Recent Advances in DNA Nanomaterials
Abstract
:1. Introduction
2. Goals of DNA-NMs Development
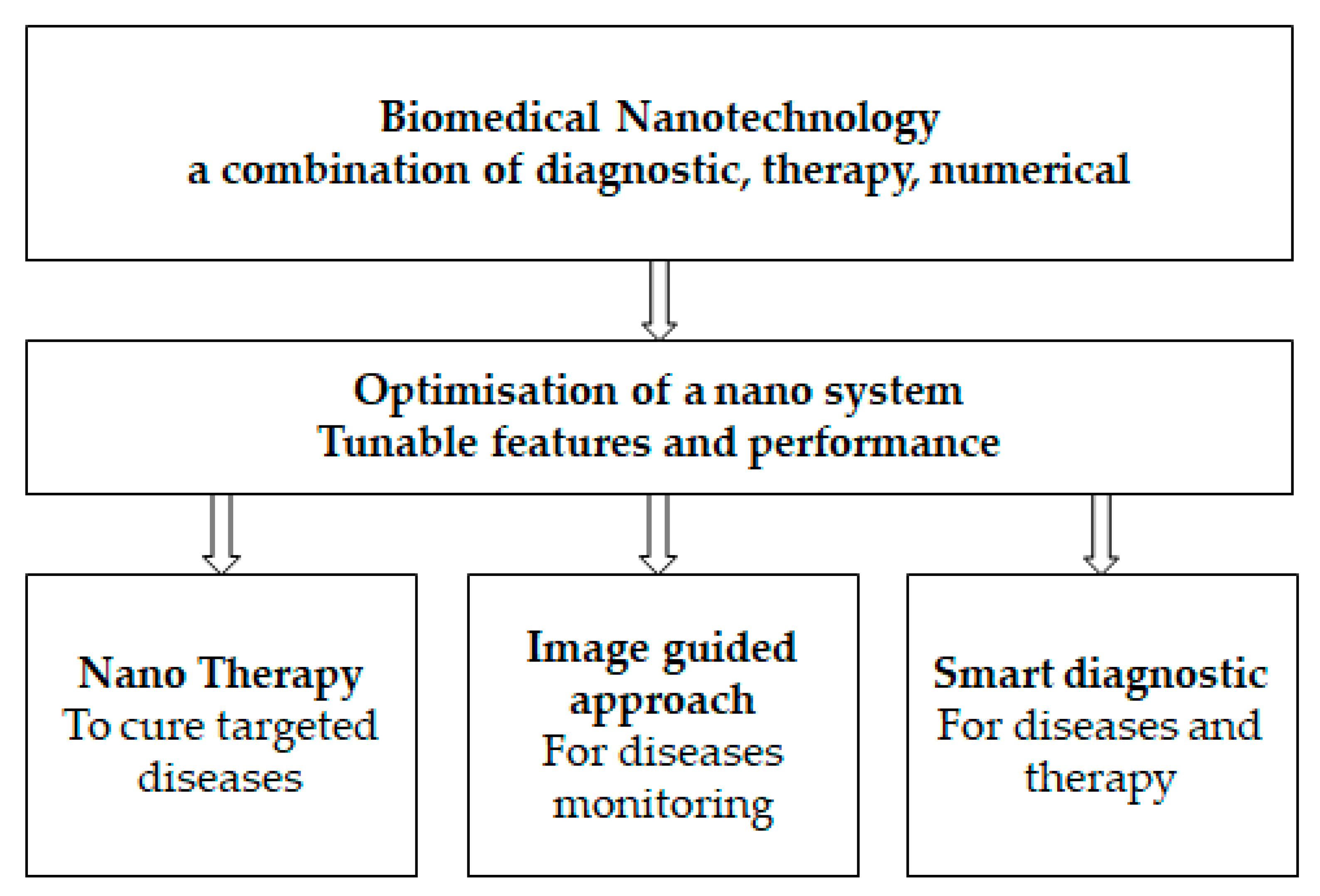
2.1. Biomedical Goals
2.2. Nanotechnological Goals
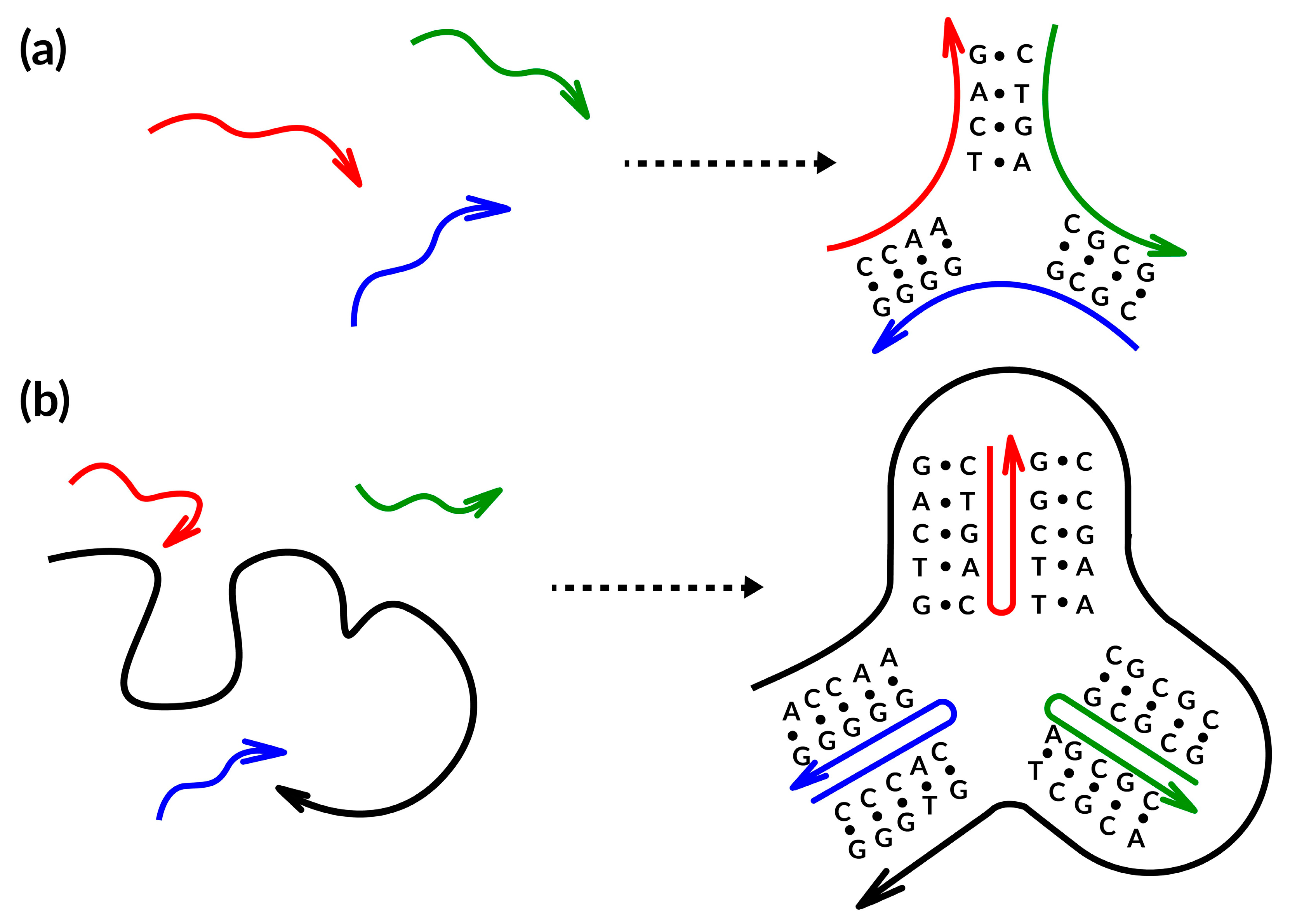
3. Technology of DNA-NMs
3.1. In Vitro Applications
3.1.1. Enzymes and Their Application In Vitro
3.1.2. Chemical Application In Vitro
Aptamers
Integration of Aptamers in DNA-NMs
3.2. In Vivo Applications
3.3. In Situ Applications
