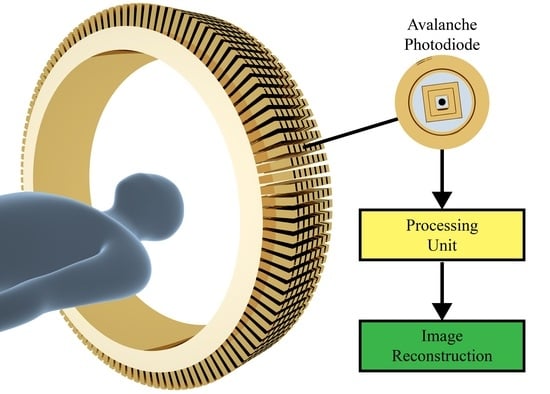
Silicon-Based Avalanche Photodiodes: Advancements and Applications in Medical Imaging
Abstract
:1. Introduction
2. Avalanche Photodiodes: Principles and Features
2.1. Operating Principles of Avalanche Photodiodes
2.1.1. Photodetection Mechanism
2.1.2. Avalanche Gain and Responsivity
2.1.3. Noise Characteristics
2.1.4. Timing Resolution
2.2. Key Features of Avalanche Photodiodes
- High responsivity. APDs are capable of detecting low-level optical signals with high responsivity due to the avalanche multiplication effect. The internal gain mechanism amplifies the initial photo-generated carriers, allowing for the detection of weak signals that would otherwise be challenging to detect with traditional detectors;
- Fast response time. APDs exhibit fast response times, typically in the order of picoseconds or nanoseconds. This fast response enables the detection and measurement of rapid optical events, making APDs suitable for applications that require precise timing information or fast real-time imaging capabilities to capture dynamic processes in medical visualization;
- Wide spectral range. APDs are available in various semiconductor materials, such as silicon, germanium, and III-V compounds, allowing for a wide spectral range of operation. This versatility enables APDs to be utilized across different wavelengths, including visible, near-infrared, and even X-ray regions, depending on the choice of the semiconductor material;
- Hybridization and integration with imaging systems. APDs can be integrated with other imaging components to enhance their capabilities and enable hybrid imaging systems. For instance, the combination of APDs with scintillator crystals enables the development of PET detectors, where APDs detect the scintillation light produced by the interaction of positrons with the scintillator material. The compatibility of APDs with various imaging modalities, including PET, SPECT, and time-resolved imaging, allows for the integration of APDs into versatile and multimodal imaging systems.
- Higher responsivity. APDs provide a higher responsivity compared to traditional photodetectors due to the avalanche multiplication effect. This allows for the detection of weaker optical signals and enhances the overall signal detection efficiency in medical-imaging systems;
- Compact size. APDs are typically smaller in size and have a more compact form factor compared to traditional photodetectors. This compactness enables their integration into miniaturized imaging devices and facilitates the development of portable and handheld medical-imaging systems;
- Lower voltage operation. APDs operate at lower bias voltages compared to PMTs, making them more energy-efficient and reducing the power requirements of the imaging system. This advantage is particularly beneficial for battery-powered or mobile medical-imaging applications;
- Enhanced stability. APDs exhibit better long-term stability and reduced aging effects compared to PMTs. This stability ensures a consistent performance over time, reducing the need for frequent recalibration and maintenance in imaging systems.
3. Applications of APDs in Medical Imaging
3.1. Positron Emission Tomography (PET)
3.2. Single-Photon Emission Computed Tomography (SPECT)
3.3. Time-of-Flight Positron Emission Tomography (TOF-PET)
3.4. Computed Tomography (CT)
3.5. Fluorescence Imaging
3.6. Bioluminescence Imaging
3.7. Optical Coherence Tomography (OCT)
4. Progress in Silicon-Based Avalanche Photodiodes
5. Advancements and Challenges
6. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khalil, M.M. Basic Sciences of Nuclear Medicine; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ozana, N.; Zavriyev, A.I.; Mazumder, D.; Robinson, M.; Kaya, K.; Blackwell, M.; Carp, S.A.; Franceschini, M.A. Superconducting nanowire single-photon sensing of cerebral blood flow. Neurophotonics 2021, 8, 035006. [Google Scholar] [CrossRef] [PubMed]
- Renker, D. Properties of avalanche photodiodes for applications in high energy physics, astrophysics and medical imaging. Nucl. Instrum. Methods Phys. Res. A 2002, 486, 164–169. [Google Scholar] [CrossRef]
