Application of Zeolites and Zeolitic Imidazolate Frameworks in Dentistry—A Narrative Review
Abstract
:1. Introduction
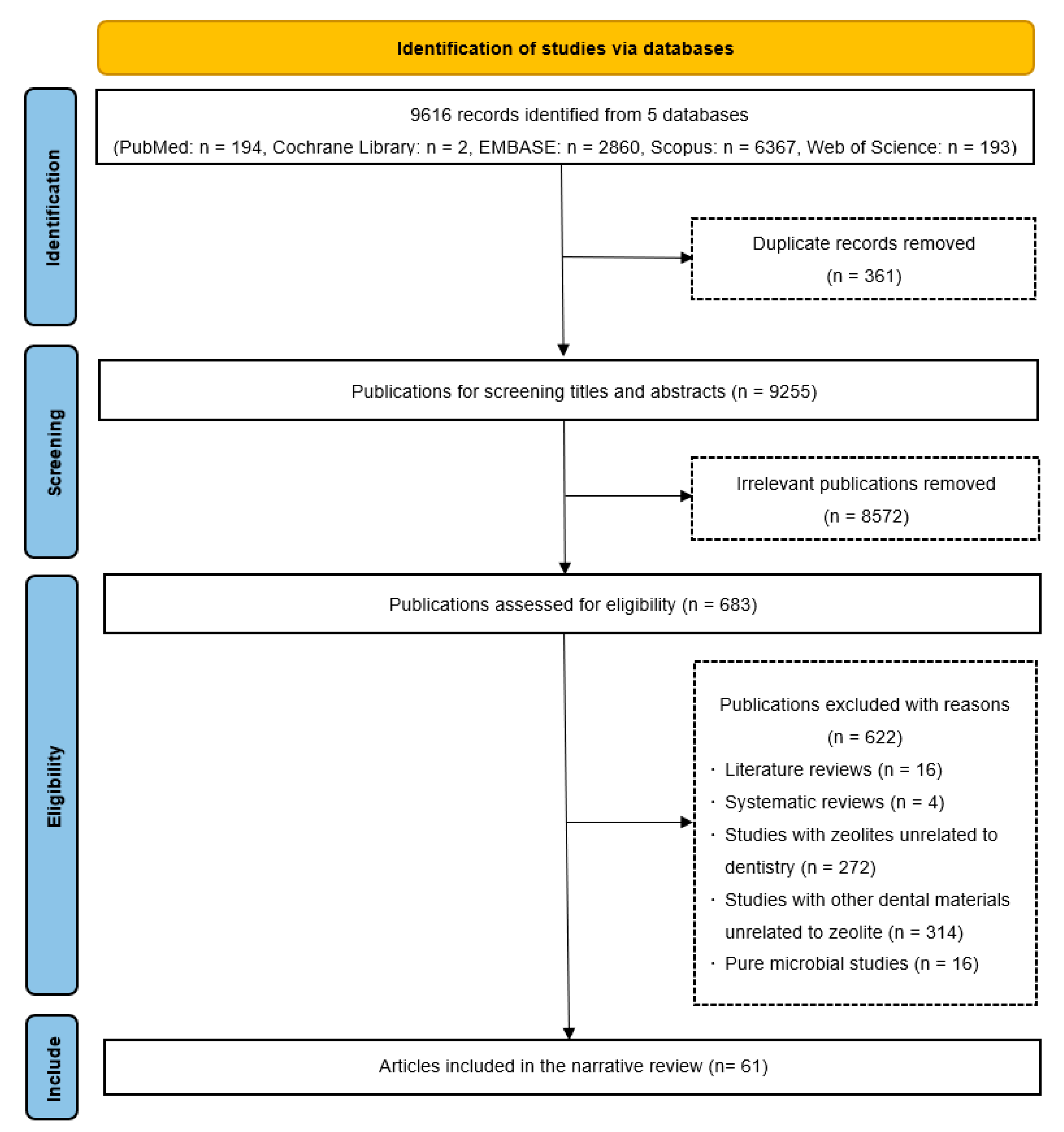
2. Literature Search
3. Zeolites for Dental Application
3.1. Silver Zeolite
3.2. Zinc Zeolite
3.3. Calcium Zeolite
3.4. Strontium Zeolite
4. ZIFs for Dental Application
4.1. ZIF-8
4.2. ZIF-67
5. Dental Applications of Zeolites and ZIFs
5.1. Restorative Dentistry
5.1.1. Zeolite/ZIF-Modified Adhesives
5.1.2. Zeolite-Loaded Restorative Materials
5.2. Endodontics
5.2.1. Zeolite-Incorporated Materials for Root-End Fillings
5.2.2. Zeolite-Incorporated Materials for Root Canal Irrigants
5.2.3. Zeolite-Incorporated Materials for Root Canal Sealers
5.3. Prosthodontics
5.3.1. Zeolite-Infiltrated All-Ceramic Dental Prostheses
5.3.2. Zeolite-Incorporated Tissue Conditioners
5.3.3. Zeolite-Loaded Denture Bases
5.3.4. Zeolite-Incorporated Soft Denture Liners
5.4. Implantology
5.4.1. Zeolite/ZIF-Coated Implants
5.4.2. ZIF-Coated Bone Graft Materials
5.4.3. ZIF-Modified Bone Adhesives
5.4.4. ZIF-Loaded Drug Delivery System
5.4.5. ZIF-Modified PCL Electrospinning
5.4.6. ZIF-Modified Post-Implantation Drugs
5.5. Periodontics
5.5.1. Zeolite/ZIF-Loaded Drugs for Deep Periodontal Pockets
5.5.2. ZIF-Embedded Guided Tissue/Bone Regeneration Membranes
5.6. Orthodontics
5.6.1. Zeolite-Modified Orthodontic Brackets
5.6.2. Zeolite-Based aPDT Photosensitizer
5.7. Oral Surgery
5.7.1. Zeolite-Modified Bone Matrix Scaffold
5.7.2. Zeolite-Loaded Oral Cancer Detection Membrane
