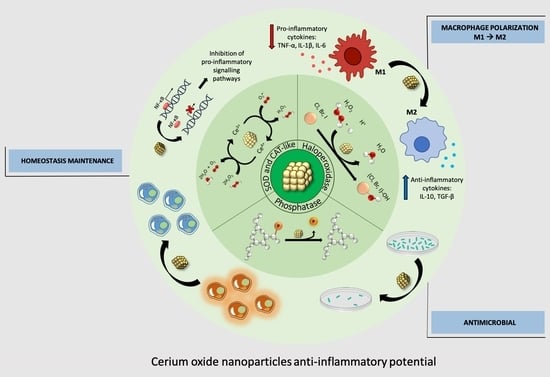
The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More than Redox?
Abstract
:1. Introduction
2. Anti-Inflammatory Application of Cerium Oxide Nanoparticles
2.1. Neurodegenerative Diseases
2.2. Inflammatory Autoimmune Diseases
2.3. Liver Inflammation
2.4. Gastrointestinal Inflammatory Disorders
2.5. Ocular Inflammation
2.6. Pathogen-Induced Inflammation
2.7. Inflammation in Tissue-Engineering-Treated Traumatic Injuries: Premises and Promises of CNPs
3. CNPs: Nanotoxicology vs. Nanomedicine
4. Conclusions: Anti-Inflammatory = Antioxidant?
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gómez de las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A Key Player in the Inflammatory Response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Biophys. Acta—Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Cristofanon, S.; Nuccitelli, S.; D’Alessio, M.; Dicato, M.; Diederich, M.; Ghibelli, L. Oxidation-Dependent Maturation and Survival of Explanted Blood Monocytes via Bcl-2 up-Regulation. Biochem. Pharmacol. 2008, 76, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Vegliante, R.; Ghibelli, L. Redox Modulation of the DNA Damage Response. Biochem. Pharmacol. 2012, 84, 1292–1306. [Google Scholar] [CrossRef]
- Griffith, B.; Pendyala, S.; Hecker, L.; Lee, P.J.; Natarajan, V.; Thannickal, V.J. NOX Enzymes and Pulmonary Disease. Antioxid. Redox Signal 2009, 11, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Hu, S.; Qiao, C.; Yuan, Z.; Li, M.I.N.; Ye, J.; Ma, H.; Wang, J.; Xin, S.; Zhang, J. Therapy with High-Dose Long-Term Antioxidant Free Radicals for Severe Paraquat Poisoning: A Pilot Study. Exp. Ther. Med. 2018, 16, 5149–5155. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food Macromolecule Based Nanodelivery Systems for Enhancing the Bioavailability of Polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Caputo, F.; De Nicola, M.; Ghibelli, L. Pharmacological Potential of Bioactive Engineered Nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological Potential of Cerium Oxide Nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The Role of Cerium Redox State in the SOD Mimetic Activity of Nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef]
- Dowding, J.M.; Seal, S.; Self, W.T. Cerium Oxide Nanoparticles Accelerate the Decay of Peroxynitrite (ONOO-). Drug Deliv. Transl. Res. 2013, 3, 375–379. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria Exhibit Redox State-Dependent Catalase Mimetic Activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium Oxide Nanoparticles Protect Cardiac Progenitor Cells from Oxidative Stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; De Nicola, M.; Mandoli, C.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Ce3+ Ions Determine Redox-Dependent Anti-Apoptotic Effect of Cerium Oxide Nanoparticles. ACS Nano 2011, 5, 4537–4549. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; De Nicola, M.; Sienkiewicz, A.; Giovanetti, A.; Bejarano, I.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium Oxide Nanoparticles, Combining Antioxidant and UV Shielding Properties, Prevent UV-Induced Cell Damage and Mutagenesis. Nanoscale 2015, 7, 15643–15656. