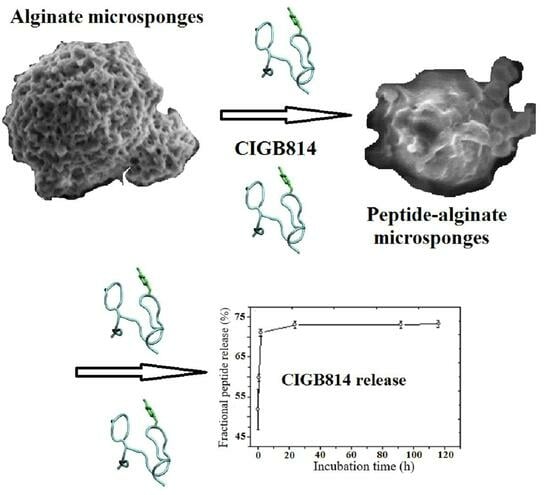
Alginate Microsponges as a Scaffold for Delivery of a Therapeutic Peptide against Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
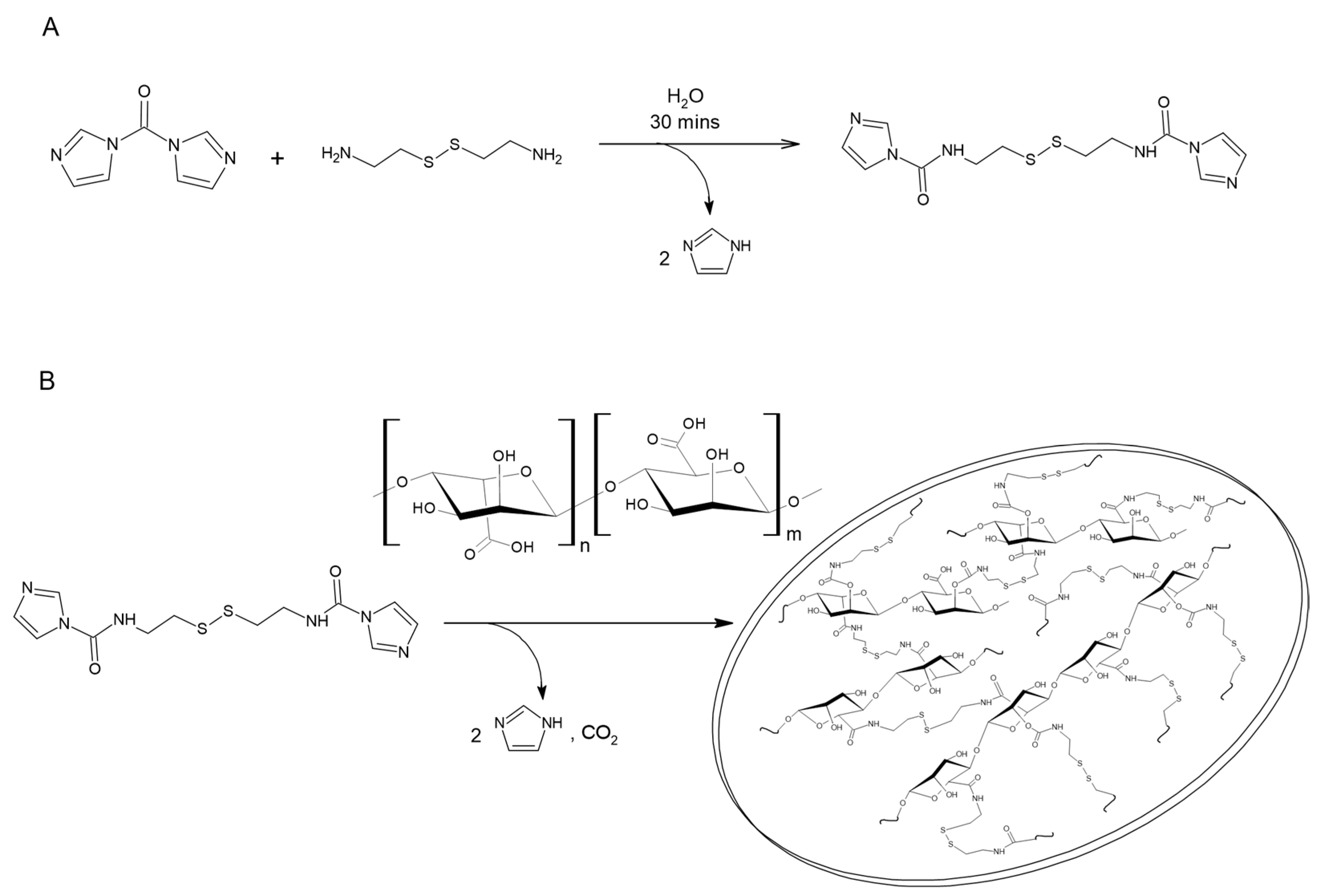
2.2. Experimental Methods
2.3. Statistical Methods: DoE Factorial Design Approach for the Estimate of PMS Diameter
3. Results and Discussion
3.1. Characterization of Alginate Microsponges
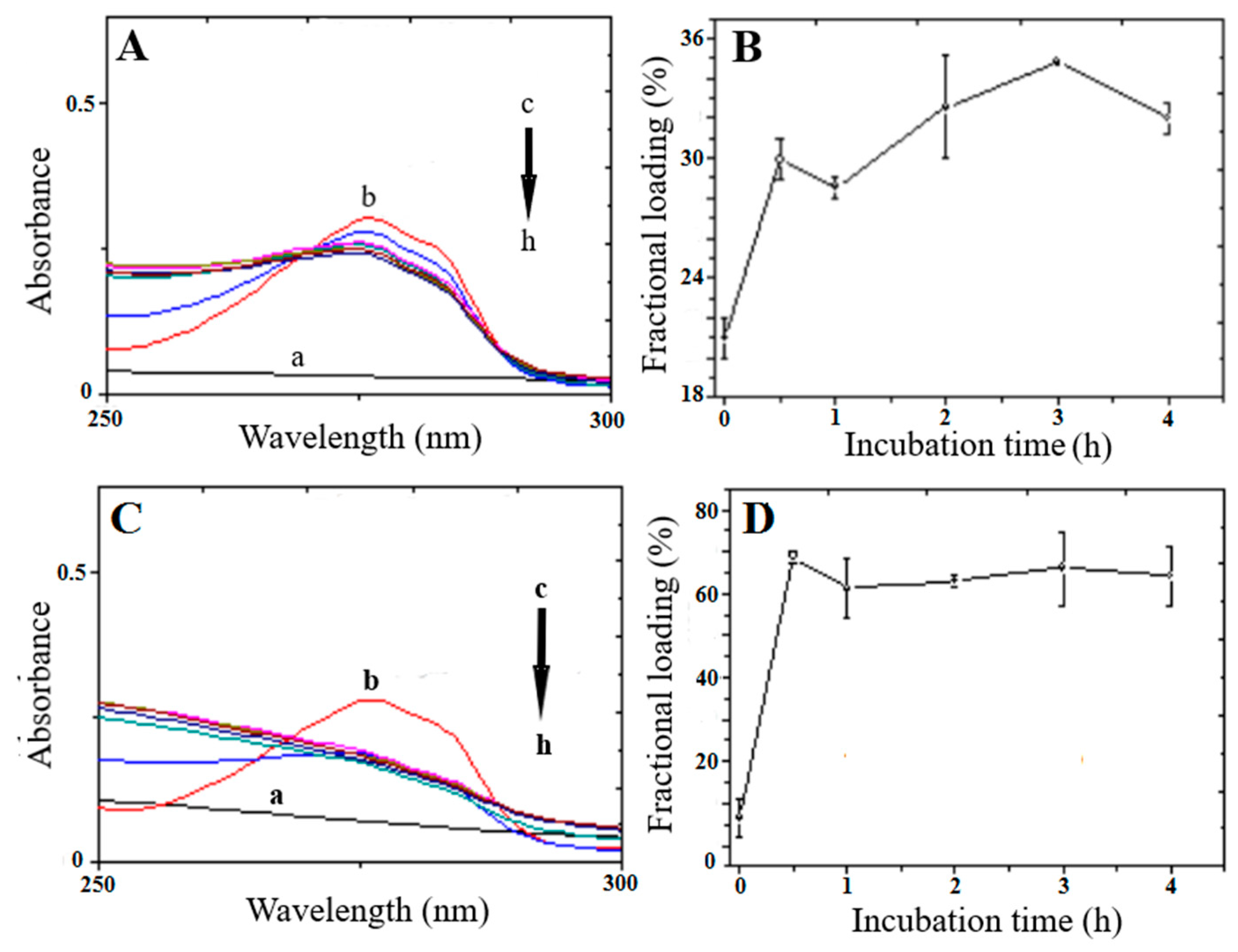
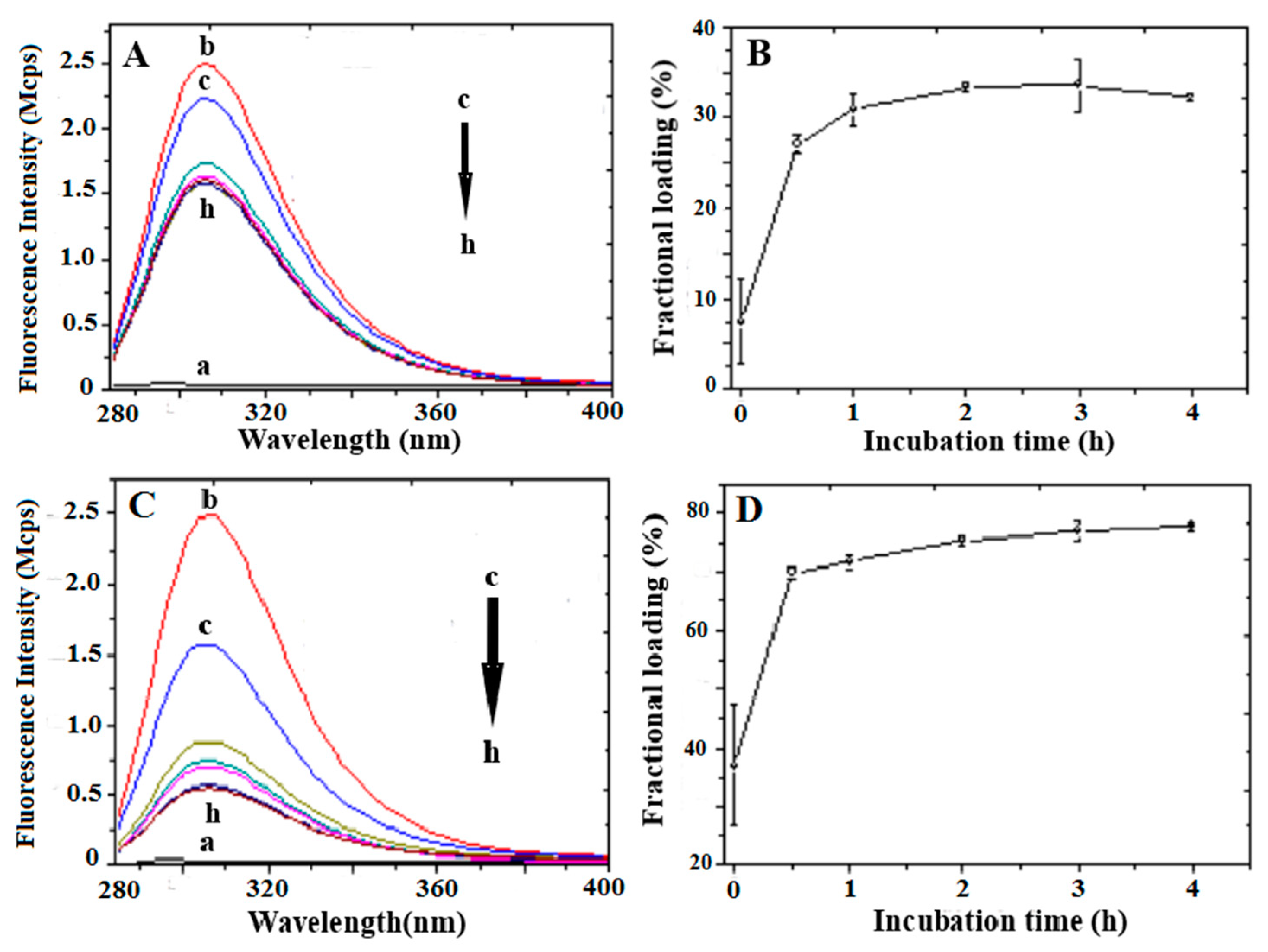
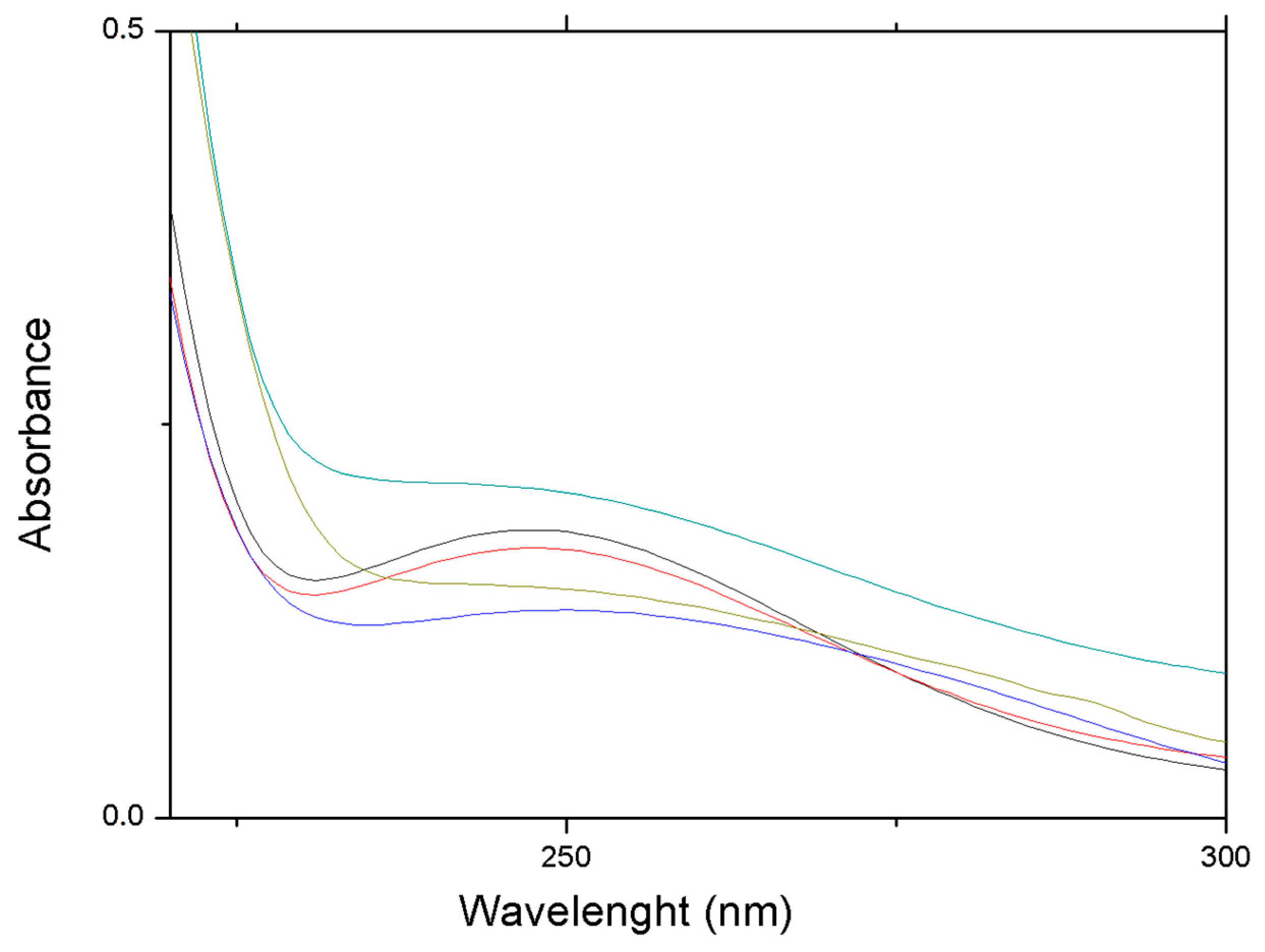