5.7.3. Zeolites Act as a Drug after Tooth Extraction
5.7.4. Zeolite-Modified Maxillofacial Silicone Elastomers
5.7.5. ZIF-Incorporated Osteogenic Glue
5.7.6. ZIF-Coated Antitumour Drugs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Mayoral, A.; Li, J.; Ruan, J.; Alfredsson, V.; Ma, Y.; Yu, J.; Terasaki, O. Electron Microscopy Studies of Local Structural Modulations in Zeolite Crystals. Angew. Chem. 2020, 59, 19403–19413. [Google Scholar] [CrossRef] [PubMed]
- Heard, C.J.; Grajciar, L.; Uhlík, F.; Shamzhy, M.; Opanasenko, M.; Čejka, J.; Nachtigall, P. Zeolite (In)Stability under Aqueous or Steaming Conditions. Adv. Mater. 2020, 32, e2003264. [Google Scholar] [CrossRef]
- Boscoboinik, J.; Yu, X.; Yang, B.; Shaikhutdinov, S.; Freund, H.-J. Building blocks of zeolites on an aluminosilicate ultra-thin film. Microporous Mesoporous Mater. 2013, 165, 158–162. [Google Scholar] [CrossRef]
- Derbe, T.; Temesgen, S.; Bitew, M. A Short Review on Synthesis, Characterization, and Applications of Zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898. [Google Scholar] [CrossRef]
- Saint-Cricq, P.; Kamimura, Y.; Itabashi, K.; Sugawara-Narutaki, A.; Shimojima, A.; Okubo, T. Antibacterial Activity of Silver-Loaded “Green Zeolites”. Eur. J. Inorg. Chem. 2012, 2012, 3398–3402. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Moshoeshoe, M.; Nadiye-Tabbiruka, M.S.; Obuseng, V. A Review of the Chemistry, Structure, Properties and Applications of Zeolites. Am. J. Mater. Sci. 2017, 7, 196–221. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 10 July 2023).