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Di Meo, E.; Lulli, D.; Deidda Tarquini, G.; Capradossi, F.; Bruni, E.; Pelliccia, A.; Traversa, E.; Dellambra, E.; Failla, C.M.; et al. Safe-Shields: Basal and Anti-UV Protection of Human Keratinocytes by Redox-Active Cerium Oxide Nanoparticles Prevents UVB-Induced Mutagenesis. Antioxidants 2023, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic Strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-Inflammatory Properties of Cerium Oxide Nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar] [CrossRef]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Review: Alzheimer’s Amyloid β-Peptide-Associated Free Radical Oxidative Stress and Neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Bartley, M.G.; Marquardt, K.; Kirchhof, D.; Wilkins, H.M.; Patterson, D.; Linseman, D.A. Overexpression of Amyloid-β Protein Precursor Induces Mitochondrial Oxidative Stress and Activates the Intrinsic Apoptotic Cascade. J. Alzheimer’s Dis. 2012, 28, 855–868. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, B.; Santucci, S.; Benedetti, E.; Di Loreto, S.; Phani, R.; Falone, S.; Amicarelli, F.; Ceru, M.; Cimini, A. Cerium Oxide Nanoparticles Trigger Neuronal Survival in a Human Alzheimer Disease Model By Modulating BDNF Pathway. Curr. Nanosci. 2009, 5, 167–176. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimeŕs Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef]
- Jia, J.; Li, C.; Zhang, T.; Sun, J.; Peng, S.; Xie, Q.; Huang, Y.; Yi, L. CeO2@PAA-LXW7 Attenuates LPS-Induced Inflammation in BV2 Microglia. Cell. Mol. Neurobiol. 2019, 39, 1125–1137. [Google Scholar] [CrossRef]
- MacHhi, J.; Yeapuri, P.; Markovic, M.; Patel, M.; Yan, W.; Lu, Y.; Cohen, J.D.; Hasan, M.; Abdelmoaty, M.M.; Zhou, Y.; et al. Europium-Doped Cerium Oxide Nanoparticles for Microglial Amyloid Beta Clearance and Homeostasis. ACS Chem. Neurosci. 2022, 13, 1232–1244. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Zhu, Y.F.; Chang, H.F.; Liang, Y. Nanoceria Restrains PM2.5-Induced Metabolic Disorder and Hypothalamus Inflammation by Inhibition of Astrocytes Activation Related NF-ΚB Pathway in Nrf2 Deficient Mice. Free Radic. Biol. Med. 2016, 99, 259–272. [Google Scholar] [CrossRef] [PubMed]
- DeCoteau, W.; Heckman, K.L.; Estevez, A.Y.; Reed, K.J.; Costanzo, W.; Sandford, D.; Studlack, P.; Clauss, J.; Nichols, E.; Lipps, J.; et al. Cerium Oxide Nanoparticles with Antioxidant Properties Ameliorate Strength and Prolong Life in Mouse Model of Amyotrophic Lateral Sclerosis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic Stress and Oxidative Stress as Common Factors of the Pathogenesis of Depression and Alzheimer’s Disease; the Role of Antioxidants in Prevention and Treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Zavvari, F.; Nahavandi, A.; Shahbazi, A. Neuroprotective Effects of Cerium Oxide Nanoparticles on Experimental Stress-Induced Depression in Male Rats. J. Chem. Neuroanat. 2020, 106, 101799. [Google Scholar] [CrossRef]
- Youn, D.H.; Tran, N.M.; Kim, B.J.; Kim, Y.; Jeon, J.P.; Yoo, H. Shape Effect of Cerium Oxide Nanoparticles on Mild Traumatic Brain Injury. Sci. Rep. 2021, 11, 15571. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Chung, S.J.; Nafiujjaman, M.; Hill, M.L.; Siziba, M.E.; Contag, C.H.; Kim, T. Ceria-Based Nanotheranostic Agent for Rheumatoid Arthritis. Theranostics 2020, 10, 11863–11880. [Google Scholar] [CrossRef]
- Kim, J.; Hong, G.; Mazaleuskaya, L.; Hsu, J.C.; Rosario-Berrios, D.N.; Grosser, T.; Cho-Park, P.F.; Cormode, D.P. Ultrasmall Antioxidant Cerium Oxide Nanoparticles for Regulation of Acute Inflammation. ACS Appl. Mater. Interfaces 2021, 13, 60852–60864. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Y.; Zhu, S.; Tong, Y.; Ji, L.; Zhang, W.; Zhang, Q.; Bi, Q. The Application Prospect of Metal/Metal Oxide Nanoparticles in the Treatment of Osteoarthritis. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Fang, C.H.; Meng, F.Q.; Ke, C.J.; Lin, F.H. Hyaluronic Acid Loaded with Cerium Oxide Nanoparticles as Antioxidant in Hydrogen Peroxide Induced Chondrocytes Injury: An in Vitro Osteoarthritis Model. Molecules 2020, 25, 4407. [Google Scholar] [CrossRef]