3.2. Peptide Loading
3.3. Peptide Release
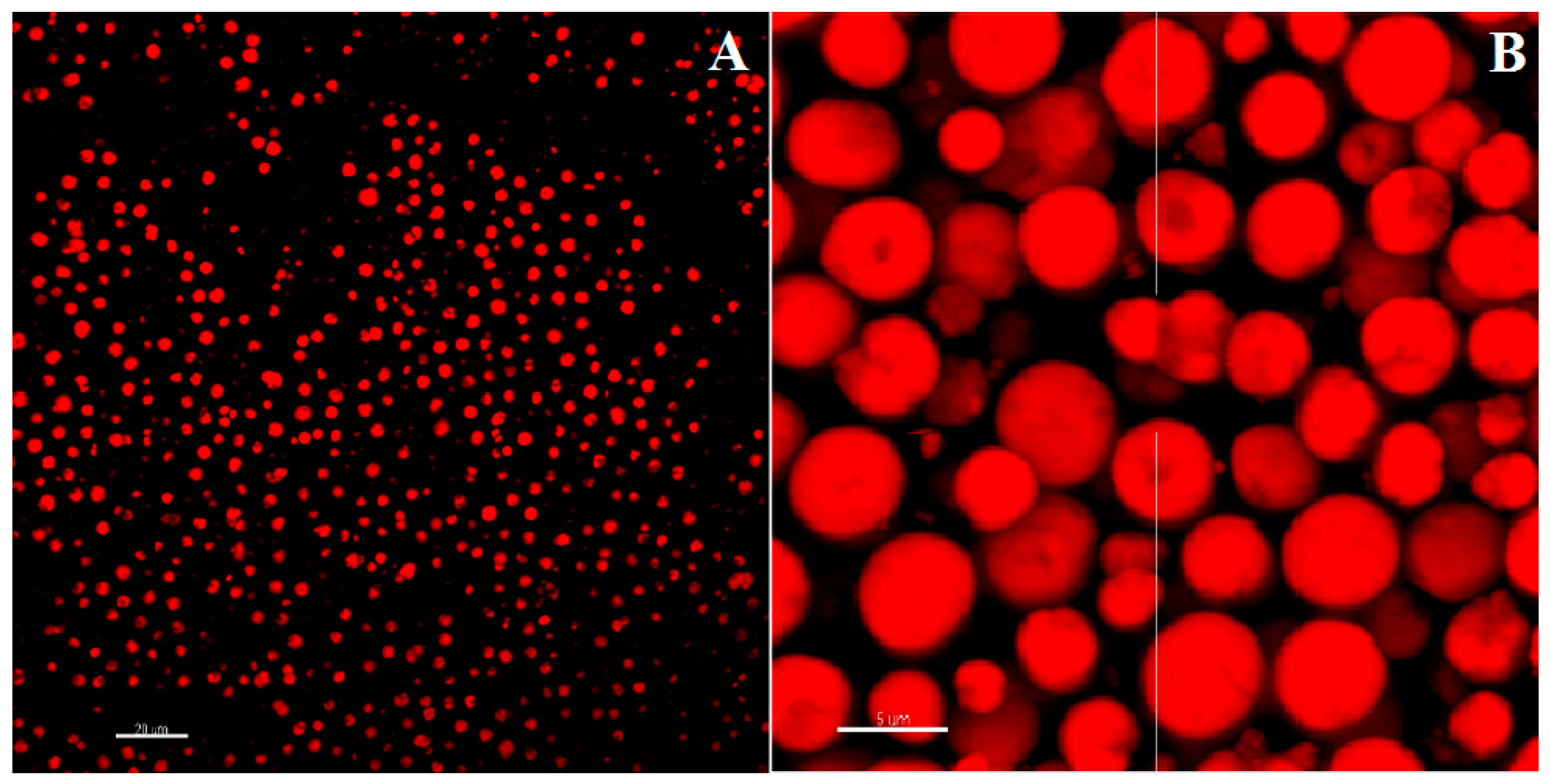
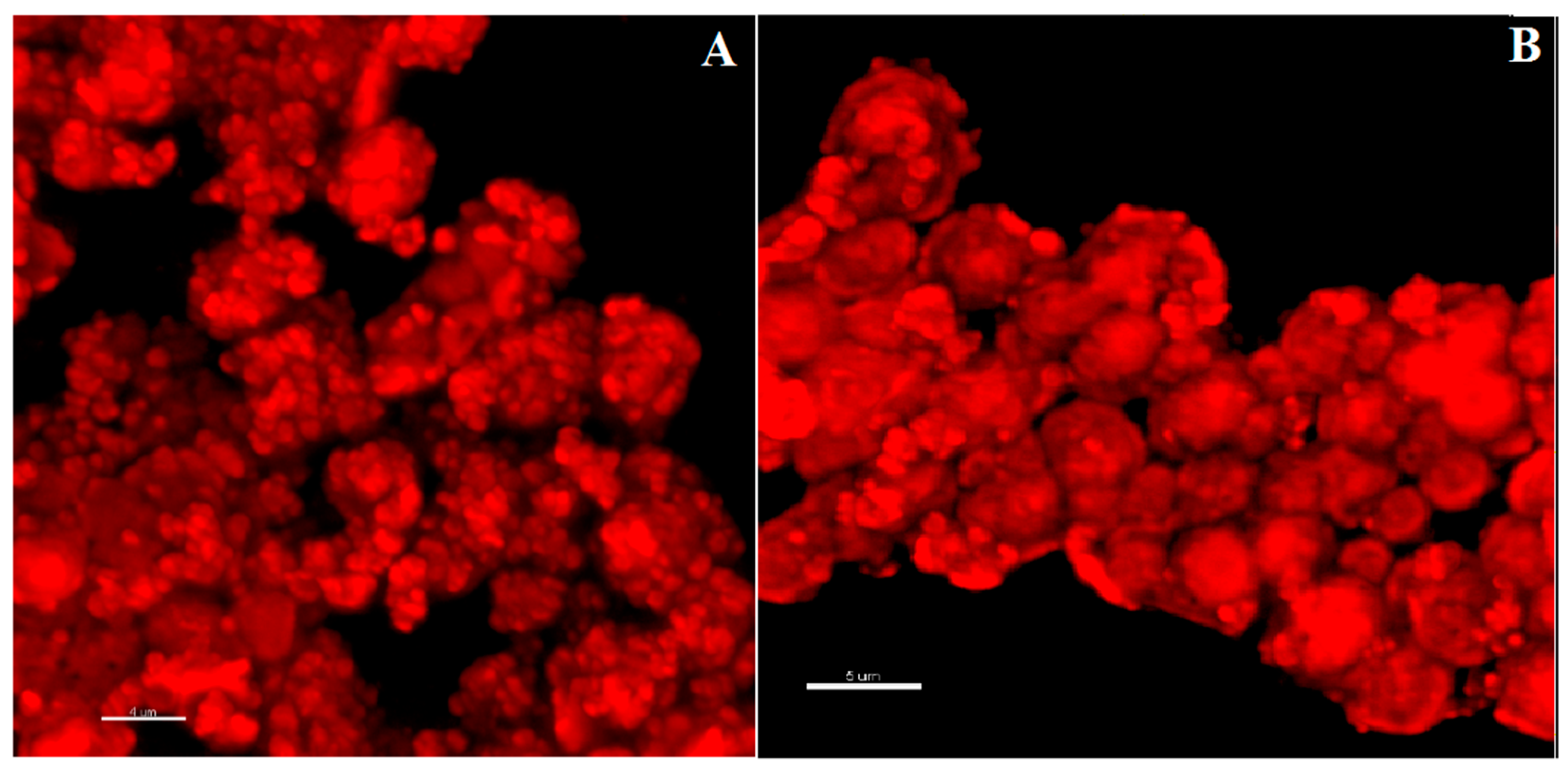
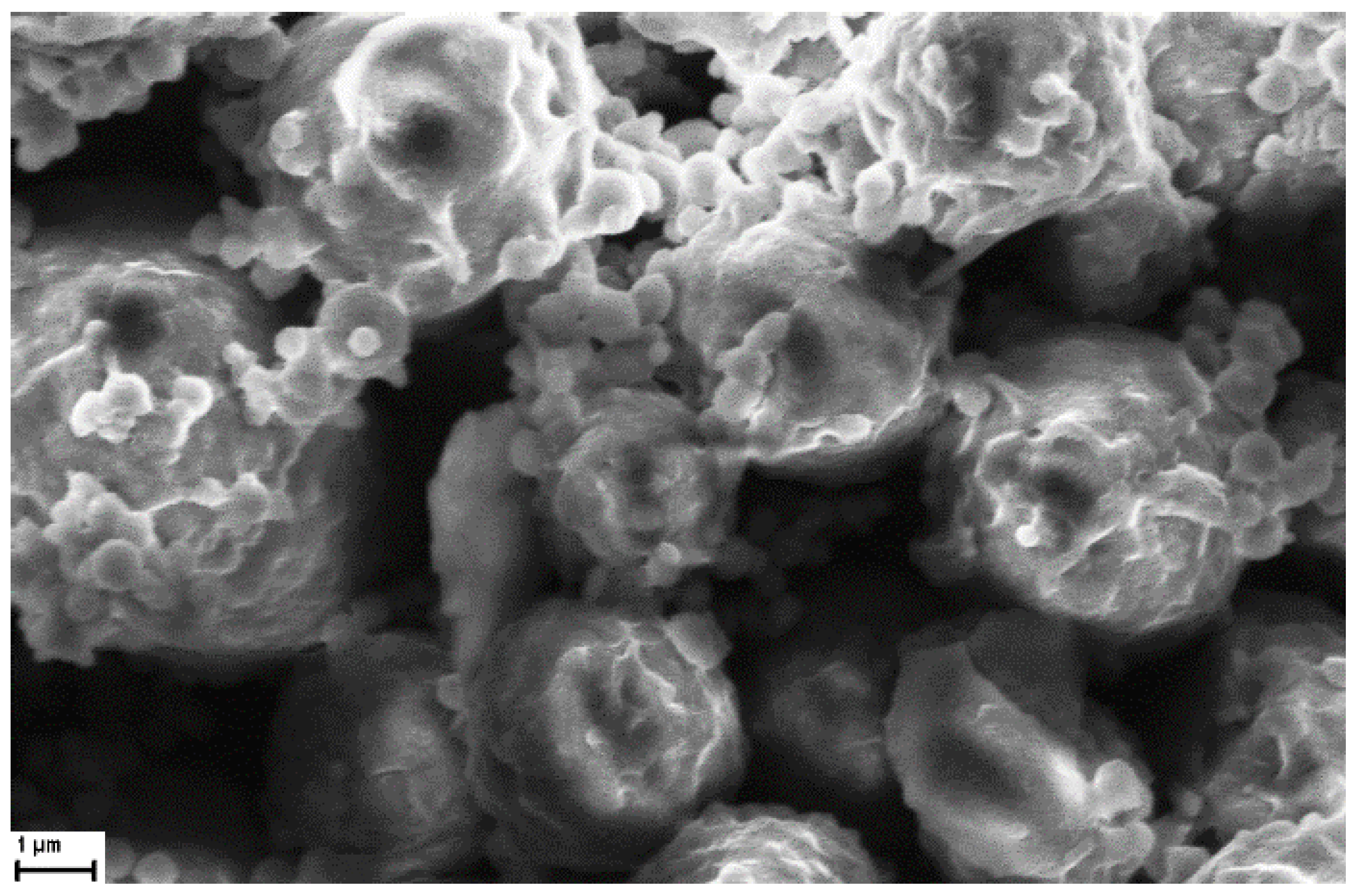
3.4. Imaging of CIGB814/NaAlg PMS
3.4.1. Fluorescence Confocal Microscopy
3.4.2. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Tang, J.; Lee, D.; Tice, T.R.; Schwendeman, S.P.; Prausnitz, M.R. Clinical translation of long-acting drug delivery formulations. Nat. Rev. Mater. 2022, 7, 406. [Google Scholar] [CrossRef]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release 2022, 342, 53. [Google Scholar] [CrossRef]