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tanaka, Y. A Simple Step toward Enhancing Hydrothermal Stability of ZIF-8. ACS Omega 2019, 4, 19905–19912. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Paul, A.; Banga, I.K.; Muthukumar, S.; Prasad, S. Engineering the ZIF-8 Pore for Electrochemical Sensor Applications—A Mini Review. ACS Omega 2022, 7, 26993–27003. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.A.; Suleiman, M.A.; Al-Amrani, W.A.; Usman, A.K.; Onaizi, S.A. Adsorptive removal of organic pollutants from contaminated waters using zeolitic imidazolate framework Composites: A comprehensive and Up-to-date review. Sep. Purif. Technol. 2023, 318, 123765. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A review of the features and applications of ZIF-8 and its derivatives for separating CO2 and isomers of C3- and C4- hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Daniel, M.; Arianee, S.-V.; Isabel, G.; Eduardo, F.; Carles, C.; José, S. Improving Sensitivity of a Chemoresistive Hydrogen Sensor by Combining ZIF-8 and ZIF-67 Nanocrystals. Proceedings 2017, 1, 462. [Google Scholar] [CrossRef]
- Cui, L.; Han, R.; Yang, L.; Wu, Y.; Pei, R.; Li, F. Synthesis and characterization of mesoporous sodalite and investigation of the effects of inorganic salts on its structure and properties. Microporous Mesoporous Mater. 2020, 306, 110385. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef]
- Tan, L.; Yuan, G.; Wang, P.; Feng, S.; Tong, Y.; Wang, C. pH-responsive Ag-Phy@ZIF-8 nanoparticles modified by hyaluronate for efficient synergistic bacteria disinfection. Int. J. Biol. Macromol. 2022, 206, 605–613. [Google Scholar] [CrossRef]
- Chebbi, M.; Azambre, B.; Cantrel, L.; Huvé, M.; Albiol, T. Influence of structural, textural and chemical parameters of silver zeolites on the retention of methyl iodide. Microporous Mesoporous Mater. 2017, 244, 137–150. [Google Scholar] [CrossRef]
- Saengmee-anupharb, S.; Srikhirin, T.; Thaweboon, B.; Thaweboon, S.; Amornsakchai, T.; Dechkunakorn, S.; Suddhasthira, T. Antimicrobial effects of silver zeolite, silver zirconium phosphate silicate and silver zirconium phosphate against oral microorganisms. Asian Pac. J. Trop. Biomed. 2013, 3, 47–52. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Matijasevic, D.; Niksic, M.; Rajic, N. Bactericidal activity of Cu-, Zn-, and Ag-containing zeolites toward Escherichia coli isolates. Environ. Sci. Pollut. Res. Int. 2017, 24, 20273–20281. [Google Scholar] [CrossRef]
- Mallette, A.J.; Hong, S.; Freeman, E.E.; Saslow, S.A.; Mergelsberg, S.; Motkuri, R.K.; Neeway, J.J.; Mpourmpakis, G.; Rimer, J.D. Heteroatom Manipulation of Zeolite Crystallization: Stabilizing Zn-FAU against Interzeolite Transformation. JACS Au 2022, 2, 2295–2306. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, Z.; Sandomierski, M.; Voelkel, A. Calcium-Rich 13X Zeolite as a Filler with Remineralizing Potential for Dental Composites. ACS Biomater. Sci. Eng. 2020, 6, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Cai, X.; Feltrin, A.C.; Feng, P.; Kaiser, A.; Akhtar, F. Calcium/strontium chloride impregnated zeolite A and X granules as optimized ammonia sorbents. RSC Adv. 2022, 12, 3491–34917. [Google Scholar] [CrossRef] [PubMed]
- Okulus, Z.; Sandomierski, M.; Zielińska, M.; Buchwald, T.; Voelkel, A. Zeolite fillers for resin-based composites with remineralizing potential. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Dotta, T.C.; Hayann, L.; de Padua Andrade Almeida, L.; Nogueira, L.F.B.; Arnez, M.M.; Castelo, R.; Cassiano, A.F.B.; Faria, G.; Martelli-Tosi, M.; Bottini, M.; et al. Strontium Carbonate and Strontium-Substituted Calcium Carbonate Nanoparticles Form Protective Deposits on Dentin Surface and Enhance Human Dental Pulp Stem Cells Mineralization. J. Funct. Biomater. 2022, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhai, X.; Zhao, L. Synthesis of ZIF-8 and ZIF-67 nanocrystals with well-controllable size distribution through reverse microemulsions. Chem. Eng. J. 2016, 289, 59–64. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Kolmykov, O.; Commenge, J.-M.; Alem, H.; Girot, E.; Mozet, K.; Medjahdi, G.; Schneider, R. Microfluidic reactors for the size-controlled synthesis of ZIF-8 crystals in aqueous phase. Mater. Des. 2017, 122, 31–41. [Google Scholar] [CrossRef]