- Izhnin, I.I.; Lozovoy, K.A.; Kokhanenko, A.P.; Khomyakova, K.I.; Douhan, R.M.H.; Dirko, V.V.; Voitsekhovskii, A.V.; Fitsych, O.I.; Akimenko, N.Y. Single-photon avalanche diode detectors based on group IV materials. Appl. Nanosci. 2021, 12, 253–263. [Google Scholar] [CrossRef]
- Renker, D.; Lorenz, E. Advances in solid state photon detectors. J. Instrum. 2009, 4, 04004. [Google Scholar] [CrossRef]
- Bartolo-Perez, C.; Chandiparsi, S.; Mayet, A.S.; Cansizoglu, H.; Gao, Y.; Qarony, W.; Ahamed, A.; Wang, S.-Y.; Cherry, S.R.; Islam, M.S.; et al. Avalanche photodetectors with photon trapping structures for biomedical imaging applications. Opt. Express 2021, 29, 19024–19033. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S. Avalanche Photodiodes and Silicon Photomultipliers of Non-Planar Designs. Sensors 2023, 23, 5369. [Google Scholar] [CrossRef] [PubMed]
- Dello Russo, S.; Elefante, A.; Dequal, D.; Pallotti, D.K.; Amato, L.S.; Sgobba, L.; de Cumis, M.S. Advances in Mid-Infrared Single-Photon Detection. Photonics 2022, 9, 470. [Google Scholar] [CrossRef]
- Bailey, D.L.; Townsend, D.W.; Valk, P.E.; Maisey, M.N. Positron Emission Tomography: Basic Sciences; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Llosa, G.; Battiston, R.; Belcari, N.; Boscardin, M.; Collazuol, G.; Corsi, F.; Dalla Betta, G.-F.; Del Guerra, A.; Dinu, N.; Levi, G.; et al. Novel Silicon Photomultipliers for PET Applications. IEEE Trans. Nucl. Sci. 2008, 55, 877–881. [Google Scholar] [CrossRef]
- Cherry, S.R. Multimodality imaging: Beyond PET/CT and SPECT/CT. Semin. Nucl. Med. 2009, 39, 348–353. [Google Scholar] [CrossRef]
- Cesca, N.; Auricchio, N.; Di Domenico, G.; Zavattini, G.; Malaguti, R.; Andritschke, R.; Kanbach, G.; Schopper, F. SiliPET: Design of an ultra-high resolution small animal PET scanner based on stacks of semi-conductor detectors. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 572, 225–227. [Google Scholar] [CrossRef]
- Acerbi, F.; Paternoster, G.; Capasso, M.; Marcante, M.; Mazzi, A.; Regazzoni, V.; Zorzi, N.; Gola, A. Silicon Photomultipliers: Technology Optimizations for Ultraviolet, Visible and Near-Infrared Range. Instruments 2019, 3, 15. [Google Scholar] [CrossRef]
- Sanaat, A.; Zafarghandi, M.S.; Ay, M.R. Design and performance evaluation of high resolution small animal PET scanner based on monolithic crystal: A simulation study. J. Instrum. 2019, 14, 01005. [Google Scholar] [CrossRef]
- Wernick, M.N.; Aarsvold, J.N. Emission Tomography—The Fundamentals of PET and SPECT; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Nieman, K.; Gaemperli, O.; Lancellotti, P.; Plein, S. Advanced Cardiac Imaging; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- LaBella, A.; Zhao, W.; Goldan, A.H. Multi-Well Avalanche Selenium Detector for Time-of-Flight PET. In Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Manchester, UK, 26 October–2 November 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Daube-Witherspoon, M.E.; Surti, S.; Perkins, A.E.; Karp, J.S. Determination of Accuracy and Precision of Lesion Uptake Measurements in Human Subjects with Time-of-Flight PET. J. Nucl. Med. 2014, 55, 602–607. [Google Scholar] [CrossRef]
- Jiang, W.; Chalich, Y.; Jamal Deen, M. Sensors for Positron Emission Tomography Applications. Sensors 2019, 19, 5019. [Google Scholar] [CrossRef] [PubMed]
- Gramuglia, F.; Wu, M.-L.; Lee, M.-J.; Bruschini, C.; Charbon, E. SPAD Microcells with 12.1 ps SPTR for SiPMs in TOF-PET Applications. In Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Piscataway, NJ, USA, 16–23 October 2021; pp. 1–2. [Google Scholar]
- Verdonck, P. Advances in Biomedical Engineering; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Bankman, I.N. Handbook of Medical Image Processing and Analysis; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Chen, Y.; Xue, L.; Zhu, Q.; Feng, Y.; Wu, M. Recent Advances in Second Near-Infrared Region (NIR-II) Fluorophores and Biomedical Applications. Front. Chem. 2021, 9, 750404. [Google Scholar] [CrossRef] [PubMed]
- Dorosz, P.; Baszczyk, M.; Kucewicz, W.; Mik, L. Silicon photomultipliers applied to the fluorescence detection of biomarkers. Nucl. Instrum. Methods Phys. Res. A 2018, 936, 70–72. [Google Scholar] [CrossRef]