- Jain, K.K. An Overview of Drug Delivery Systems. In Drug Delivery Systems. Methods in Molecular Biology; Jain, K., Ed.; Humana: New York, NY, USA, 2020; Volume 2059. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, K.M.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent progress in nanostructured smart drug delivery systems for Cancer Therapy: A Review. ACS Appl. BioMater. 2022, 5, 971. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Johnson, A.P.; Sabu, C.; Nivitha, K.P.; Sankar, R.; Ameena Shirin, V.K.; Henna, T.K.; Raphey, V.R.; Gangadharappa, H.V.; Kotta, S.; Pramod, K. Bioinspired and biomimetic micro- and nanostructures in biomedicine. J. Control. Release 2022, 343, 724. [Google Scholar] [CrossRef]
- Embil, K.; Nacht, S. The Microsponge® Delivery System (MDS): A topical delivery system with reduced irritancy incorporating multiple triggering mechanisms for the release of actives. J. Microencapsul. 1996, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Kaity, S.; Maiti, S.; Ghosh, A.K.; Pal, D.; Ghosh, A.; Banerjee, S. Microsponges: A novel strategy for drug delivery system. J. Adv. Pharm. Technol. Res. 2010, 1, 283. [Google Scholar] [CrossRef]
- Pradhan, S.K. Microsponges as the versatile tool for drug delivery system. Int. J. Res. Pharm. Chem. 2011, 1, 243. [Google Scholar]
- Aloorkar, N.K.; Kulkarni, A.S.; Ingale, D.J.; Patil, R.A. Microsponges as Innovative Drug Delivery Systems. Int. J. Pharm. Sci. Nanotechn. 2012, 5, 1597. [Google Scholar]