- Shuai, C.; Zan, J.; Deng, F.; Yang, Y.; Peng, S.; Zhao, Z. Core–shell-Structured ZIF-8@PDA-HA with Controllable Zinc Ion Release and Superior Bioactivity for Improving a Poly-l-lactic Acid Scaffold. ACS Sustain. Chem. Eng. 2021, 9, 1814–1825. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, J.; Ma, Z.; Feng, H.; Chen, S.; Cai, H.; Xue, Y.; Pei, X.; Wang, J.; Wan, Q. 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 2020, 12, 24437–24449. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xue, Y.; Zhu, Z.; Chen, J.; Liu, Y.; Cheng, X.; Zhang, X.; Wang, J.; Pei, X.; Wan, Q. Nanoscale Zeolitic Imidazolate Framework-8 Activator of Canonical MAPK Signaling for Bone Repair. ACS Appl. Mater. Interfaces 2021, 13, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chu, C.-C.; Liu, G.; Wáng, Y.-X.J. Metal-Organic Framework-Based Nanomedicine Platforms for Drug Delivery and Molecular Imaging. Small 2015, 11, 4806–4822. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts. J. Mater. Chem. 2018, 6, 1887–1899. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Zhao, C.; Yuan, Z.; Zhang, D.; Zhao, H.; Yang, N.; Guo, K.; He, Y.; He, Y.; et al. A pH-responsive MOF for site-specific delivery of fungicide to control citrus disease of Botrytis cinerea. Chem. Eng. J. 2022, 431, 133351. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Liu, S.; Cao, X.; Wu, K.; Cheong, W.-C.; Chen, Z.; Wang, Y.; Li, Y.; Liu, Y.; et al. Core–Shell ZIF-8@ZIF-67-Derived CoP Nanoparticle-Embedded N-Doped Carbon Nanotube Hollow Polyhedron for Efficient Overall Water Splitting. J. Am. Chem. Soc. 2018, 140, 2610–2618. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wang, S.; Wang, B.; Wang, X.; Mi, Z.; Fu, J.; Zhang, Z.; Yan, W. Enhancing the Stability of the Resin-Dentin Bonding Interface with Ag+- and Zn2+-Exchanged Zeolite A. ACS Biomater. Sci. Eng. 2022, 8, 1717–1725. [Google Scholar] [CrossRef]
- Reddy, Y.N.; De, A.; Paul, S.; Pujari, A.K.; Bhaumik, J. In Situ Nanoarchitectonics of a MOF Hydrogel: A Self-Adhesive and pH-Responsive Smart Platform for Phototherapeutic Delivery. Biomacromolecules 2023, 24, 1717–1730. [Google Scholar] [CrossRef]
- El-Guindy, J.; Selim, M.; El-Agroudi, M. Alternative pretreatment modalities with a self-adhesive system to promote dentin/alloy shear bond strength. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2010, 19, 205–211. [Google Scholar] [CrossRef]
- Bim-Júnior, O.; Gaglieri, C.; Bedran-Russo, A.K.; Bueno-Silva, B.; Bannach, G.; Frem, R.; Ximenes, V.F.; Lisboa-Filho, P.N. MOF-Based Erodible System for On-Demand Release of Bioactive Flavonoid at the Polymer-Tissue Interface. ACS Biomater. Sci. Eng. 2020, 6, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Bim-Junior, O.; Alania, Y.; Tabatabaei, F.S.; Frem, R.; Bedran-Russo, A.K.; Lisboa-Filho, P.N. Biomimetic Growth of Metal-Organic Frameworks for the Stabilization of the Dentin Matrix and Control of Collagenolysis. Langmuir 2022, 38, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Rüttermann, S.; Trellenkamp, T.; Bergmann, N.; Raab, W.H.; Ritter, H.; Janda, R. A new approach to influence contact angle and surface free energy of resin-based dental restorative materials. Acta Biomater. 2011, 7, 1160–1165. [Google Scholar] [CrossRef]
- Sandomierski, M.; Buchwald, Z.; Koczorowski, W.; Voelkel, A. Calcium forms of zeolites A and X as fillers in dental restorative materials with remineralizing potential. Microporous Mesoporous Mater. 2020, 294, 109899. [Google Scholar] [CrossRef]
