrystals and their applications. Nano Today 2015, 10, 240–267. [Google Scholar] [CrossRef]
- Mruthunjayappa, M.H.; Kotrappanavar, N.S.; Mondal, D. New prospects on solvothermal carbonisation assisted by organic solvents, ionic liquids and eutectic mixtures—A critical review. Prog. Mater. Sci. 2022, 126, 100932. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Sun, X.; Liu, P.; Yang, D.; Zhao, X. ZnO-Layered Double Hydroxide@Graphitic Carbon Nitride Composite for Consecutive Adsorption and Photodegradation of Dyes under UV and Visible Lights. Materials 2016, 9, 927. [Google Scholar] [CrossRef]
- Ahmed, N.; Morikawa, M.; Izumi, Y. Photocatalytic conversion of carbon dioxide into methanol using optimized layered double hydroxide catalysts. Catal. Today 2012, 185, 263–269. [Google Scholar] [CrossRef]
- Nong, J.; Jin, Y.; Tan, J.; Ma, H.; Lian, Y. Construction of NiCo-LDH/g-C3N4 heterojunctions as efficient photocatalysts for enhanced degradation of tetracycline hydrochloride and hydrogen evolution. New J. Chem. 2022, 46, 22830–22840. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Zhang, C.; Zeng, G.; Tan, X.; Yu, Z.; Zhong, Y.; Wang, H.; Cui, F. Utilization of LDH-based materials as potential adsorbents and photocatalysts for the decontamination of dyes wastewater: A review. RSC Adv. 2016, 6, 79415–79436. [Google Scholar] [CrossRef]
- Van Vaerenbergh, B.; De Vlieger, K.; Claeys, K.; Vanhoutte, G.; De Clercq, J.; Vermeir, P.; Verberckmoes, A. The effect of the hydrotalcite structure and nanoparticle size on the catalytic performance of supported palladium nanoparticle catalysts in Suzuki cross-coupling. Appl. Catal. A Gen. 2018, 550, 236–244. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Huang, Q.; Zhang, H.; Zhu, J.; Qiu, Y.; Yin, D.; Zhao, J. Targeted modulation of g-C3N4 photocatalytic performance for pharmaceutical pollutants in water using ZnFe-LDH derived mixed metal oxides: Structure-activity and mechanism. Sci. Total Environ. 2019, 650, 1112–1121. [Google Scholar] [CrossRef]
- Shi, J.; Li, S.; Wang, F.; Gao, L.; Li, Y.; Zhang, X.; Lu, J. 2D/2D g-C3N4/MgFe MMO nanosheet heterojunctions with enhanced visible-light photocatalytic H2 production. J. Alloys Compd. 2018, 769, 611–619. [Google Scholar] [CrossRef]
- Ali Khan, A.; Tahir, M. Constructing S-Scheme Heterojunction of CoAlLa-LDH/g-C3N4 through Monolayer Ti3C2-MXene to Promote Photocatalytic CO2 Re-forming of Methane to Solar Fuels. ACS Appl. Energy Mater. 2022, 5, 784–806. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Lee, S.L.J.; Lin, S. Facile fabrication of a 2D/2D CoFe-LDH/g-C3N4 nanocomposite with enhanced photocatalytic tetracycline degradation. Environ. Sci. Poll. Res. 2023, 30, 4709–4720. [Google Scholar] [CrossRef]
- Gandamalla, A.; Manchala, S.; Verma, A.; Fu, Y.-P.; Shanker, V. Microwave-assisted synthesis of ZnAl-LDH/g-C3N4 composite for degradation of antibiotic ciprofloxacin under visible-light illumination. Chemosphere 2021, 283, 131182. [Google Scholar] [CrossRef]