- Balamurugan, K.; Kshirasagar, N.; Govardhan, P. Microsponges as a drug delivery system. Pharma Innov. J. 2019, 8, 139. [Google Scholar]
- Tiwari, A.; Tiwari, V.; Palaria, B.; Kumar, M.; Kaushik, D. Microsponges: A breakthrough tool in pharmaceutical research. Fut. J. Pharmaceut. Sci. 2022, 8, 31. [Google Scholar] [CrossRef]
- Anshika, C.; Semimul, A.M. Microsponge drug delivery system: Emerging technique in novel drug delivery system and recent advances. Res. J. Pharm. Technol. 2022, 15, 4835. [Google Scholar] [CrossRef]
- Caso, M.F.; Carotenuto, F.; Di Nardo, P.; Migliore, A.; Aguilera, A.; Lopez, C.M.; Venanzi, M.; Cavalieri, F.; Rinaldi, A. Nanoporous Microsponge Particles (NMP) of Polysaccharides as Universal Carriers for Biomolecules Delivery. Nanomaterials 2020, 10, 1075. [Google Scholar] [CrossRef]
- Cavalieri, F. Hyaluronic Acid Micro-Sponges and Method for the Production Thereof. Patent PCT/IB2016/052792, 17 November 2016. Available online: https://patents.google.com/patent/WO2016181365A1/en (accessed on 1 August 2023).