- Xiong, L.; Bao, H.; Li, S.; Gu, D.; Li, Y.; Yin, Q.; Li, W.; Miao, L.; Liu, C. Cerium Oxide Nanoparticles Protect against Chondrocytes and Cartilage Explants from Oxidative Stress via Nrf2/HO-1 Pathway in Temporomandibular Joint Osteoarthritis. Front. Bioeng. Biotechnol. 2023, 11, 1076240. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Oksenberg, J.R. The Neurobiology of Multiple Sclerosis: Genes, Inflammation, and Neurodegeneration. Neuron 2006, 52, 61–76. [Google Scholar] [CrossRef]
- Eitan, E.; Hutchison, E.R.; Greig, N.H.; Tweedie, D.; Celik, H.; Ghosh, S.; Fishbein, K.W.; Spencer, R.G.; Sasaki, C.Y.; Ghosh, P.; et al. Combination Therapy with Lenalidomide and Nanoceria Ameliorates CNS Autoimmunity. Exp. Neurol. 2015, 273, 151–160. [Google Scholar] [CrossRef]
- Seki, E.; Schwabe, R.F. Hepatic Inflammation and Fibrosis: Functional Links and Key Pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T.; Kondro, M.; Beregova, T.; Bodnar, P.; Shcherbakov, O.; Bubnov, R.; Caprnda, M.; Delev, D.; et al. Cerium Dioxide Nanoparticles Possess Anti-Inflammatory Properties in the Conditions of the Obesity-Associated NAFLD in Rats. Biomed. Pharmacother. 2017, 90, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Vafaei, S.A.; Naseri, N.; Darini, A.; Azandaryani, M.T.; Ara, F.K.; Mirzaei, F. Protective Effects of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease (NAFLD) and Carbon Tetrachloride-Induced Liver Damage in Rats: Study on Intestine and Liver. Metab. Open 2021, 12, 100151. [Google Scholar] [CrossRef]
- Lebda, F.M.; El Agaty, S.M.; Ali, R.H.; Hamam, G.G.; El-Monem, A.M.A.; Lasheen, N.N. Cerium Oxide Nanoparticles Modulate Liver X Receptor and Short Heterodimer Partner, and Attenuate Liver Steatosis and Steatohepatitis in a Rat Model of Postmenopausal Obesity. Gen. Physiol. Biophys. 2022, 41, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver Fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Oró, D.; Yudina, T.; Fernández-Varo, G.; Casals, E.; Reichenbach, V.; Casals, G.; De La Presa, B.G.; Sandalinas, S.; Carvajal, S.; Puntes, V.; et al. Cerium Oxide Nanoparticles Reduce Steatosis, Portal Hypertension and Display Anti-Inflammatory Properties in Rats with Liver Fibrosis. J. Hepatol. 2016, 64, 691–698. [Google Scholar] [CrossRef]
- Godugu, C.; Khurana, A.; Saifi, M.A. Rare Earth Cerium Oxide Nanoparticles Attenuated Liver Fibrosis in Bile Duct Ligation Mice Model. J. Trace Elem. Med. Biol. 2023, 75, 127102. [Google Scholar] [CrossRef] [PubMed]
- Manne, N.D.P.K.; Arvapalli, R.; Graffeo, V.A.; Bandarupalli, V.V.K.; Shokuhfar, T.; Patel, S.; Rice, K.M.; Ginjupalli, G.K.; Blough, E.R. Prophylactic Treatment with Cerium Oxide Nanoparticles Attenuate Hepatic Ischemia Reperfusion Injury in Sprague Dawley Rats. Cell. Physiol. Biochem. 2017, 42, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, F.; Xia, Y.; Dai, W.; Chen, K.; Li, S.; Liu, T.; Zheng, Y.; Wang, J.; Lu, W.; et al. Astaxanthin Pretreatment Attenuates Hepatic Ischemia Reperfusion-Induced Apoptosis and Autophagy via the ROS/MAPK Pathway in Mice. Mar. Drugs 2015, 13, 3368–3387. [Google Scholar] [CrossRef]