- Hotta, M.; Nakajima, H.; Yamamoto, K.; Aono, M. Antibacterial temporary filling materials: The effect of adding various ratios of Ag-Zn-Zeolite. J. Oral Rehabil. 1998, 25, 485–489. [Google Scholar] [CrossRef]
- Ghatole, K.; Patil, A.; Giriyappa, R.H.; Singh, T.V.; Jyotsna, S.V.; Rairam, S. Evaluation of Antibacterial Efficacy of MTA with and without Additives Like Silver Zeolite and Chlorhexidine. J. Clin. Diagn. Res. JCDR 2016, 10, Zc11–Zc14. [Google Scholar] [CrossRef]
- Ghasemi, N.; Rahimi, S.; Samiei, M.; Mohamadi, M.; Rezaei, Y.; Divband, B.; Farhangi, N. Effect of the of Zeolite Containing Silver-Zinc Nanoparticles on the Push out Bond Strength of Mineral Trioxide Aggregate in Simulated Furcation Perforation. J. Dent. 2019, 20, 102–106. [Google Scholar] [CrossRef]
- Samiei, M.; Ghasemi, N.; Asl-Aminabadi, N.; Divband, B.; Golparvar-Dashti, Y.; Shirazi, S. Zeolite-silver-zinc nanoparticles: Biocompatibility and their effect on the compressive strength of mineral trioxide aggregate. J. Clin. Exp. Dent. 2017, 9, e356–e360. [Google Scholar] [CrossRef]
- Ghivari, S.B.; Bhattacharya, H.; Bhat, K.G.; Pujar, M.A. Antimicrobial activity of root canal irrigants against biofilm forming pathogens- An in vitro study. J. Conserv. Dent. JCD 2017, 20, 147–151. [Google Scholar] [CrossRef]
- Chung, H.A.; Titley, K.; Torneck, C.D.; Lawrence, H.P.; Friedman, S. Adhesion of glass-ionomer cement sealers to bovine dentin conditioned with intracanal medications. J. Endod. 2001, 27, 85–88. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Vakhnovetsky, J.; Vakhnovetsky, A.; Samadi, E.; Samadi, F. Volume and Power of Expansion of Novel Polyurethane-based Sealers. J. Endod. 2023, 49, 1020–1026. [Google Scholar] [CrossRef]
- Patel, V.; Santerre, J.P.; Friedman, S. Suppression of bacterial adherence by experimental root canal sealers. J. Endod. 2000, 26, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Cinar, C.; Ulusu, T.; Ozçelik, B.; Karamüftüoğlu, N.; Yücel, H. Antibacterial effect of silver-zeolite containing root-canal filling material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Padachey, N.; Patel, V.; Santerre, P.; Cvitkovitch, D.; Lawrence, H.P.; Friedman, S. Resistance of a novel root canal sealer to bacterial ingress in vitro. J. Endod. 2000, 26, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Naji, G.A.-H.; Omar, R.A.; Yahya, R. The effect of sodalite zeolite infiltrated material on the fracture toughness, elastic modulus and optical properties of all-ceramic dental prostheses. Ceram. Int. 2016, 42, 18737–18746. [Google Scholar] [CrossRef]
- Naji, G.A.; Omar, R.A.; Yahya, R. Influence of sodalite zeolite infiltration on the coefficient of thermal expansion and bond strength of all-ceramic dental prostheses. J. Mech. Behav. Biomed. Mater. 2017, 67, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Abe, Y.; Sato, Y.; Okamoto, K.; Ueshige, M.; Akagawa, Y. Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J. Dent. 1997, 25, 373–377. [Google Scholar] [CrossRef]
- Ueshige, M.; Abe, Y.; Sato, Y.; Tsuga, K.; Akagawa, Y.; Ishii, M. Dynamic viscoelastic properties of antimicrobial tissue conditioners containing silver-zeolite. J. Dent. 1999, 27, 517–522. [Google Scholar] [CrossRef]
- Casemiro, L.A.; Martins, C.H.G.; Pires-de-Souza, F.d.C.P.; Panzeri, H. Antimicrobial and mechanical properties of acrylic resins with incorporated silver-zinc zeolite-part I. Gerodontology 2008, 25, 187–194. [Google Scholar] [CrossRef]
- Malic, S.; Rai, S.; Redfern, J.; Pritchett, J.; Liauw, C.M.; Verran, J.; Tosheva, L. Zeolite-embedded silver extends antimicrobial activity of dental acrylics. Colloids Surf. B Biointerfaces 2019, 173, 52–57. [Google Scholar] [CrossRef]
- Aljafery, A.M.; Al-Jubouri, O.M.; Wally, Z.J.; Almusawi, R.M.; Abdulrudha, N.H.; Haider, J. The Effects of Incorporating Ag-Zn Zeolite on the Surface Roughness and Hardness of Heat and Cold Cure Acrylic Resins. J. Compos. Sci. 2022, 6, 85. [Google Scholar] [CrossRef]