3.4. Other Examples of Self-Assembled DNA-NMs
3.5. DNA Adsorption over the Nanoparticles
4. Products of DNA Nano-Tuning
4.1. Plasmids
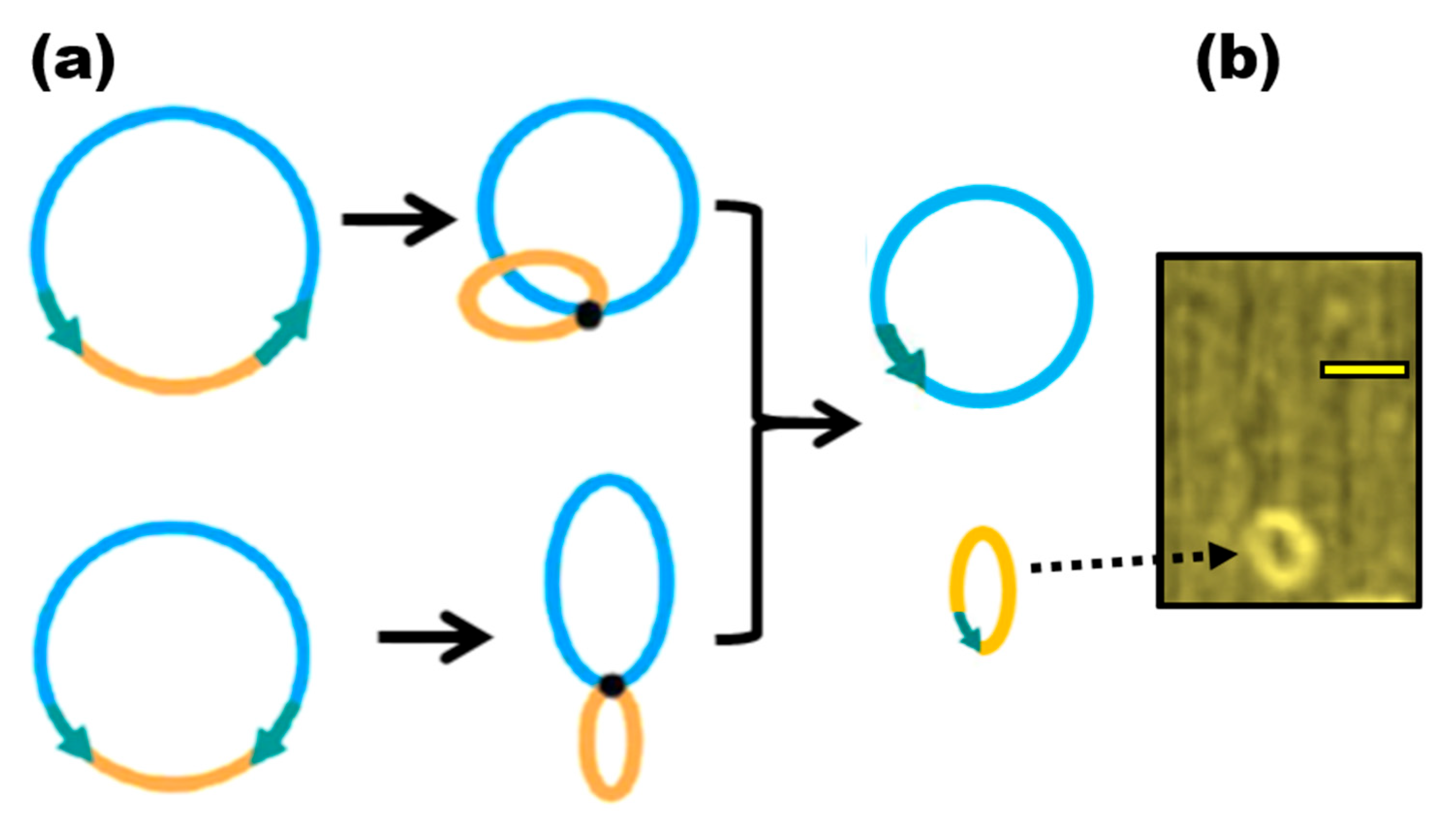
4.1.1. Plasmid Oligomers
4.1.2. Plasmid Microcircles Excision
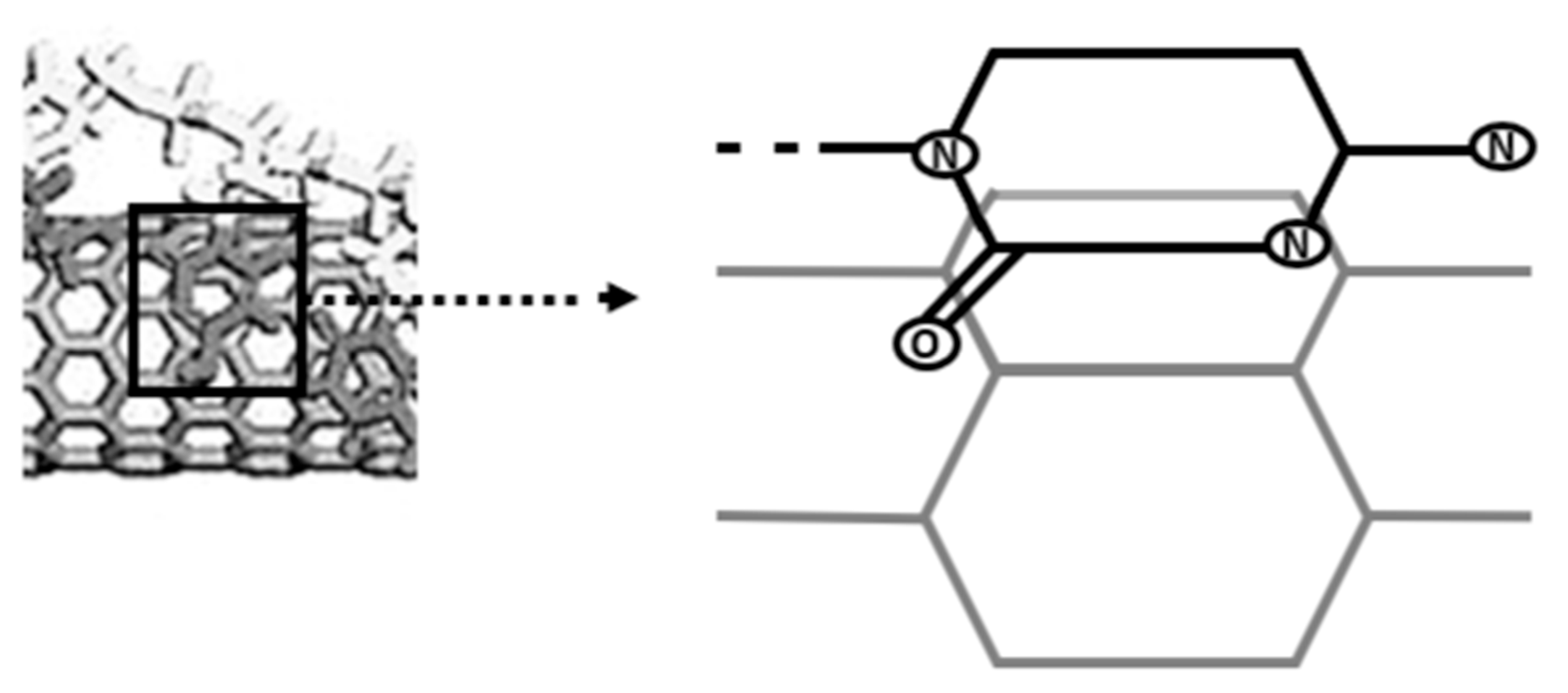
4.2. DNA-CNT Hybrids
4.3. DNA Origami “Capsidisation”
4.4. Spherical NA
5. Conclusions
- The stability of DNA nanostructures should be improved with great care. It is also necessary to develop techniques for examining the stability of DNA nanostructures in vivo. It is known that unprotected DNA experiences degradation in vivo [150].
- It is necessary to make it easier to manipulate ligands on DNA nanostructures, increasing the efficiency of DNA–ligand conjugation and identifying the distribution of receptors on the cell surface. The creation of ligand-conjugated DNA nanostructures requires the development of effective preparation methods.