- Cheshmeh Soltani, R.D.; Abolhasani, E.; Mashayekhi, M.; Jorfi, N.; Boczkaj, G.; Khataee, A. Degradation of tetracycline antibiotic utilizing light driven-activated oxone in the presence of g-C3N4/ZnFe LDH binary heterojunction nanocomposite. Chemosphere 2022, 303, 135201. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chi, D.; Yang, Z.; Xing, Z.; Yin, J.; Li, Z.; Zhu, Q.; Zhou, W. Mesoporous g-C3N4/Zn–Ti LDH laminated van der Waals heterojunction nanosheets as remarkable visible-light-driven photocatalysts. Int. J. Hydrog. Energy 2019, 44, 16348–16358. [Google Scholar] [CrossRef]
- Xu, H.; Shan, C.; Wu, X.; Sun, M.; Huang, B.; Tang, Y.; Yan, C.-H. Fabrication of layered double hydroxide microcapsules mediated by cerium doping in metal–organic frameworks for boosting water splitting. Energy Environ. Sci. 2020, 13, 2949–2956. [Google Scholar] [CrossRef]
- Yuan, N.; Deng, Y.-R.; Wang, S.-H.; Gao, L.; Yang, J.-L.; Zou, N.-C.; Liu, B.-X.; Zhang, J.-Q.; Liu, R.-P.; Zhang, L. Towards superior lithium–sulfur batteries with metal–organic frameworks and their derivatives. Tungsten 2022, 4, 269–283. [Google Scholar] [CrossRef]
- Huang, X.; Xu, X.; Yang, R.; Fu, X. Synergetic adsorption and photocatalysis performance of g-C3N4/Ce-doped MgAl-LDH in degradation of organic dye under LED visible light. Colloid. Surf. A Physicochem. Eng. Asp. 2022, 643, 128738. [Google Scholar] [CrossRef]
- Huang, Z.; Song, H.; Li, A.; An, Z.; Zhang, K.; Xiang, X.; Shu, X.; He, J. Z-Scheme ZnM-LDHs/g-C3N4 (M = Al, Cr) Photocatalysts: Their Desulfurization Performance and Mechanism for Model Oil with Air. Energy Fuels 2020, 34, 14676–14687. [Google Scholar] [CrossRef]
- Beigi, F.; Mahjoub, A.R.; Khavar, A.H.C. Design and synthesis of Bi-doped NiAl-LDH/g-C3N4 heterostructure; a novel 2D/2D system for simultaneous enhanced photocatalytic degradation and fluorescence sensing of ciprofloxacin. Appl. Surf. Sci. 2023, 637, 157972. [Google Scholar] [CrossRef]
- Tripathi, A.; Hussain, C.M. ZnAl-LDH and B-impregnated polymeric semiconductor (g-C3N4) for solar light-driven photocatalysis to treat phenolic effluent. Sustain. Mater. Technol. 2021, 28, e00266. [Google Scholar] [CrossRef]
- Hu, J.; Sun, C.; Wu, L.-X.; Zhao, G.-Q.; Liu, H.-Y.; Jiao, F.-P. Halogen doped g-C3N4/ZnAl-LDH hybrid as a Z-scheme photocatalyst for efficient degradation for tetracycline in seawater. Sep. Purif. Technol. 2023, 309, 123047. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Su, B.; Ma, J. Hydrothermal construction of flower-like g-C3N4/NiZnAl-LDH S-scheme heterojunction with oxygen vacancies for enhanced visible-light triggered photocatalytic performance. J. Alloys Compd. 2022, 922, 166098. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, G.-Q.; Wu, L.-X.; Sun, C.; Long, X.; Long, X.-Q.; Jiao, F.-P. Designing and Fabricating a Vulcanized ZnAl LDH-Modified g-C3N4 Heterojunction for Enhanced Visible-Light-Driven Photocatalytic Degradation Activity. Ind. Eng. Chem. Res. 2022, 61, 15225–15239. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tonda, S. Novel CoAl-LDH/g-C3N4/RGO ternary heterojunction with noTable 2D/2D/2D configuration for highly efficient visible-light-induced photocatalytic elimination of dye and antibiotic pollutants. J. Hazard. Mater. 2019, 368, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, R.; Aliyan, H.; Richeson, D.; Li, Y. A comparison increasing the photodegradation power of a Ag/g–C3N4/CoNi–LDH nanocomposite: Photocatalytic activity toward water treatment. J. Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Bhuvaneswari, K.; Palanisamy, G.; Pazhanivel, T.; Maiyalagan, T. r-GO supported g-C3N4/NiMgAl layered triple hydroxide hybrid as a Visible Light photocatalyst for organic dye removal. Colloid. Surf. A Physicochem. Eng. Asp. 2020, 602, 125078. [Google Scholar] [CrossRef]
- Zhao, G.-Q.; Long, X.; Hu, J.; Zou, J.; Jiao, F.-P.; Research, E.C. NiFe-layered double hydroxides as a novel hole repository layer for reinforced visible-light photocatalytic activity for degradation of refractory pollutants. Ind. Eng. Chem. Res. 2021, 60, 13834–13845. [Google Scholar] [CrossRef]
- Salehi, G.; Abazari, R.; Mahjoub, A.R. Visible-Light-Induced Graphitic–C3N4@Nickel–Aluminum Layered Double Hydroxide Nanocomposites with Enhanced Photocatalytic Activity for Removal of Dyes in Water. Inorg. Chem. 2018, 57, 8681–8691. [Google Scholar] [CrossRef]
- He, Y.; Zhou, S.; Wang, Y.; Jiang, G.; Jiao, F. Fabrication of g-C3N4@NiFe-LDH heterostructured nanocomposites for highly efficient photocatalytic removal of rhodamine B. J. Mater. Sci.: Mater. Electron. 2021, 32, 21880–21896. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Xu, W.; Fang, J.; Sun, J.; Liu, Z.; Chen, Y.; Liang, Y.; Fang, Z. Synergistic adsorption-photocatalytic degradation of different antibiotics in seawater by a porous g-C3N4/calcined-LDH and its application in synthetic mariculture wastewater. J. Hazard. Mater. 2021, 416, 126183. [Google Scholar] [CrossRef]
- Bhuvaneswari, K.; Palanisamy, G.; Bharathi, G.; Pazhanivel, T.; Upadhyaya, I.R.; Kumari, M.L.A.; Rajesh, R.P.; Govindasamy, M.; Ghfar, A.; Al-Shaalan, N.H. Visible light driven reduced graphene oxide supported ZnMgAl LTH/ZnO/g-C3N4 nanohybrid photocatalyst with notable two-dimension formation for enhanced photocatalytic activity towards organic dye degradation. Environ. Res. 2021, 197, 111079. [Google Scholar] [CrossRef]
- Sheikhpoor, H.; Saljooqi, A.; Shamspur, T.; Mostafavi, A. Co-Al Layered double hydroxides decorated with CoFe2O4 nanoparticles and g-C3N4 nanosheets for efficient photocatalytic pesticide degradation. Environ. Technol. Innov. 2021, 23, 101649. [Google Scholar] [CrossRef]