- Palubiak, D.; El-Desouki, M.M.; Marinov, O.; Deen, M.J.; Fang, Q. High-Speed, Single-Photon Avalanche-Photodiode Imager for Biomedical Applications. IEEE Sens. J. 2011, 11, 2401–2412. [Google Scholar] [CrossRef]
- Bruschini, C.; Homulle, H.; Antolovic, I.M.; Burri, S.; Charbon, E. Single-photon avalanche diode imagers in biophotonics: Review and outlook. Light: Sci. Appl. 2019, 8, 87. [Google Scholar] [CrossRef]
- He, X.; Gao, J.; Gambhir, S.S.; Cheng, Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: Status and challenges. Trends Mol. Med. 2010, 16, 574–583. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, B.; Xu, Y.; Yang, Y.; Ji, J.; Cao, W.; Lu, J.; Ding, J.; Cao, H.; Chu, B.; et al. In vivo bioluminescence imaging of natural bacteria within deep tissues via ATP-binding cassette sugar transporter. Nat. Commun. 2023, 14, 2331. [Google Scholar] [CrossRef]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Contag, C.H.; Bachmann, M.H. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 2002, 4, 235–260. [Google Scholar] [CrossRef]
- Wang, T.; Brewer, M.; Zhu, Q. An overview of optical coherence tomography for ovarian tissue imaging and characterization. WIREs Nanomed. Nanobiotechnol. 2015, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kak, A.C.; Slaney, M. Principles of Computerized Tomographic Imaging; IEEE Press: Piscataway, NJ, USA, 2001. [Google Scholar]
- Koch, E.; Hammer, D.; Wang, S.; Cuevas, M.; Walther, J. Resonant Doppler Imaging with Common Path OCT. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 14–18 June 2009; Volume 7372, p. 737220. [Google Scholar]
- Choma, M.A.; Sarunic, M.V.; Yang, C.; Izatt, J.A. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 2003, 11, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Povazay, B.; Bizheva, K.; Unterhuber, A.; Hermann, B.; Sattmann, H.; Fercher, A.F.; Drexler, W.; Apolonski, A.; Wadsworth, W.J.; Knight, J.C.; et al. Submicrometer axial resolution optical coherence tomography. Opt. Lett. 2002, 27, 1800–1802. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Hitzenberger, C.K.; Fercher, A.F. Performance of fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Bruschini, C.; Charbon, E. CMOS-based single-photon detectors: Technology and applications. In Proceedings of the 23rd Opto-Electronics and Communications Conference (OECC 2018), Cheju, Republic of Korea, 2–6 July 2018; pp. 1–2. [Google Scholar] [CrossRef]
- Voitsekhovskii, A.; Kokhanenko, A.; Korotaev, A.; Nesmelov, S. Photoelectric characteristics of PtSi-Si Schottky barrier with heavily boron-doped nanolayer. Proc. SPIE 2001, 4413, 387–390. [Google Scholar] [CrossRef]
- Hadfield, R.H. Single-photon detectors for optical quantum information applications. Nat. Photonics 2009, 3, 696–705. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Tang, B.; Wang, W.; Li, Z. High-Performance Waveguide-Integrated Ge/Si Avalanche Photodetector with Lateral Multiplication Region. Micromachines 2022, 13, 649. [Google Scholar] [CrossRef]
- Thorburn, F.E.; Huddleston, L.L.; Kirdoda, J.; Millar, R.W.; Ferre-Llin, L.; Yi, X.; Paul, D.J.; Buller, G.S. High efficiency planar geometry germanium-on-silicon single-photon avalanche diode detectors. In Advanced Photon Counting Techniques XIV; SPIE: Bellingham, WA, USA, 2020; Volume 11386, pp. 28–36. [Google Scholar]
- Ceccarelli, F.; Acconcia, G.; Gulinatti, A.; Ghioni, M.; Rech, I.; Osellame, R. Recent Advances and Future Perspectives of Single-Photon Avalanche Diodes for Quantum Photonics Applications. Adv. Quantum Technol. 2021, 4, 2000102. [Google Scholar] [CrossRef]
- Douhan, R.; Lozovoy, K.; Kokhanenko, A.; Deeb, H.; Dirko, V.; Khomyakova, K. Recent Advances in Si-Compatible Nanostructured Photodetectors. Technologies 2023, 11, 17. [Google Scholar] [CrossRef]
- Shin, D.; Park, B.; Chae, Y.; Yun, I. The effect of a deep virtual guard ring on the device characteristics of silicon single photon avalanche diodes. IEEE Trans. Electron Devices 2019, 66, 2986–2991. [Google Scholar] [CrossRef]