- Yadav, N.S.; Saraf, S.; Mishra, S.K.; Hazari, P. Effects of fluconazole, chlorhexidine gluconate, and silver-zinc zeolite on flexural strength of heat-cured polymethyl methacrylate resin. J. Nat. Sci. Biol. Med. 2015, 6, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Hummudi, I.M.; Sadeq, H.A.A. Influence of Silver-Zinc Zeolite Incorporation on Shear Bond Strength of Silicon Cold Cure Soft Liner. J. Tech. 2021, 3, 31–36. [Google Scholar] [CrossRef]
- Ferreira, A.N.; D’Souza, K.; Aras, M.; Chitre, V.; Parsekar, S.; Pinto, M.J.W. Long term antifungal efficacy of silver-zinc zeolite nanoparticles incorporated in two soft denture liners—An in vitro assessment. Dent. Res. J. 2022, 19, 12. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Li, D.; Zhang, Z.Y.; Liu, G.; Liang, H.; Qin, Y.; Yu, J.; Li, Y. Fabrication of bioactive 3D printed porous titanium implants with Sr ion-incorporated zeolite coatings for bone ingrowth. J. Mater. Chem. B 2018, 6, 3254–3261. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Pei, X.; Wang, J.; Wan, Q.; Jiang, S.; Huang, C.; Pei, X. Enhanced Osseointegration of Porous Titanium Modified with Zeolitic Imidazolate Framework-8. ACS Appl. Mater. Interfaces 2017, 9, 25171–25183. [Google Scholar] [CrossRef]
- Bedi, R.S.; Beving, D.E.; Zanello, L.P.; Yan, Y. Biocompatibility of corrosion-resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater. 2009, 5, 3265–3271. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Geng, Z.; Xu, X.; Han, X.; Chen, L.; Ji, P.; Liu, Y. Novel pH-Responsive CaO2@ZIF-67-HA-ADH Coating That Efficiently Enhances the Antimicrobial, Osteogenic, and Angiogenic Properties of Titanium Implants. ACS Appl. Mater. Interfaces 2023, 15, 42965–42980. [Google Scholar] [CrossRef]
- Oheix, E.; Reicher, C.; Nouali, H.; Michelin, L.; Josien, L.; Daou, T.J.; Pieuchot, L. Rational Design and Characterisation of Novel Mono- and Bimetallic Antibacterial Linde Type A Zeolite Materials. J. Funct. Biomater. 2022, 13, 73. [Google Scholar] [CrossRef]
- Wang, L.; Dai, F.; Yang, Y.; Zhang, Z. Zeolitic Imidazolate Framework-8 with Encapsulated Naringin Synergistically Improves Antibacterial and Osteogenic Properties of Ti Implants for Osseointegration. ACS Biomater. Sci. Eng. 2022, 8, 3797–3809. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Li, M.; Jiang, X.; Yu, H.; Sun, D. An efficient metal-organic framework-based drug delivery platform for synergistic antibacterial activity and osteogenesis. J. Colloid Interface Sci. 2023, 640, 521–539. [Google Scholar] [CrossRef]
- Fardjahromi, M.A.; Ejeian, F.; Razmjou, A.; Vesey, G.; Mukhopadhyay, S.C.; Derakhshan, A.; Warkiani, M.E. Enhancing osteoregenerative potential of biphasic calcium phosphates by using bioinspired ZIF8 coating. Mater. Sci. Eng. C 2021, 123, 111972. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.; Pei, X.; Zhang, X.; Cheng, X.; Hu, S.; Gao, X.; Wang, J.; Chen, J.; Wan, Q. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36978–36995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Zhang, X.; Li, Y.; Zhang, S.; Yang, L.; Li, R.; Wan, Q.; Pei, X.; Chen, J.; et al. Drug-Delivery Nanoplatform with Synergistic Regulation of Angiogenesis-Osteogenesis Coupling for Promoting Vascularized Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 17543–17561. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.Y.; Pei, X.; Li, Y.H.; Feng, H.; He, Z.H.; Xie, W.J.; Pei, X.B.; Zhu, Z.; Wan, Q.B.; et al. One-Pot Facile Encapsulation of Dimethyloxallyl Glycine by Nanoscale Zeolitic Imidazolate Frameworks-8 for Enhancing Vascularized Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2202317. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W.; et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv. Healthc. Mater. 2021, 10, e2001369. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fang, J.; Wang, Y.; Sun, J.; Sun, Y.; Sun, X.; Qi, M.; Li, W.; Li, C.; Zhou, Y.; et al. Antibacterial Zeolite Imidazole Frameworks with Manganese Doping for Immunomodulation to Accelerate Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, e2101515. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 2000, 16, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xie, L.; Wu, Y.; Wu, Y.; Liu, Y.; Gao, Y.; Yang, J.; Zhang, X.; Jiang, L. Dexamethasone-loaded zeolitic imidazolate frameworks nanocomposite hydrogel with antibacterial and anti-inflammatory effects for periodontitis treatment. Mater. Today Bio 2022, 16, 100360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, M.; Li, C.; Dong, B.; Wang, J.; Weir, M.D.; Imazato, S.; Du, L.; Lynch, C.D.; Xu, L.; et al. Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis. J. Mater. Chem. B 2019, 7, 6955–6971. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Sun, M.; Cheng, Z.; Jia, W.; Jiao, K.; Wang, S.; Jiang, K.; Yang, Y.; Dai, Z.; et al. ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis. Acta Biomater. 2022, 146, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, Y.; Jiao, K.; Jia, W.; Jiang, K.; Cheng, Z.; Liu, G.; Luo, Y. A periodontal tissue regeneration strategy via biphasic release of zeolitic imidazolate framework-8 and FK506 using a uniaxial electrospun Janus nanofiber. J. Mater. Chem. B Mater. Biol. Med. 2022, 10, 765–778. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, C.; Yan, L.; Lei, H.; Peng, C.; Liu, S.; Fan, L.; Chu, Y. Antibacterial and osteoconductive polycaprolactone/polylactic acid/nano-hydroxyapatite/Cu@ZIF-8 GBR membrane with asymmetric porous structure. Int. J. Biol. Macromol. 2023, 224, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Ejeian, F.; Razmjou, A.; Nasr-Esfahani, M.H.; Mohammad, M.; Karamali, F.; Ebrahimi Warkiani, M.; Asadnia, M.; Chen, V. ZIF-8 Modified Polypropylene Membrane: A Biomimetic Cell Culture Platform with a View to the Improvement of Guided Bone Regeneration. Int. J. Nanomed. 2020, 15, 10029–10043. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Divband, B.; Ranjkesh, B.; Pournaghi Azar, F.; Yeganeh Sefidan, F.; Kachoei, M.; Karimzadeh, B. Antimicrobial and Mechanical Properties of Orthodontic Acrylic Resin Containing Zinc Oxide and Titanium Dioxide Nanoparticles Supported on 4A Zeolite. Int. J. Dent. 2022, 2022, 8155971. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Bahador, A. Enhanced reduction of polymicrobial biofilms on the orthodontic brackets and enamel surface remineralization using zeolite-zinc oxide nanoparticles-based antimicrobial photodynamic therapy. BMC Microbiol. 2021, 21, 273. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Li, S.; Zhang, P.; Yao, Q. Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv. 2020, 1, 376–3762. [Google Scholar] [CrossRef]
- Xie, H.; Liu, X.; Huang, Z.; Xu, L.; Bai, R.; He, F.; Wang, M.; Han, L.; Bao, Z.; Wu, Y.; et al. Nanoscale Zeolitic Imidazolate Framework (ZIF)–8 in Cancer Theranostics: Current Challenges and Prospects. Cancers 2022, 14, 3935. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Pazarçeviren, A.E.; Keskin, D.; Tezcaner, A.; Hussain, R.; Evis, Z. Porous clinoptilolite-nano biphasic calcium phosphate scaffolds loaded with human dental pulp stem cells for load bearing orthopedic applications. Biomed. Mater. 2019, 14, 055010. [Google Scholar] [CrossRef]
- Alipour, M.; Aghazadeh, M.; Akbarzadeh, A.; Vafajoo, Z.; Aghazadeh, Z.; Raeisdasteh Hokmabad, V. Towards osteogenic differentiation of human dental pulp stem cells on PCL-PEG-PCL/zeolite nanofibrous scaffolds. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3431–3437. [Google Scholar] [CrossRef]
- Ninan, N.; Grohens, Y.; Elain, A.; Kalarikkal, N.; Thomas, S. Synthesis and characterisation of gelatin/zeolite porous scaffold. Eur. Polym. J. 2013, 49, 2433–2445. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Hosseini, A.; Taghavi, F.; Jafari, S.; Lotfabadi, A.; Ejtehadi, M.R.; Shahbazi, S.; Fattahi, A.; Ghasemi, A.; Barzegari, E.; et al. Molecular interaction of fibrinogen with zeolite nanoparticles. Sci. Rep. 2019, 9, 1558. [Google Scholar] [CrossRef] [PubMed]