- Arjomandi-Behzad, L.; Alinejad, Z.; Zandragh, M.R.; Golmohamadi, A.; Vojoudi, H. Facile synthesis of hollow spherical g-C3N4@LDH/NCQDs ternary nanostructure for multifunctional antibacterial and photodegradation activities. iScience 2023, 26, 106213. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Parida, K.M. Deciphering Z-scheme Charge Transfer Dynamics in Heterostructure NiFe-LDH/N-rGO/g-C3N4 Nanocomposite for Photocatalytic Pollutant Removal and Water Splitting Reactions. Sci. Rep. 2019, 9, 2458. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, L.; Hu, T.; Lian, Q.; Liu, Y.; Hou, J.; Yu, Y.; Cao, Y.; Ma, F. In2S3/g-C3N4/CoZnAl-LDH composites with the lamellar dual S-scheme heterostructure and its enhanced photocatalytic performance. Colloid. Surf. A Physicochem. Eng. Asp. 2023, 658, 130744. [Google Scholar] [CrossRef]
- Lestari, P.R.; Takei, T.; Kumada, N. Novel ZnTi/C3N4/Ag LDH heterojunction composite for efficient photocatalytic phenol degradation. J. Solid State Chem. 2021, 294, 121858. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Che, H.; Ao, Y.; Wang, P. Rationally constructing of a novel composite photocatalyst with multi charge transfer channels for highly efficient sulfamethoxazole elimination: Mechanism, degradation pathway and DFT calculation. Chem. Eng. J. 2021, 426, 131585. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]



| Synthesis Strategy | Advantages | Disadvantages |
|---|---|---|
| Electrostatic self-assembly method | Very suitable for the preparation of 2D/2D LDHs/g-C3N4 | Time- and cost-consuming, complicated preparation process, and low efficiency |
| In situ coprecipitation method | Facile, eco-friendly, and highly efficient | Easy to agglomerate |
| Hydrothermal and solvothermal method | (i) Facile, eco-friendly, and highly efficient (ii) Produced photocatalysts with a relatively high crystallinity, small size distribution, and controllable architecture | Complex preparation process |
| Calcination method | Facile, eco-friendly, and effective, and reduced aggregation | Relatively poor interface contacts between two semiconductors |
| Photocatalysts | Preparation Strategy | Mass (mg) | Light Source | Target Pollutant/Initial Concentration | Degradation Time (min) and Rate (%) | Kapp a [10−2 min−1] | Ref. |
|---|---|---|---|---|---|---|---|
| ZnAl-LDH/ g-C3N4 | Microwave-assisted method | 30 | 35 W Xe arc lamp | CIP/20 mg L−1 | 140 (~84.1) | 1.22 | [80] |
| g-C3N4/ZnFe LDH | Mechanical stirrer method | 50–300 | 6-W UVC lamp | TC/10–55 μM | 30 (~92.4) | - | [81] |
| g-C3N4/Zn-Ti LDH | Hydrothermal method | 100 | 300 W xenon lamp, >420 nm | Ceftriaxone sodium/10 mg L−1 | 240 (~97) | 1.14 | [82] |
| g-C3N4/ MgAl0.80Ce0.20-LDH | Solvothermal method | 20 | 5 W LED lamp, 400–760 nm | CR/50 mg L−1 | 180 (~90) | 1.01 | [85] |
| g-C3N4@Ni-Ti LDH | Hydrothermal method | - | 400 W Hg lamp | AMX/1000 mg L−1 | 75 (~99.5) | - | [41] |
| ZnM-LDH/ g-C3N4 (M = Al, Cr) | Electrostatic self-assembly method | 180 | 500 W mercury lamp | Model oil/90 mL | 180 (~99.8) | - | [86] |
| Bi-doped NiAl-LDH/ g-C3N4 | Annealing method | 50 | 400 W Mercury-vapor lamp, >400 nm | Cipro/10 mg L−1 | 180 (~86) | - | [87] |