- Richardson, J.A.; Webster, E.A.G.; Grant, L.A.; Henderson, R.K. Scaleable single-photon avalanche diode structures in nanometer CMOS technology. IEEE Trans. Electron Devices 2011, 58, 2028–2035. [Google Scholar] [CrossRef]
- Finkelstein, H.; Hsu, M.J.; Esener, S.C. STI-bounded single-photon avalanche diode in a deep-submicrometer CMOS technology. IEEE Electron Device Lett. 2006, 27, 887–889. [Google Scholar] [CrossRef]
- Leitner, T.; Feiningstein, A.; Turchetta, R.; Coath, R.; Chick, S.; Visokolov, G.; Savuskan, V.; Javitt, M.; Gal, L.; Brouk, I.; et al. Measurements and simulations of low dark count rate single photon avalanche diode device in a low voltage 180-nm CMOS image sensor technology. IEEE Trans. Electron Devices 2013, 60, 1982–1988. [Google Scholar] [CrossRef]
- Malass, I.; Uhring, W.; Le Normand, J.-P.; Dumas, N.; Dadouche, F. A single photon avalanche detector in a 180 nm standard CMOS technology. In Proceedings of the 2015 IEEE 13th International New Circuits and Systems Conference (NEWCAS), Grenoble, France, 7–10 June 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Accarino, C.; Al-Rawhani, M.; Shah, Y.D.; Maneuski, D. Low noise and high photodetection probability SPAD in 180 nm standard CMOS technology. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; He, Y.; Huang, M.; Yang, H.; Wang, G.; Yang, Z.; Yuan, J. The design and characterization of high photon detection efficiency CMOS single-photon avalanche diode. Mod. Phys. Lett. B 2018, 32, 1850302. [Google Scholar] [CrossRef]
- Michel, J.; Liu, J.; Kimerling, L.C. High-performance Ge-on-Si photodetectors. Nat. Photonics 2010, 4, 527–534. [Google Scholar] [CrossRef]
- Lozovoy, K.A.; Voitsekhovskiy, A.V.; Kokhanenko, A.P.; Satdarov, V.G.; Pchelyakov, O.P.; Nikiforov, A.I. Heterostructures with self-organized quantum dots of Ge on Si for optoelectronic devices. Opto-Electron. Rev. 2014, 22, 171–177. [Google Scholar] [CrossRef]
- Virot, L.; Crozat, P.; Fedeli, J.-M.; Hartmann, J.-M.; Marris-Morini, D.; Cassan, E.; Boeuf, F.; Vivien, L. Germanium avalanche receiver for low power interconnects. Nat. Commun. 2014, 5, 4957. [Google Scholar] [CrossRef]
- Huang, M.; Li, S.; Cai, P.; Hou, G.; Su, T.-I.; Chen, W.; Hong, C.; Pan, D. Germanium on silicon avalanche photodiode. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 3800911. [Google Scholar] [CrossRef]
- Sood, A.; Zeller, J.; Ghuman, P.; Babu, S.; Dupuis, R.; Efstathiadis, H. Development of high performance ultraviolet and near-infrared detector technologies. In Infrared Sensors, Devices, and Applications VIII; SPIE: Bellingham, WA, USA, 2018; Volume 10766, pp. 47–55. [Google Scholar]
- Izhnin, I.I.; Fitsych, O.I.; Voitsekhovskii, A.V.; Kokhanenko, A.P.; Lozovoy, K.A.; Dirko, V.V. Nanostructures with Ge–Si quantum dots for infrared photodetectors. Opto-Electron. Rev. 2018, 26, 195–200. [Google Scholar] [CrossRef]
- Hall, D.; Liu, Y.-H.; Lo, Y.-H. Single photon avalanche detectors: Prospects of new quenching and gain mechanisms. Nanophotonics 2015, 4, 397–412. [Google Scholar] [CrossRef]
- Loudon, A.Y.; Hiskett, P.A.; Buller, G.S.; Carline, R.T.; Herbert, D.C.; Leong, W.Y.; Rarity, J.G. Enhancement of the infrared detection efficiency of silicon photon-counting avalanche photodiodes by use of silicon germanium absorbing layers. Opt. Lett. 2002, 27, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Kang, Y.; Hu, C.; Zhou, Q.; Liu, H.-D.; Campbell, J.C. Geiger-mode operation of Ge-on-Si avalanche photodiodes. IEEE J. Quantum Electron. 2011, 47, 731–735. [Google Scholar] [CrossRef]
- Warburton, R.E.; Intermite, G.; Myronov, M.; Allred, P.; Lealey, D.R.; Gallacher, K.; Paul, D.J.; Pilgrim, N.J.; Lever, L.J.M.; Ikonic, Z.; et al. Ge-on-Si single-photon avalanche diode detectors: Design, modeling, fabrication, and characterization at wavelengths 1310 and 1550 nm. IEEE Trans. Electron Devices 2013, 60, 3807–3813. [Google Scholar] [CrossRef]
- Vines, P.; Kuzmenko, K.; Kirdoda, J.; Dumas, D.C.S.; Mirza, M.M.; Millar, R.W.; Paul, D.J.; Buller, G.S. High performance planar germanium-on-silicon single-photon avalanche diode detectors. Nat. Commun. 2019, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kirdoda, J.; Dumas, D.C.S.; Millar, R.W.; Mirza, M.M.; Paul, D.J.; Kuzmenko, K.; Vines, P.; Greener, Z.; Buller, G.S. Geiger mode Ge-on-Si single-photon avalanche diode detectors. In Proceedings of the 2019 IEEE 16th International Conference on Group IV Photonics (GFP), Singapore, 28–30 August 2019; pp. 1–2. [Google Scholar] [CrossRef]