- It is important to assess the in vivo pharmacodynamics and pharmacokinetics of ligand-functionalized DNA nanostructures. The application of therapeutic ligand modification on DNA nanostructures is common. Nonetheless, in vitro research is the main emphasis of the effort.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, S.M.; Zhang, S.; Seeman, N.C. DNA junctions, antijunctions, and mesojunctions. Biochemistry 1992, 31, 10955–10963. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Birktoft, J.J.; Chen, Y.; Wang, T.; Sha, R.; Constantinou, P.E.; Ginell, S.L.; Mao, C.; Seeman, N.C. From molecular to macroscopic via the rational design of a self-assembled 3D DNA crystal. Nature 2009, 461, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 2011, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Yang, Y.; Suzuki, Y.; Hidaka, K.; Sugiyama, H. Single-Molecule Visualization of the Hybridization and Dissociation of Photoresponsive Oligonucleotides and Their Reversible Switching Behavior in a DNA Nanostructure. Angew. Chem. Int. Ed. 2012, 51, 10518–10522. [Google Scholar] [CrossRef] [PubMed]
- Liedl, T.; Simmel, F.C. Switching the Conformation of a DNA Molecule with a Chemical Oscillator. Nano Lett. 2005, 5, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. A reversible pH-driven DNA nanoswitch array. J. Am. Chem. Soc 2006, 128, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- Yurke, B.; Turberfield, A.J.; Mills, A.P., Jr.; Simmel, F.C.; Neumann, J.L. A DNA-fuelled molecular machine made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [CrossRef]
- Saccà, B.; Siebers, B.; Meyer, R.; Bayer, M.; Niemeyer, C.M. Nanolattices of Switchable DNA-Based Motors. Small 2012, 8, 3000–3008. [Google Scholar] [CrossRef]
- Sannohe, Y.; Endo, M.; Katsuda, Y.; Hidaka, K.; Sugiyama, H. Visualization of Dynamic Conformational Switching of the G-Quadruplex in a DNA Nanostructure. J. Am. Chem. Soc. 2010, 132, 16311–16313. [Google Scholar] [CrossRef]
- Seeman, N.C. Nanomaterials Based on DNA. Annu. Rev. Biochem. 2010, 79, 65–87. [Google Scholar] [CrossRef]
- Pan, P.; Wang, W.; Ru, C.; Sun, Y.; Liu, X. MEMS-based platforms for mechanical manipulation and characterization of cells. J. Micromech. Microeng. 2017, 27, 123003. [Google Scholar] [CrossRef]
- Diller, E.; Sitti, M. Control of multiple heterogeneous magnetic micro-robots on non-specialized surfaces. In Proceedings of the IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; IEEE: Piscataway, NJ, USA, 2011. [Google Scholar]
- Chakraborty, K.; Veetil, A.T.; Jaffrey, S.R.; Krishnan, Y. Nucleic Acid–Based Nanodevices in Biological Imaging. Annu. Rev. Biochem. 2016, 85, 349–373. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Q.; Beziere, N.; Song, L.; Zhang, Q.; Peng, D.; Chi, C.; Yang, X.; Guo, H.; Diot, G.; et al. DNA-Nanostructure-Gold-Nanorod Hybrids for Enhanced In Vivo Optoacoustic Imaging and Photothermal Therapy. Adv. Mater. 2016, 28, 10000–10007. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A. Biomedical nanotechnology related grand challenges and perspectives. Front. Nanotechnol. 2019, 1, 1. [Google Scholar] [CrossRef]
- Kaushik, A.; Dixit, C.K. (Eds.) Nanobiotechnology for Sensing Applications: From Lab to Field; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- Kaushik, A.; Jayant, R.D.; Nair, M. (Eds.) Advances in Personalized Nanotherapeutics; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Kaushik, A.; Mujawar, M.A. Point of Care Sensing Devices: Better Care for Everyone. Sensors 2018, 18, 4303. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Jayant, R.D.; Kaushik, A.; Sagar, V. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev. 2016, 103, 202–217. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Nair, M. Nanomedicine for neuroHIV/AIDS management. Nanomedicine 2018, 13, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, W.J. Informatics, data science, and artificial intelligence. JAMA 2018, 320, 1103–1104. [Google Scholar] [CrossRef]
- Greene, C.S.; Tan, J.; Ung, M.; Moore, J.H.; Cheng, C. Big Data Bioinformatics. J. Cell. Physiol. 2014, 229, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.B.; Levitt, M. What is biomedical data science, and do we need an annual review of it? Annu. Rev. Biomed. Data Sci. 2018, 1, 1–3. [Google Scholar] [CrossRef]
- Lesk, A. Introduction to Bioinformatics; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Chiuchisan, I.; Chiuchisan, I.; Dimian, M. Internet of Things for e-Health: An approach to medical applications. In Proceedings of the 2015 International Workshop on Computational Intelligence for Multimedia Understanding (IWCIM), Prague, Czech Republic, 29–30 October 2015; IEEE: Piscataway, NJ, USA, 2015; Volume 105. [Google Scholar]
- Yin, Y.; Zeng, Y.; Chen, X.; Fan, Y. The internet of things in healthcare: An overview. J. Ind. Inf. Integr. 2016, 1, 3–13. [Google Scholar] [CrossRef]
- Rodrigues, J.J.P.C.; Segundo, D.B.D.R.; Junqueira, H.A.; Sabino, M.H.; Prince, R.M.; Al-Muhtadi, J.; De Albuquerque, V.H.C. Enabling Technologies for the Internet of Health Things. IEEE Access 2018, 6, 13129–13141. [Google Scholar] [CrossRef]
- Rej, S.; Ano, Y.; Chatani, N. Bidentate Directing Groups: An Efficient Tool in C–H Bond Functionalization Chemistry for the Expedient Construction of C–C Bonds. Chem. Rev. 2020, 120, 1788–1887. [Google Scholar] [CrossRef] [PubMed]
- Domoto, Y.; Abe, M.; Kikuchi, T.; Fujita, M. Self-Assembly of Coordination Polyhedra with Highly Entangled Faces Induced by Metal–Acetylene Interactions. Angew. Chem. Int. Ed. 2019, 59, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-temperature laser synthesis in liquid of oxide, metal-oxide core-shells, and doped oxide nanoparticles. Chem.-Eur. J. 2020, 26, 9206. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Suma, T.; Richardson, J.J.; Ejima, H. Modular Assembly of Biomaterials Using Polyphenols as Building Blocks. ACS Biomater. Sci. Eng. 2019, 5, 5578–5596. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for Dynamic Functional Materials from Atomic-/Molecular-Level Manipulation to Macroscopic Action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef]
- European Comission. Regulation (EC) No 1907/2006—Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH)—EU-OSHA. Available online: https://osha.europa.eu/de/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council (accessed on 29 August 2023).