- Kavya, K.C.; Dixit, R.; Jayakumar, R.; Nair, S.V.; Chennazhi, K.P. Synthesis and characterization of chitosan/chondroitin sulfate/nano-SiO2 composite scaffold for bone tissue engineering. J. Biomed. Nanotechnol. 2012, 8, 149–160. [Google Scholar] [CrossRef]
- Shigeyama, H.; Wang, T.; Ichinose, M.; Ansai, T.; Lee, S.W. Identification of volatile metabolites in human saliva from patients with oral squamous cell carcinoma via zeolite-based thin-film microextraction coupled with GC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1104, 49–58. [Google Scholar] [CrossRef]
- Çelikbaş, İ.; Mavi, E.; Hepokur, C. The evaluation of the effects of natural zeolite (Clinoptilolite) in diabetic rats on bone healing in dental extracting socket. J. Oral Biol. Craniofacial Res. 2023, 13, 36–40. [Google Scholar] [CrossRef]
- Barman, A.; Rashid, F.; Farook, T.H.; Jamayet, N.B.; Dudley, J.; Yhaya, M.F.B.; Alam, M.K. The Influence of Filler Particles on the Mechanical Properties of Maxillofacial Prosthetic Silicone Elastomers: A Systematic Review and Meta-Analysis. Polymers 2020, 12, 1536. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; He, Q.; Li, D.; Xin, L.; Xu, C.; Zhu, X.; Mei, L.; Cannon, R.D.; Ji, P.; et al. A Mechanically Reinforced Super Bone Glue Makes a Leap in Hard Tissue Strong Adhesion and Augmented Bone Regeneration. Adv. Sci. 2023, 10, e2206450. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wu, Q.; Tan, L.; Huang, Z.; Fu, C.; Ren, X.; Xia, N.; Chen, Z.; Ma, X.; Lan, X.; et al. High Biocompatible ZIF-8 Coated by ZrO2 for Chemo-microwave Thermal Tumor Synergistic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 10520–10531. [Google Scholar] [CrossRef]

| Dental Application of Zeolites | Type of Zeolite/ZIF | Properties of Zeolites in Materials | Functions of Zeolites in Materials |
|---|---|---|---|
| Restorative Dentistry | |||
| Zeolite/ZIF-modified adhesives |
|
|
|
| Zeolite-loaded restorative materials |
|
|
|
| Endodontics | |||
| Zeolite-incorporated root filling |
|
|
|
| Zeolite-incorporated irrigants |
|
|
|
| Zeolite-incorporated sealers |
|
|
|
| Prosthodontics | |||
| Zeolite-infiltrated all-ceramic dental prostheses |
|
|
|
| Zeolite-incorporated tissue conditioners |
|
|
|
| Zeolite-loaded denture bases |
|
|
|
| Zeolite-incorporated denture liners |
|
|
|
| Implantology | |||
| Zeolite/ZIF-coated implant |
|
|
|
| ZIF-coated bone graft materials |
|
|
|
| ZIF-modified bone adhesive |
|
|
|
| ZIF-loaded drug delivery system |
|
|
|
| ZIF-modified PCL electrospinning |
|
|
|
| ZIF-modified post-implantation drug |
|
|
|
| Periodontics | |||
| Zeolite/ZIF-loaded deep periodontal pocket drugs |
|
|
|
| ZIF-embedded guided tissue regeneration membranes |
|
|
|
| ZIF-embedded guided bone regeneration membranes |
|
|
|
| Orthodontics | |||
| Zeolite-modified orthodontic bracket |
|
|
|
| Zeolite-based PDT photosensitizer |
|
|
|
| Oral surgery | |||
| Zeolite-modified bone matrix scaffold |
|
|
|
| Zeolite-loaded oral cancer detection membrane |
|
|
|
| Zeolite acted as the drug after tooth extraction |
|
|
|
| Zeolite-modified maxillofacial silicone elastomer |
|
|
|
| ZIF-incorporated osteogenic glue |
|
|
|
| ZIF-coated antitumour drugs |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.J.; Chu, C.-H.; Yu, O.Y. Application of Zeolites and Zeolitic Imidazolate Frameworks in Dentistry—A Narrative Review. Nanomaterials 2023, 13, 2973. https://doi.org/10.3390/nano13222973
Li LJ, Chu C-H, Yu OY. Application of Zeolites and Zeolitic Imidazolate Frameworks in Dentistry—A Narrative Review. Nanomaterials. 2023; 13(22):2973. https://doi.org/10.3390/nano13222973
Chicago/Turabian StyleLi, Laura Jiaxuan, Chun-Hung Chu, and Ollie Yiru Yu. 2023. "Application of Zeolites and Zeolitic Imidazolate Frameworks in Dentistry—A Narrative Review" Nanomaterials 13, no. 22: 2973. https://doi.org/10.3390/nano13222973