- Chen, H.T.; Verbist, J.; Verheyen, P.; De Heyn, P.; Lepage, G.; De Coster, J.; Absil, P.; Yin, X.; Bauwelinck, J.; Van Campenhout, J.; et al. High sensitivity 10 Gbp/s Si photonic receiver based on a low-voltage waveguide-coupled Ge avalanche photodetector. Opt. Express 2015, 23, 815–822. [Google Scholar] [CrossRef]
- Martinez, N.J.D.; Gehl, M.; Derose, C.T.; Starbuck, A.L.; Pomerene, A.T.; Lentine, A.L.; Trotter, D.C.; Davids, P.S. Single photon detection in a waveguide-coupled Ge-on-Si lateral avalanche photodiode. Opt. Express 2017, 25, 16130–16139. [Google Scholar] [CrossRef] [PubMed]
- Soref, R.A.; De Leonardis, F.; Passaro, V.M.N. Simulations of nanoscale room temperature waveguide-coupled single-photon avalanche detectors for silicon photonic sensing and quantum applications. Appl. Nano Mater. 2019, 2, 7503–7512. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, Z.; Wang, B.; Liang, D.; Fiorentino, M.; Beausoleil, R.G. Silicon–germanium avalanche photodiodes with direct control of electric field in charge multiplication region. Optica 2019, 6, 772–777. [Google Scholar] [CrossRef]
- Srinivasan, S.A.; Berciano, M.; De Heyn, P.; Lardenois, S.; Pantouvaki, M.; Van Campenhout, J. 27 GHz Silicon-contacted waveguide-coupled Ge/Si avalanche photodiode. J. Light. Technol. 2020, 38, 3044–3050. [Google Scholar] [CrossRef]
- Stepina, N.P.; Val’kovskii, V.V.; Dvurechenskii, A.V.; Nikiforov, A.I.; Moers, J.; Gruetzmacher, D. Mesoscopic structures with Ge quantum dots in Si for single-photon detectors. Optoelectron. Instrum. Data Process. 2014, 50, 266–270. [Google Scholar] [CrossRef]
- Ali, D.; Richardson, C.J.K. Strain-balanced Si/SiGe type-II superlattices for near-infrared photodetection. Appl. Phys. Lett. 2014, 105, 031116. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, M.; Yu, Z.; Jiang, X.; Huo, Y.; Zang, K.; Zhang, J.; Harris, J.S.; Jin, G.; Zhang, Q.; et al. Simulation of a high-efficiency and low-jitter nanostructured silicon single-photon avalanche diode. Optica 2015, 2, 974–979. [Google Scholar] [CrossRef]
- Zang, K.; Jiang, X.; Huo, Y.; Ding, X.; Morea, M.; Chen, X.; Lu, C.-Y.; Ma, J.; Zhou, M.; Xia, Z.; et al. Silicon single-photon avalanche diodes with nanostructured light trapping. Nat. Commun. 2017, 8, 628. [Google Scholar] [CrossRef]
- Yanikgonul, S.; Leong, V.; Ong, J.R.; Png, C.E.; Krivitsky, L. 2D Monte Carlo simulation of silicon waveguide-based single-photon avalanche diode for visible wavelengths. Opt. Express 2018, 26, 15232–15246. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Liu, D.; Lin, X.; Wang, W.; Ding, Z.; Wang, Z.; Cheng, B.; Xue, C. GeSn on Si avalanche photodiodes for short wave infrared detection. In Optical Sensing and Imaging Technologies and Applications; SPIE: Bellingham, WA, USA, 2018; Volume 10846, pp. 327–334. [Google Scholar]
- Hamamatsu Photonics. Available online: https://www.hamamatsu.com/eu/en.html (accessed on 1 December 2023).
- OSI Optoelectronics. Available online: https://www.osioptoelectronics.com/ (accessed on 1 December 2023).
- Ushio America, Inc. Available online: https://www.ushio.com/ (accessed on 1 December 2023).
- AMS Technologies. Available online: https://www.amstechnologies-webshop.com/ (accessed on 1 December 2023).
- Excelitas. Available online: https://www.excelitas.com/ (accessed on 1 December 2023).
- Micro Photon Devices. Available online: http://www.micro-photon-devices.com/home (accessed on 1 December 2023).
- Onsemi. Available online: https://www.onsemi.com/ (accessed on 1 December 2023).
- Sony Semiconductor Solutions Group. Available online: https://www.sony-semicon.com/en/index.html (accessed on 1 December 2023).
- STMicroelectronics. Available online: https://www.st.com/content/st_com/en.html (accessed on 1 December 2023).
- Advanced Photonix. Available online: https://www.advancedphotonix.com/ (accessed on 1 December 2023).