- Crespilho, F.N.; Iost, R.M.; Travain, S.A.; Oliveira, O.N., Jr.; Zucolotto, V. Enzyme immobilization on Ag nanoparticles/polyaniline nanocomposites. Biosens. Bioelectron. 2009, 24, 3073–3077. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Montemagno, C.D. Artificial Organelle: ATP Synthesis from Cellular Mimetic Polymersomes. Nano Lett. 2005, 5, 2538–2542. [Google Scholar] [CrossRef]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Seeman, N.C. Macromolecular Design, Nucleic Acid Junctions, and Crystal Formation. J. Biomol. Struct. Dyn. 1985, 3, 11–34. [Google Scholar] [CrossRef]
- Jaekel, A.; Stegemann, P.; Saccà, B. Manipulating enzymes properties with DNA nanostructures. Molecules 2019, 24, 3694. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C.; Kallenbach, N.R. DNA branched junctions. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C.; Kallenbach, N.R. Design of immobile nucleic acid junctions. Biophys. J. 1983, 44, 201–209. [Google Scholar] [CrossRef]
- Feldkamp, U.; Niemeyer, C.M. Rational engineering of dynamic DNA systems. Angew. Chem. Int. Ed. 2008, 47, 3871–3873. [Google Scholar] [CrossRef] [PubMed]
- Simmel, F.C. Programming the dynamics of biochemical reaction networks. ACS Nano 2013, 7, 6–10. [Google Scholar] [CrossRef]
- Zhang, D.Y. Engineering entropy-driven reactions and networks catalyzed by DNA. Science 2007, 318, 1121–1125. [Google Scholar] [CrossRef]
- Stephanopoulos, N. Hybrid Nanostructures from the Self-Assembly of Proteins and DNA. Chem 2020, 6, 364–405. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Walls, D.J. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 2003, 302, 1545. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jaworski, A.; Ohshima, K.; Wells, R.D. Expansion and deletion of CTG repeats from human disease genes are determined by the direction of replication in E. coli. Nat. Genet. 1995, 10, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Gellibolian, R.; Oostra, B.A.; Wells, R.D. Cloning, characterization and properties of plasmids containing CGG triplet repeats from the FMR-1 gene. J. Mol. Biol 1996, 258, 614. [Google Scholar] [CrossRef]
- Maurer, D.J.; O’Callaghan, B.L.; Livingston, D.M. Orientation Dependence of Trinucleotide CAG Repeat Instability in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996, 16, 6617–6622. [Google Scholar] [CrossRef] [PubMed]
- Miret, J.J.; Pessoa-Brandão, L.; Lahue, R.S. Instability of CAG and CTG trinucleotide repeats in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997, 17, 3382–3387. [Google Scholar] [CrossRef] [PubMed]
- Freudenreich, C.H.; Stavenhagen, J.B.; Zakian, V.A. Stability of a CTG/CAG trinucleotide repeat in yeast is dependent on its orientation in the genome. Mol. Cell. Biol. 1997, 17, 2090. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.I.; Sutherland, G.R. Dynamic mutation: Possible mechanisms and significance in human disease. Trends Biochem. Sci. 1997, 22, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Graeber, M.B.; Haberhausen, G.; Köhler, A. Molecular basis and diagnosis of neurogenetic disorders. J. Neurol. Sci. 1994, 124, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, V.; Montermini, L.; Molto, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Pandolfo, M. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996, 271, 1423. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Greenwell, P.W.; Liu, C.-P.; Arnheim, N.; Petes, T.D. Triplet repeats form secondary structures that escape DNA repair in yeast. Proc. Natl. Acad. Sci. USA 1999, 96, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Darlow, J.M.; Leach, D.R. The effects of trinucleotide repeats found in human inherited disorders on palindrome inviability in Escherichia coli suggest hairpin folding preferences in vivo. Genetics 1995, 141, 825–832. [Google Scholar] [CrossRef]
- Petruska, J.; Hartenstine, M.J.; Goodman, M.F. Analysis of Strand Slippage in DNA Polymerase Expansions of CAG/CTG Triplet Repeats Associated with Neurodegenerative Disease. J. Biol. Chem. 1998, 273, 5204–5210. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.G.; Ochman, H. Production of single-stranded DNA templates by exonuclease digestion following the polymerase chain reaction. Nucleic Acids Res. 1989, 17, 5865. [Google Scholar] [CrossRef]
- Hultman, T.; Stahl, S.; Homes, E.; Uhlén, M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989, 17, 4937–4946. [Google Scholar] [CrossRef]
- Holland, M.M.; Fisher, D.L.; Mitchell, L.G.; Rodriquez, W.C.; Canik, J.J.; Merril, C.R.; Weedn, V.W. Mitochondrial DNA Sequence Analysis of Human Skeletal Remains: Identification of Remains from the Vietnam War. J. Forensic Sci. 1993, 38, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.W.; Ellsworth, D.L.; Honeycutt, R.L. The production of single-stranded DNA suitable for sequencing using the polymerase chain reaction. Biotechniques 1991, 10, 24. [Google Scholar]
- Gyllensten, U.B.; Erlich, H.A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc. Natl. Acad. Sci. USA 1988, 85, 7652–7656. [Google Scholar] [CrossRef]