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; WonWoo Lee, W.W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Domínguez-Horta, M.C.; Serrano-Díaz, A.; Hernández-Cedeño, M.; Martíınez-Donato, G.; Guillén-Nieto, G. A peptide derived from HSP60 reduces proinflammatory cytokines and soluble mediators: A therapeutic approach to inflammation. Front. Immunol. 2023, 14, 1162739. [Google Scholar] [CrossRef]
- Prada, D.; Gómez, J.; Lorenzo, N.; Corrales, O.; López, A.; González, E.; Cabrales, A.; Reyes, Y.; Bermudez, Y.; Avila, Y.; et al. Phase I Clinical Trial with a Novel Altered Peptide Ligand Derived from Human Heat-Shock Protein 60 for Treatment of Rheumatoid Arthritis: Safety, Pharmacokinetics and Preliminary Therapeutic Effects. J. Clin. Trials 2018, 8, 1. [Google Scholar] [CrossRef]
- Cimino, R.; Savioli, M.; Carrante, N.F.; Placidi, E.; Garay-Perez, H.; López-Abad, M.; Lasa, A.M.; Del Carmen Domínguez-Horta, M.; Gatto, E.; Cavalieri, F.; et al. Aggregation properties of a therapeutic peptide for rheumatoid arthritis: A spectroscopic and molecular dynamics study. ChemPhysMater 2022, 1, 62. [Google Scholar] [CrossRef]
- Gatto, E.; Toniolo, C.; Venanzi, M. Peptide Self-Assembled Nanostructures: From Models to Therapeutic Peptides. Nanomaterials 2022, 12, 466. [Google Scholar] [CrossRef]
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Seyedmahmoud, R.; Rainer, A.; Mozetic, P.; Giannitelli, S.M.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly(L-lactide) scaffolds for tissue engineering. I. Design of experiments. J. Biomed. Mater. Res. Part A 2014, 103, 91–102. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Mozetic, P.; Rainer, A.; Giannitelli, S.M.; Basoli, F.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly(L-lactide) scaffolds for tissue engineering. II: Regression. J. Biomed. Mater. Res. Part A 2015, 103, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814. [Google Scholar] [CrossRef]
- Thomas, J.C. The determination of log normal particle size distributions by dynamic light scattering. J. Colloid Interface Sci. 1987, 117, 187. [Google Scholar] [CrossRef]
- Xu, J.X.; Vithanage, B.C.N.; Athukorale, S.; Zhang, D. Scattering and Absorption Differ Drastically in Their Inner Filter Effects on Fluorescence, Resonance Synchronous, and Polarized Resonance Synchronous Spectroscopic Measurements. Analyst 2018, 143, 3382. [Google Scholar] [CrossRef]
- Mendoza García, M.A.; Izadifar, M.; Chen, X. Evaluation of PBS Treatment and PEI Coating Effects on Surface Morphology and Cellular Response of 3D-Printed Alginate Scaffolds. J. Funct. Biomater. 2017, 8, 48. [Google Scholar] [CrossRef]
- Birtalan, E.; Rudat, B.; Kölmel, D.K.; Fritz, D.; Vollrath, S.B.L.; Schepers, U.; Bräse, S. Investigating rhodamine B-labeled peptoids: Scopes and limitations of its applications. Biopolymers 2011, 96, 694. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Label | Unit | Low Level (−1) | High Level (+1) | |
|---|---|---|---|---|---|
| X1 | Cys amount | Cys | mg | 20 | 30 |
| X2 | CDI amount | CDI | mg | 20 | 30 |
| X3 | H2O amount | H2O | mL | 200 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariaudo, D.; Cavalieri, F.; Rinaldi, A.; Aguilera, A.; Lopez, M.; Perez, H.G.; Felipe, A.; del Carmen Dominguez, M.; Ruiz, O.; Martinez, G.; et al. Alginate Microsponges as a Scaffold for Delivery of a Therapeutic Peptide against Rheumatoid Arthritis. Nanomaterials 2023, 13, 2709. https://doi.org/10.3390/nano13192709
Ariaudo D, Cavalieri F, Rinaldi A, Aguilera A, Lopez M, Perez HG, Felipe A, del Carmen Dominguez M, Ruiz O, Martinez G, et al. Alginate Microsponges as a Scaffold for Delivery of a Therapeutic Peptide against Rheumatoid Arthritis. Nanomaterials. 2023; 13(19):2709. https://doi.org/10.3390/nano13192709
Chicago/Turabian StyleAriaudo, Daniela, Francesca Cavalieri, Antonio Rinaldi, Ana Aguilera, Matilde Lopez, Hilda Garay Perez, Ariel Felipe, Maria del Carmen Dominguez, Odalys Ruiz, Gillian Martinez, and et al. 2023. "Alginate Microsponges as a Scaffold for Delivery of a Therapeutic Peptide against Rheumatoid Arthritis" Nanomaterials 13, no. 19: 2709. https://doi.org/10.3390/nano13192709