- Palubiak, D.P.; Deen, M.J. CMOS SPADs: Design Issues and Research Challenges for Detectors, Circuits, and Arrays. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 6000718. [Google Scholar] [CrossRef]
- Lee, M.-J.; Choi, W.-Y. Performance Optimization and Improvement of Silicon Avalanche Photodetectors in Standard CMOS Technology. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 3801013. [Google Scholar] [CrossRef]
- Pavia, J.M.; Wolf, M.; Charbon, E. Single-Photon Avalanche Diode Imagers Applied to Near-Infrared Imaging. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 3800908. [Google Scholar]
- Liu, C.; Ye, H.-F.; Shi, Y.-L. Advances in near-infrared avalanche diode single-photon detectors. Chip 2022, 1, 5. [Google Scholar] [CrossRef]
- Miao, Y.; Lin, H.; Li, B.; Dong, T.; He, C.; Du, J.; Zhao, X.; Zhou, Z.; Su, J.; Wang, H.; et al. Review of Ge(GeSn) and InGaAs Avalanche Diodes Operating in the SWIR Spectral Region. Nanomaterials 2023, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, M.; Gasparini, L.; Perenzoni, M.; Pancheri, L. High Dynamic Range Imaging with TDC-Based CMOS SPAD Arrays. Instruments 2019, 3, 38. [Google Scholar] [CrossRef]
- Chen, D.; March, S.D.; Jones, A.H.; Shen, Y.; Dadey, A.A.; Sun, K.; McArthur, J.A.; Skipper, A.M.; Xue, X.; Guo, B.; et al. Photon-trapping-enhanced avalanche photodiodes for mid-infrared applications. Nat. Photonics 2023, 17, 594–600. [Google Scholar] [CrossRef]
- Vinke, R.; Lohner, H.; Schaart, D.R.; van Dam, H.T.; Seifert, S.; Beekman, F.J.; Dendooven, P. Optimizing the timing resolution of SiPM sensors for use in TOF-PET detectors. Nucl. Instrum. Methods Phys. Res. A 2009, 610, 188–191. [Google Scholar] [CrossRef]
- Naseem; Ahmad, Z.; Liao, Y.-M.; Chao, R.-L.; Wang, P.-S.; Lee, Y.-S.; Yang, S.; Wang, S.-Y.; Chang, H.-S.; Chen, H.-S.; et al. Avalanche Photodiodes with Dual Multiplication Layers for High-Speed and Wide Dynamic Range Performances. Photonics 2021, 8, 98. [Google Scholar] [CrossRef]
- Isidori, T.; McCavana, P.; McClean, B.; Minafra, N.; Raab, N.; Rock, L.; Royon, C. Performance of a low gain avalanche detector in a medical linac and characterisation of the beam profile. Phys. Med. Biol. 2021, 66, 135002. [Google Scholar] [CrossRef]
- Puszka, A.; Di Sieno, L.; Mora, A.D.; Pifferi, A.; Contini, D.; Planat-Chretien, A.; Koenig, A.; Boso, G.; Tosi, A.; Herve, L.; et al. Spatial resolution in depth for time-resolved diffuse optical tomography using short source-detector separations. Biomed. Opt. Express 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Nam, J.H.; Buttafava, M.; Villa, F.; Velten, A.; Tosi, A. Fast-Gated 16 × 1 SPAD Array for Non-Line-of-Sight Imaging Applications. Instruments 2020, 4, 14. [Google Scholar] [CrossRef]
- Britvitch, I.; Johnson, I.; Renker, D.; Stoykov, A.; Lorenz, E. Characterisation of Geiger-mode avalanche photodiodes for medical imaging applications. Nucl. Instrum. Methods Phys. Res. A 2007, 571, 308–311. [Google Scholar] [CrossRef]
- Zaidi, H. Future Trends in Nuclear Medical Imaging. In Recent Advances in Multidisciplinary Applied Physics; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2005; pp. 657–664. [Google Scholar]
- Bisogni, M.G.; Del Guerra, A.; Belcari, N. Medical applications of silicon photomultipliers. Nucl. Instrum. Methods Phys. Res. A 2019, 926, 118–128. [Google Scholar] [CrossRef]
- Aull, B.F.; Duerr, E.K.; Frechette, J.P.; McIntosh, K.A.; Schuette, D.R.; Younger, R.D. Large-Format Geiger-Mode Avalanche Photodiode Arrays and Readout Circuits. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 3800510. [Google Scholar] [CrossRef]
- Moses, W.W. Photodetectors for nuclear medical imaging. Nucl. Instrum. Methods Phys. Res. A 2009, 610, 11–15. [Google Scholar] [CrossRef]
- Yokota, H.; Fukasawa, A.; Hirano, M.; Ide, T. Low-Light Photodetectors for Fluorescence Microscopy. Appl. Sci. 2021, 11, 2773. [Google Scholar] [CrossRef]
- Ota, Y.; Morimoto, K.; Sasago, T.; Shinohara, M.; Kuroda, Y.; Endo, W.; Maehashi, Y.; Maekawa, S.; Tsuchiya, H.; Abdelahafar, A.; et al. A 0.37W 143dB-Dynamic-Range 1Mpixel Backside-Illuminated Charge-Focusing SPAD Image Sensor with Pixel-Wise Exposure Control and Adaptive Clocked Recharging. In Proceedings of the IEEE International Solid-State Circuits Conference (ISSCC), San Francisco, CA, USA, 20–26 February 2022; pp. 94–96. [Google Scholar] [CrossRef]