- Shyamala, V.; Ames, G.F. Amplification of bacterial genomic DNA by the polymerase chain reaction and direct sequencing after asymmetric amplification: Application to the study of periplasmic permeases. J. Bacteriol. 1989, 171, 1602–1608. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Lund, K.; Williams, B.; Ke, Y.; Liu, Y.; Yan, H. DNA Nanotechnology: A Rapidly Evolving Field. Curr. Nanosci. 2006, 2, 113–122. [Google Scholar] [CrossRef]
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 A Resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef]
- Mcfarland, A.D.; Duyne, R.P.V. Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity. Nano Lett. 2003, 3, 1057–1062. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Sun, L.; Yu, L.; Shen, W. DNA nanotechnology and its applications in biomedical research. J. Biomed. Nanotechnol. 2014, 10, 2350–2370. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.H.; Zhang, T.; Luo, H.; Yen, T.M.; Chen, P.W.; Han, Y.; Lo, Y.H. Nucleic acid aptamers: An emerging tool for biotechnology and biomedical sensing. Sensors 2015, 15, 16281–16313. [Google Scholar] [CrossRef] [PubMed]
- Shamah, S.M.; Healy, J.M.; Cload, S.T. Complex target SELEX. Acc. Chem. Res 2008, 41, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Neumann, O.; Zhang, D.; Tam, F.; Lal, S.; Wittung-Stafshede, P.; Halas, N.J. Direct optical detection of aptamer conformational changes induced by target molecules. Anal. Chem. 2009, 81, 10002–10006. [Google Scholar] [CrossRef]
- Mok, W.; Li, Y. Recent progress in nucleic acid aptamer-based biosensors and bioassays. Sensors 2008, 8, 7050–7084. [Google Scholar] [CrossRef]
- Baker, B.R.; Lai, R.Y.; Wood, M.S.; Doctor, E.H.; Heeger, A.J.; Plaxco, K.W. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J. Am. Chem. Soc. 2006, 128, 3138–3139. [Google Scholar] [CrossRef]
- Radi, A.E.; Acero Sánchez, J.L.; Baldrich, E.; O’Sullivan, C.K. Reagentless, reusable, ultrasensitive electrochemical molecular beacon aptasensor. J. Am. Chem. Soc. 2006, 128, 117–124. [Google Scholar] [CrossRef]
- Cai, H.; Lee, T.M.-H.; Hsing, I.-M. Label-free protein recognition using an aptamer-based impedance measurement assay. Sens. Actuators B Chem. 2006, 114, 433–437. [Google Scholar] [CrossRef]
- Floch, F.L.; Ho, H.A.; Leclerc, M. Label-free electrochemical detection of protein based on a ferrocene-bearing cationic polythiophene and aptamer. Anal. Chem. 2006, 78, 4727–4731. [Google Scholar] [CrossRef]
- Shangguan, D.; Li, Y.; Tang, Z.; Cao, Z.C.; Chen, H.W.; Mallikaratchy, P.; Sefah, K.; Yang, C.J.; Tan, W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA 2006, 103, 11838–11843. [Google Scholar] [CrossRef] [PubMed]
- Mallikaratchy, P. Evolution of complex target SELEX to identify aptamers against mammalian cell-surface antigens. Molecules 2017, 22, 215. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yan, J.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z. Investigations on the interface of nucleic acid aptamers and binding targets. Analyst 2018, 143, 5317–5338. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 0076. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Tan, W. Aptamers generated from cell-SELEX for molecular medicine: A chemical biology approach. Acc. Chem. Res. 2010, 43, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.M.; Liu, H.; Kuai, H.; Peng, R.; Mo, L.; Zhang, X.B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef]
- Duan, M.; Long, Y.; Yang, C.; Wu, X.; Sun, Y.; Li, J.; Hu, X.; Lin, W.; Han, D.; Zhao, Y. Selection and characterization of DNA aptamer for metastatic prostate cancer recognition and tissue imaging. Oncotarget 2016, 7, 36436–36446. [Google Scholar] [CrossRef]
- Tang, M.S.L.; Shiu, S.C.; Godonoga, M.; Cheung, Y.W.; Liang, S.; Dirkzwager, R.M.; Kinghorn, A.B.; Fraser, L.A.; Heddle, J.G.; Tanner, J.A. An aptamer-enabled DNA nanobox for protein sensing. Nanomedicine 2018, 14, 1161–1168. [Google Scholar] [CrossRef]
- Dai, B.; Hu, Y.; Duan, J.; Yang, X.D. Aptamer-guided DNA tetrahedron as a novel targeted drug delivery system for MUC1-expressing breast cancer cells in vitro. Oncotarget 2016, 7, 38257–38269. [Google Scholar] [CrossRef]
- Zhou, Z.; Sohn, Y.S.; Nechushtai, R.; Willner, I. DNA tetrahedra modules as versatile optical sensing platforms for multiplexed analysis of miRNAs, endonucleases, and aptamer-ligand complexes. ACS Nano 2020, 14, 9021–9031. [Google Scholar] [CrossRef]
- Pan, Q.; Nie, C.; Hu, Y.; Yi, J.; Liu, C.; Zhang, J.; He, M.M.; He, M.Y.; Chen, T.; Chu, X. Aptamer-functionalized DNA origami for targeted codelivery of antisense oligonucleotides and doxorubicin to enhance therapy in drug-resistant cancer cells. ACS Appl. Mater. Interfaces 2020, 12, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Vengut-Climent, E.; de Rochambeau, D.; Sleiman, H.F. Uptake and fate of fluorescently labeled DNA nanostructures in cellular environments: A cautionary tale. ACS Cent. Sci. 2019, 5, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Kuai, H.; Zhao, Z.; Mo, L.; Liu, H.; Hu, X.; Fu, T.; Zhang, X.; Tan, W. Circular bivalent aptamers enable in vivo stability and recognition. J. Am. Chem. Soc. 2017, 139, 9128–9131. [Google Scholar] [CrossRef] [PubMed]