| ZnAl-LDH/ g-C3N4 | Thermal condensation | - | - | Phenol/700 mg L−1 | 270 (~62.38) | - | [88] |
| CoFe-LDH/ g-C3N4 | Coprecipitation method | - | 5 W LED light, >420 nm | TC/40 mg L−1 | 180 (~83.8) | - | [79] |
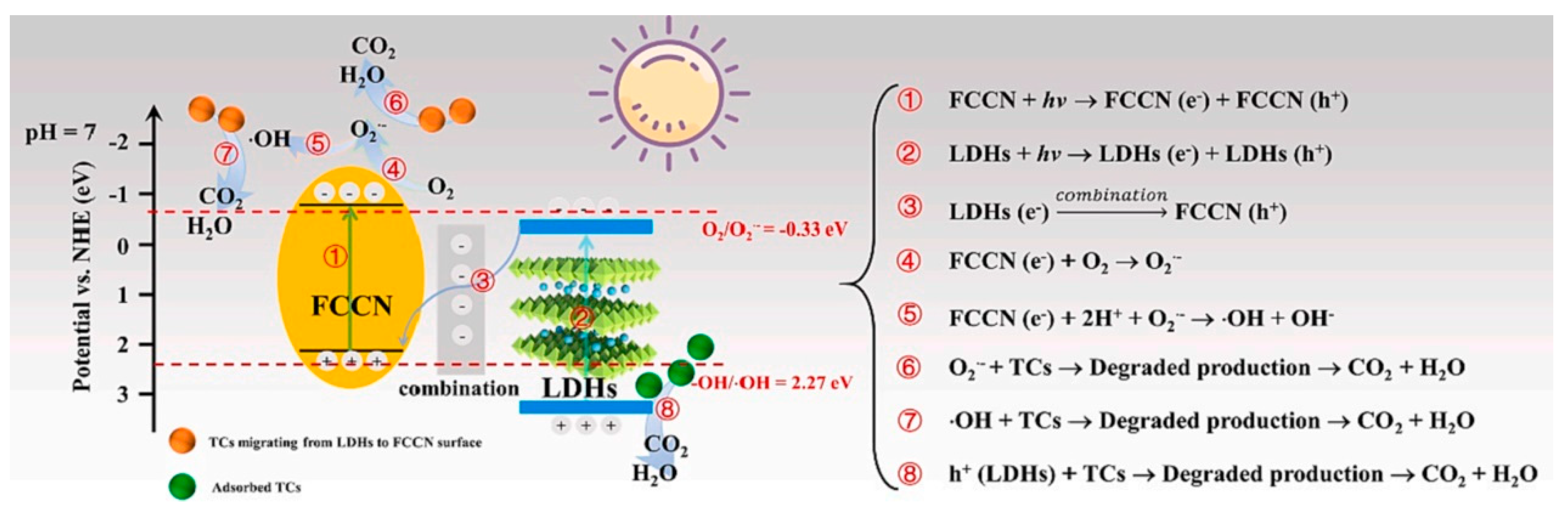
| FCCN/LDH | Coprecipitation process | 50 | 300 W Xe lamp | TC/20 mg L−1 | 120 (~72.13) | 2.314 | [89] |
| g-C3N4/ NiZnAl-LDH | Hydrothermal method | 25 | 500 W Xenon lamp, >400 nm | TC/10 mg L−1 | 120 (>99) | 2.329 | [90] |
| ZnAlSx@ g-C3N4 | Hydrothermal method | 50 | 300 W Xe lamp | TC/20 mg L−1 | 180 (~94.05) | - | [91] |
| Ag/g-C3N4/ CoNi-LDH | Hydrothermal method | 40 | 100 W Xe lamp | TC/30 mg L−1 | 100 (~86.3) | - | [93] |
| RGO/g-C3N4/ NiMgAl LDH | Hydrothermal method | 20 | 250 W mercury lamp | MB/50 mg L−1 | 75 (~95.14) | 1.8 | [94] |
| LDH/CN/RGO | Hydrothermal method | 50 | 300-W halogen lamp | TC/20 mg L−1 | 30 (>99) | - | [92] |
| NiFe-LDH/ g-C3N4 | Hydrothermal method | 20 | 500 W Xenon lamp, >420 nm | - | 60 (~96.81) | 5.457 | [95] |
| g-C3N4@ NiAl-LDH | Hydrothermal method | 600 | 500W high-pressure Hg lamp. | - | 180 (~99) | - | [96] |
| g-C3N4@ NiFe-LDH | Hydrothermal method | 50 | 500 W Xenon lamp, >420 nm | - | 240 (~99) | 1.52 | [97] |
| g-C3N4/MgZnAl-calcined LDH | Template method | 250 | 300 W Xenon lamp, >420 nm | - | 240 (>99) | - | [98] |
| ZnMgAl LTH/ ZnO/g-C3N4 | Stirring strategy | 10 | 300 W Xenon lamp | MB/50 mg L−1 | 75 (>99) | - | [99] |
| Co-Al LDH/g-C3N4- CoFe2O4 | Stirring strategy | - | 50 W LED lamp | - | - | 2.4 | [100] |
| g-C3N4/ ZnFeMMO | Stirring strategy | 25 | 500 W Xenon lamp, >300 nm | Sulfadiazine/5 mg L−1 | 240 (~96.4) | 1.317 | [76] |
| g-C3N4@LDH/NCQDs | Hydrothermal method | 100 | 300 W Xenon lamp, >400 nm | TC/20 mg L−1 | 120 (~90) | - | [101] |
| NiFe-LDH/ NRGO/g-C3N4 | Calcination- electrostatic self-assembly method | 20 | - | RhB/20 mg L−1 | 120 (~97) | - | [102] |
| ZnCr-LDH/ g-C3N4-C(N) | Coprecipitation method | 50 | 500 W Xenon lamp, >400 nm | CR/20 mg L−1 | 60 (~70) | 1.924 | [57] |
| In2S3/g-C3N4/ CoZnAl-LDH | Hydrothermal method | 150 | 300 W Xe lamp, 320–780 nm | MO/50 mg L−1 | 120 (~90.75) | - | [103] |
| ZnTi/C3N4/ Ag LDH | Self-assembly method | 150 | 300 W Xe lamp | Phenol/20 mg L−1 | 210 (~76.6) | - | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Xu, J.; Ding, G.; He, D.; Zhang, H.; Qiu, W.; Li, C.; Liao, G. Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal. Nanomaterials 2023, 13, 3066. https://doi.org/10.3390/nano13233066
Du C, Xu J, Ding G, He D, Zhang H, Qiu W, Li C, Liao G. Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal. Nanomaterials. 2023; 13(23):3066. https://doi.org/10.3390/nano13233066
Chicago/Turabian StyleDu, Cheng, Jialin Xu, Guixiang Ding, Dayong He, Hao Zhang, Weibao Qiu, Chunxue Li, and Guangfu Liao. 2023. "Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal" Nanomaterials 13, no. 23: 3066. https://doi.org/10.3390/nano13233066