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting X-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef]
- Yoon, J. Hyperspectral Imaging for Clinical Applications. BioChip J. 2022, 16, 1–12. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Cai, S.; Han, Z.; Liu, X.; Wang, F.; Cao, Y.; Wang, Z.; Li, H.; Chen, Y.; et al. Flexible Hybrid Electronics for Digital Healthcare. Adv. Mater. 2020, 32, 1902062. [Google Scholar] [CrossRef]
- Yu, Y.; Nyein, H.Y.Y.; Gao, W.; Javey, A. Flexible Electrochemical Bioelectronics: The Rise of In Situ Bioanalysis. Adv. Mater. 2020, 32, 1902083. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Liu, Y. Wearable Electronics Based on Stretchable Organic Semiconductors. Small 2023, 19, 202206309. [Google Scholar] [CrossRef]
- Kim, K.; Kim, B.; Lee, C.H. Printing Flexible and Hybrid Electronics for Human Skin and Eye-Interfaced Health Monitoring Systems. Adv. Mater. 2020, 32, 1902051. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, L.; Yeo, J.C.; Lim, C.T. Flexible Hybrid Sensors for Health Monitoring: Materials and Mechanisms to Render Wearability. Adv. Mater. 2020, 32, 1902133. [Google Scholar] [CrossRef] [PubMed]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Pumera, M. Wearable sensors for telehealth based on emerging materials and nanoarchitectonics. NPJ Flex. Electron. 2023, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun, Y.; Wu, W.; Huang, K.; Liang, Y.; Yan, M.; Zeng, H. High-speed photon-number-resolving detection via a GHz-gated SiPM. Opt. Express 2022, 30, 7501–7510. [Google Scholar] [CrossRef] [PubMed]
- Nehra, R.; Chang, C.-H.; Yu, Q.; Beling, A.; Pfister, O. Photon-number-resolving segmented detectors based on single-photon avalanche-photodiodes. Opt. Express 2020, 28, 3660–3675. [Google Scholar] [CrossRef] [PubMed]
- Slenders, E.; Castello, M.; Buttafava, M.; Villa, F.; Tosi, A.; Lanzano, L.; Koho, S.V.; Vicidomini, G. Confocal-based fluorescence fluctuation spectroscopy with a SPAD array detector. Light Sci. Appl. 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Kitsmiller, V.J.; O’Sullivan, T.D. Next-generation frequency domain diffuse optical imaging systems using silicon photomultipliers. Opt. Lett. 2019, 44, 562–565. [Google Scholar] [CrossRef]
- Shapiro, J.H. Computational ghost imaging. Physical Review A 2008, 78, 061802. [Google Scholar] [CrossRef]
- Erkmen, B.I.; Shapiro, J.H. Ghost imaging: From quantum to classical to computational. Adv. Opt. Photonics 2010, 2, 405–450. [Google Scholar] [CrossRef]
- Padgett, M.J.; Boyd, R.W. An introduction to ghost imaging: Quantum and classical. Philos. Trans. R. Soc. A 2017, 375, 20160233. [Google Scholar] [CrossRef]
- Gariepy, G.; Krstajic, N.; Henderson, R.; Li, C.; Thomson, R.R.; Buller, G.S.; Heshmat, B.; Raskar, R.; Leach, J.; Faccio, D. Single-photon sensitive light-in-flight imaging. Nat. Commun. 2015, 6, 6021. [Google Scholar] [CrossRef] [PubMed]
- Deeb, H.; Khomykova, K.; Kokhanenko, A.; Douhan, R.; Lozovoy, K. Dependence of Ge/Si Avalanche Photodiode Performance on the Thickness and Doping Concentration of the Multiplication and Absorption Layers. Inorganics 2023, 11, 303. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y.; Fang, J.; Kang, W.; Sun, Y.; Liang, Y.; Hao, Q.; Yan, M.; Zeng, H. Mid-infrared photon counting and resolving via efficient frequency upconversion. Photonics Res. 2021, 9, 259–265. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, K.; Fang, J.; Yan, M.; Wu, E.; Zeng, H. Mid-infrared single-pixel imaging at the single-photon level. Nat. Commun. 2023, 14, 1073. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Y.; Yan, M.; Wu, E.; Huang, K.; Zeng, H. Highly sensitive detection of infrared photons by nondegenerate two-photon absorption under midinfrared pumping. Phys. Rev. Appl. 2020, 14, 064035. [Google Scholar] [CrossRef]
- Liang, Y.; Fei, Q.; Liu, Z.; Huang, K.; Zeng, H. Low-noise InGaAs/InP single-photon detector with widely tunable repetition rates. Photonics Res. 2019, 7, A1–A6. [Google Scholar] [CrossRef]
- Zhang, J.; Itzler, M.A.; Zbunden, H.; Pan, J.-W. Advances in InGaAs/InP single-photon detector systems for quantum communication. Light Sci. Appl. 2015, 4, e286. [Google Scholar] [CrossRef]