- Kabza, A.M.; Sczepanski, J.T. LDNA-based catalytic hairpin assembly circuit. Molecules 2020, 25, 947. [Google Scholar] [CrossRef] [PubMed]
- Tawarada, R.; Seio, K.; Sekine, M. Synthesis and properties of artificial base pairs by use of halogen bonds. Nucleic Acids Symp. Ser. 2006, 50, 121–122. [Google Scholar] [CrossRef]
- Kashida, H.; Doi, T.; Sakakibara, T.; Hayashi, T.; Asanuma, H. p-Stilbazole moieties as artificial base pairs for photocross-linking of DNA duplex. J. Am. Chem. Soc. 2013, 135, 7960–7966. [Google Scholar] [CrossRef] [PubMed]
- Hampton, T. Researchers create artificial DNA bases. J. Am. Med. Assoc. 2008, 299, 1251. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.; Cao, Y.; Zhu, Z.; Yang, C.; Chen, J.; Qing, F.; Tan, W. Trifluoromethylated nucleic acid analogues capable of self-assembly through hydrophobic interactions. Chem. Sci. 2014, 5, 4076–4081. [Google Scholar] [CrossRef]
- Jin, C.; Fu, T.; Wang, R.; Liu, H.; Zou, J.; Zhao, Z.; Ye, M.; Zhang, X.; Tan, W. Fluorinated molecular beacons as functional DNA nanomolecules for cellular imaging. Chem. Sci. 2017, 8, 7082–7086. [Google Scholar] [CrossRef]
- Wang, R.; Jin, C.; Zhu, X.; Zhou, L.; Xuan, W.; Liu, Y.; Liu, Q.; Tan, W. Artificial base zT as functional “element” for constructing photoresponsive DNA nanomolecules. J. Am. Chem. Soc. 2017, 139, 9104–9107. [Google Scholar] [CrossRef]
- Gerling, T.; Kube, M.; Kick, B.; Dietz, H. Sequence-programmable covalentbonding of designed DNA assemblies. Sci. Adv. 2018, 4, eaau1157. [Google Scholar] [CrossRef] [PubMed]
- Anastassacos, F.M.; Zhao, Z.; Zeng, Y.; Shih, W.M. Glutaraldehyde crosslinking of oligolysines coating DNA origami greatly reduces susceptibility to nuclease degradation. J. Am. Chem. Soc. 2020, 142, 3311–3315. [Google Scholar] [CrossRef]
- Huang, Z.; Qiu, L.; Zhang, T.; Tan, W. Integrating DNA nanotechnology with aptamers for biological and biomedical applications. Matter 2021, 4, 4461–4489. [Google Scholar] [CrossRef]
- Ko, S.; Liu, H.; Chen, Y.; Mao, C. DNA nanotubes as combinatorial vehicles for cellular delivery. Biomacromolecules 2008, 9, 3039–3043. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Q.; Li, N.; Dai, L.; Liu, Q.; Song, L.; Wang, J.; Li, Y.; Tian, J.; Ding, B.; et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 2014, 8, 6633–6643. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Burns, J.R.; Seifert, A.; Fertig, N.; Howorka, S. A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane. Nat. Nanotechnol. 2016, 11, 152–156. [Google Scholar] [CrossRef]
- Fu, J.; Liu, M.; Liu, Y.; Woodbury, N.W.; Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 2012, 134, 5516–5519. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Wickham SF, J.; Shih, W.M.; Perrault, S.D. Addressing the instability of DNA nanostructures in tissue culture. ACS Nano 2014, 8, 8765–8775. [Google Scholar] [CrossRef]
- Gait, M.J.; Komiyama, M.; Seeman, N.C.; Seitz, O.; Micklefield, J.; Liu, D.R. DNA nanostructure serum stability: Greater than the sum of its parts. Org. Biomol. Chem. 2013, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M.; Hausman, R.E. The Cell: A Molecular Approach; ASM Press: Washington, DC, USA; Sinauer Associates Inc.: Sunderland, MA, USA, 2000; Chapter 1. [Google Scholar]
- Kallenbach, N.R.; Ma, R.I.; Seeman, N.C. An immobile nucleic acid junction constructed from oligonucleotides. Nature 1983, 305, 829–831. [Google Scholar] [CrossRef]
- Kong, G.; Zhang, M.; Xiong, M.; Fu, X.; Ke, G.; Zhang, X.-B. DNA nanostructure-based fluorescent probes for cellular sensing. Anal. Methods 2020, 12, 1415–1429. [Google Scholar] [CrossRef]
- Li, L.; Xing, H.; Zhang, J.; Lu, Y. Functional DNA Molecules Enable Selective and Stimuli-Responsive Nanoparticles for Biomedical Applications. Acc. Chem. Res. 2019, 52, 2415–2426. [Google Scholar] [CrossRef]
- Yang, F.; Li, Q.; Wang, L.; Zhang, G.J.; Fan, C. Framework-Nucleic- Acid-Enabled Biosensor Development. ACS Sens. 2018, 3, 903–919. [Google Scholar] [CrossRef]
- Jarak, I.; Pereira-Silva, M.; Santos, A.C.; Veiga, F.; Cabral, H.; Figueiras, A. Multifunctional polymeric micelle-based nucleic acid delivery: Current advances and future perspectives. Appl. Mater. Today 2021, 25, 101217. [Google Scholar] [CrossRef]
- Li, Y.; Osada, K.; Chen, Q.; Tockary, T.A.; Dirisala, A.; Takeda, K.M.; Uchida, S.; Nagata, K.; Itaka, K.; Kataoka, K. Toroidal Packaging of pDNA into Block Ionomer Micelles Exerting Promoted in Vivo Gene Expression. Biomacromolecules 2015, 16, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Osada, K. Structural Polymorphism of Single pDNA Condensates Elicited by Cationic Block Polyelectrolytes. Polymers 2020, 12, 1603. [Google Scholar] [CrossRef]
- Bos, I.; Brink, E.; Michels, L.; Sprakel, J. DNA dynamics in complex coacervate droplets and micelles. Soft Matter. 2022, 18, 2012–2027. [Google Scholar] [CrossRef]
- Marras, A.E.; Vieregg, J.R.; Ting, J.M.; Rubien, J.D.; Tirrell, M.V. Polyelectrolyte Complexation of Oligonucleotides by Charged Hydrophobic—Neutral Hydrophilic Block Copolymers. Polymers 2019, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Du, X.; Bi, Y.; He, P.-P.; Mu, Y.; Liu, C.; Gao, Q.; Yin, M.; Guo, W. Smart hydrogels based on self-assembly of one short single-stranded DNA for functional surface patterning. ACS Appl. Polym. Mater. 2022, 4, 5199–5208. [Google Scholar] [CrossRef]
- Wei, B.; Dai, M.; Yin, P. Complex shapes self-assembled from single-stranded DNA tiles. Nature 2012, 485, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Adsorption of DNA onto gold nanoparticles and graphene oxide: Surface science and applications. Phys. Chem. Chem. Phys. 2012, 14, 10485–10496. [Google Scholar] [CrossRef] [PubMed]
- Erben, C.M.; Goodman, R.P.; Turberfield, A.J. A self-assembled DNA bipyramid. J. Am. Chem. Soc. 2007, 129, 6992–6993. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J. DNA adsorption by indium tin oxide nanoparticles. Langmuir 2015, 31, 371–377. [Google Scholar] [CrossRef]
- Gorbunova, E.A.; Epanchintseva, A.V.; Pyshnyi, D.V.; Pyshnaya, I.A. Noncovalent Adsorption of Single-Stranded and Double-Stranded DNA on the Surface of Gold Nanoparticles. Appl. Sci. 2023, 13, 7324. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Surface science of DNA adsorption onto citrate-capped gold nanoparticles. Langmuir 2012, 28, 3896–3902. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Freezing-Driven DNA Adsorption on Gold Nanoparticles: Tolerating Extremely Low Salt Concentration but Requiring High DNA Concentration. Langmuir 2019, 35, 6476–6482. [Google Scholar] [CrossRef]
- Wu, R.; Peng, H.; Zhu, J.J.; Jiang, L.P.; Liu, J. Attaching DNA to Gold Nanoparticles with a Protein Corona. Front. Chem. 2020, 8, 121. [Google Scholar] [CrossRef]
- Bloomfield, V.A.; Crothers, D.M.; Tinoco, I. Nucleic Acids—Structures, Properties, and Functions; University Science Books: Sausalito, CA, USA, 2000. [Google Scholar]
- Wilner, O.I.; Willner, I. Functionalized DNA Nanostructures. Chem. Rev. 2012, 112, 2528–2556. [Google Scholar] [CrossRef]
- Stanley, S.M. Climatic forcing and the origin of the human genus. In Effects of Past Global Change in Life; National Research Council: Washington, DC, USA, 1995; Volume 10, p. 233e243. [Google Scholar]
- Jakobsen, J.; Mikkelsen, J.; Nielsen, A. Elimination of the plasmid bacterial backbone in site- directed transgenesis. Biotechniques 2010, 48, 313–316. [Google Scholar] [CrossRef]
- Hoess, R.; Wierzbicki, A.; Abremski, K. Formation of small circular DNA molecules via an in vitro site-specific recombination system. Gene 1985, 40, 325–329. [Google Scholar] [CrossRef]
- Mills, A.; Gago, F. Structural landscape of the transition from an ssDNA dumbbell plus its complementary hairpin to a dsDNA microcircle via a kissing loop intermediate. Molecules 2021, 26, 3017. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, R.E. Novel plastid gene minicircles in the dinoflagellate Amphidinium operculatum. Gene 2004, 331, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Umemura, K. Hybrids of nucleic acids and carbon nanotubes for nanobiotechnology. Nanomaterials 2015, 5, 321–350. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Biomolecule-functionalized carbon nanotubes: Applications in nanobioelectronics. ChemPhysChem 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Mikkilä, J.; Eskelinen, A.P.; Niemelä, E.H.; Linko, V.; Frilander, M.J.; Törmä, P.; Kostiainen, M.A. Virus-encapsulated DNA origami nanostructures for cellular delivery. Nano Lett. 2014, 14, 2196–2200. [Google Scholar] [CrossRef]
- Seitz, I.; Saarinen, S.; Kumpula, E.P.; McNeale, D.; Anaya-Plaza, E.; Lampinen, V.; Hytönen, V.P.; Sainsbury, F.; Cornelissen, J.J.L.M.; Linko, V.; et al. DNA-origami-directed virus capsid polymorphism. Nat. Nanotechnol. 2023. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Vahidnezhad, H.; Youssefian, L.; Mosafer, J.; Baradaran, B.; Uitto, J. Applications of Spherical Nucleic Acid Nanoparticles as Delivery Systems. Trends Mol. Med. 2019, 25, 1066–1079. [Google Scholar] [CrossRef]
- Teplensky, M.H.; Distler, M.E.; Kusmierz, C.D.; Evangelopoulos, M.; Gula, H.; Elli, D.; Tomatsidou, A.; Nicolaescu, V.; Gelarden, I.; Yeldandi, A.; et al. Spherical nucleic acids as an infectious disease vaccine platform. Proc. Natl. Acad. Sci. USA 2022, 119, e2119093119. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: A qualitative study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekkouche, I.; Kuznetsova, M.N.; Rejepov, D.T.; Vetcher, A.A.; Shishonin, A.Y. Recent Advances in DNA Nanomaterials. Nanomaterials 2023, 13, 2449. https://doi.org/10.3390/nano13172449
Bekkouche I, Kuznetsova MN, Rejepov DT, Vetcher AA, Shishonin AY. Recent Advances in DNA Nanomaterials. Nanomaterials. 2023; 13(17):2449. https://doi.org/10.3390/nano13172449
Chicago/Turabian StyleBekkouche, Incherah, Maria N. Kuznetsova, Dovlet T. Rejepov, Alexandre A. Vetcher, and Alexander Y. Shishonin. 2023. "Recent Advances in DNA Nanomaterials" Nanomaterials 13, no. 17: 2449. https://doi.org/10.3390/nano13172449