- Rizvi, S.; Cao, J.; Zhang, K.; Hao, Q. DeepGhost: Real-time computational ghost imaging via deep learning. Sci. Rep. 2020, 10, 11400. [Google Scholar] [CrossRef] [PubMed]
- Morales-Curiel, L.F.; Gonzalez, A.C.; Castro-Olvera, G.; Lin, L.-C.; El-Quessny, M.; Porta-de-la-Riva, M.; Severino, J.; Morera, L.B.; Venturini, V.; Ruprecht, V.; et al. Volumetric imaging of fast cellular dynamics with deep learning enhanced bioluminescence microscopy. Commun. Biol. 2022, 5, 1330. [Google Scholar] [CrossRef]
- Jacques, S.; Christe, B. Introduction to Clinical Engineering; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Balas, V.; Jha, S.; Kumar, R.; Son, L.H.; Khari, M. Internet of Things in Biomedical Engineering; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Terreno, E.; Uggeri, F.; Aime, S. Image guided therapy: The advent of theranostic agents. J. Control. Release 2012, 161, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, N. An overview of recent advances in quantum dots for biomedical applications. Colloids Surf. B 2014, 124, 118–131. [Google Scholar] [CrossRef]
- Lee, J.S.; Youn, Y.H.; Kwon, I.K.; Ko, N.R. Recent advances in quantum dots for biomedical applications. J. Pharm. Investig. 2018, 48, 209–214. [Google Scholar] [CrossRef]
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of Nanoparticles in Biomedical Imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Wang, Q.; Wu, Z.; Zhang, X.; Li, B.; Lin, L. Recent advances in quantum dots-based biosensors for antibiotics detection. J. Pharm. Anal. 2022, 12, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, N.; Chan, V. Recent Advances in Silicon Quantum Dot-Based Fluorescent Biosensors. Biosensors 2023, 13, 311. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.-D.; Gu, X.; Ming, D. Photodetectors based on two dimensional materials for biomedical application. Biosens. Bioelectron. 2019, 143, 111617. [Google Scholar] [CrossRef] [PubMed]
- Izhnin, I.I.; Kurbanov, K.R.; Lozovoy, K.A.; Kokhanenko, A.P.; Dirko, V.V.; Voitsekhovskii, A.V. Epitaxial fabrication of 2D materials of group IV elements. Appl. Nanosci. 2020, 10, 4375–4383. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpinska, D.; Dybko, A.; Chudy, M.; Jastrzebska, A.M.; Olszyna, A.; Brzozka, Z. Future Applications of MXenes in Biotechnology, Nanomedicine, and Sensors. Trends Biotechnol. 2020, 38, 264–279. [Google Scholar] [CrossRef]
- Huang, H.; Dong, C.; Feng, W.; Wang, Y.; Huang, B.; Chen, Y. Biomedical engineering of two-dimensional MXenes. Adv. Drug Deliv. Rev. 2022, 184, 114178. [Google Scholar] [CrossRef] [PubMed]
- Lozovoy, K.A.; Izhnin, I.I.; Kokhanenko, A.P.; Dirko, V.V.; Vinarskiy, V.P.; Voitsekhovskii, A.V.; Fitsych, O.I.; Akimenko, N.Y. Single-element 2D materials beyond graphene: Methods of epitaxial synthesis. Nanomaterials 2022, 12, 2221. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Rao, S.; Liu, R.; Wang, L.; Chen, W.; Tao, W.; Kong, N. 2D materials-based nanomedicine: From discovery to applications. Adv. Drug Deliv. Rev. 2022, 185, 114268. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Du, T.; Chang, Z.; Yin, C.; Cheng, Y. On the interface between biomaterials and two-dimensional materials for biomedical applications. Adv. Drug Deliv. Rev. 2022, 186, 114314. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozovoy, K.A.; Douhan, R.M.H.; Dirko, V.V.; Deeb, H.; Khomyakova, K.I.; Kukenov, O.I.; Sokolov, A.S.; Akimenko, N.Y.; Kokhanenko, A.P. Silicon-Based Avalanche Photodiodes: Advancements and Applications in Medical Imaging. Nanomaterials 2023, 13, 3078. https://doi.org/10.3390/nano13233078
Lozovoy KA, Douhan RMH, Dirko VV, Deeb H, Khomyakova KI, Kukenov OI, Sokolov AS, Akimenko NY, Kokhanenko AP. Silicon-Based Avalanche Photodiodes: Advancements and Applications in Medical Imaging. Nanomaterials. 2023; 13(23):3078. https://doi.org/10.3390/nano13233078
Chicago/Turabian StyleLozovoy, Kirill A., Rahaf M. H. Douhan, Vladimir V. Dirko, Hazem Deeb, Kristina I. Khomyakova, Olzhas I. Kukenov, Arseniy S. Sokolov, Nataliya Yu. Akimenko, and Andrey P. Kokhanenko. 2023. "Silicon-Based Avalanche Photodiodes: Advancements and Applications in Medical Imaging" Nanomaterials 13, no. 23: 3078. https://doi.org/10.3390/nano13233078