- Zengin, A.; Erikçi, A.; Telli, G.; Gümüşel, B.; Kösemehmetoğlu, K.; Uçar, G.; Algın, M.C. Anti-Inflammatory Effects of Oral and Intraperitoneal Administration of Cerium Oxide Nanoparticles on Experimental Hepatic Ischemia-Reperfusion Injury. Turk. J. Surg. 2022, 38, 255–265. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Chan, F.K.; McColl, K. EL Peptic Ulcer Disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Kavitt, R.T.; Lipowska, A.M.; Anyane-Yeboa, A.; Gralnek, I.M. Diagnosis and Treatment of Peptic Ulcer Disease. Am. J. Med. 2019, 132, 447–456. [Google Scholar] [CrossRef]
- Prasad, R.; Davan, R.; Jothi, S.; Phani, A.R.; Raju, D.B. Cerium Oxide Nanoparticles Protects Gastrointestinal Mucosa From Ethanol Induced Gastric Ulcers in In-Vivo Animal Model. Nano Biomed. Eng. 2013, 5, 46–49. [Google Scholar] [CrossRef]
- Golyshkin, D.; Kobyliak, N.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Ostapchenko, L.; Caprnda, M.; Skladany, L.; Opatrilova, R.; Rodrigo, L.; et al. Nanocrystalline Cerium Dioxide Efficacy for Prophylaxis of Erosive and Ulcerative Lesions in the Gastric Mucosa of Rats Induced by Stress. Biomed. Pharmacother. 2016, 84, 1383–1392. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Hashemzadeh, A.; Rahmani, F.; Yaghoubi, A.; Nazari, S.E.; Avan, A.; Mehr, S.M.H.; Soleimanpour, S.; Khazaei, M. Cerium Oxide Nanoparticles Acts as a Novel Therapeutic Agent for Ulcerative Colitis through Anti-Oxidative Mechanism. Life Sci. 2021, 278, 119500. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hancock, M.L.; Grulke, E.A.; Unrine, J.M.; Dozier, A.K.; Graham, U.M. Carboxylic Acids Accelerate Acidic Environment-Mediated Nanoceria Dissolution. Nanotoxicology 2019, 13, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Hancock, M.L.; Grulke, E.A.; Yokel, R.A. Carboxylic Acids and Light Interact to Affect Nanoceria Stability and Dissolution in Acidic Aqueous Environments. Beilstein J. Nanotechnol. 2023, 14, 762–780. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hancock, M.L.; Cherian, B.; Brooks, A.J.; Ensor, M.L.; Vekaria, H.J.; Sullivan, P.G.; Grulke, E.A. Simulated Biological Fluid Exposure Changes Nanoceria’s Surface Properties but Not Its Biological Response. Eur. J. Pharm. Biopharm. 2019, 144, 252–265. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, H.; Shi, H.; Liu, W.; Sahle-Demessie, E. Fates of Au, Ag, ZnO, and CeO2Nanoparticles in Simulated Gastric Fluid Studied Using Single-Particle-Inductively Coupled Plasma-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2020, 31, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.T.; Livi, K.; Arai, Y. Effects of PH and Phosphate on CeO2 Nanoparticle Dissolution. Chemosphere 2015, 119, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative Damage-Induced Inflammation Initiates Age-Related Macular Degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative Stress and Reactive Oxygen Species: A Review of Their Role in Ocular Disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef]
- Aslan, M.; Cort, A.; Yucel, I. Oxidative and Nitrative Stress Markers in Glaucoma. Free Radic. Biol. Med. 2008, 45, 367–376. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Chan, P.S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabesity Res. 2007, 2007, 43603. [Google Scholar] [CrossRef]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare Earth Nanoparticles Prevent Retinal Degeneration Induced by Intracellular Peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; Chen, L.; Seal, S.; McGinnis, J.F. Nanoceria Inhibit Expression of Genes Associated with Inflammation and Angiogenesis in the Retina of Vldlr Null Mice. Exp. Eye Res. 2013, 116, 63–74. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, Y.; Zeng, L.; Li, X.; Chen, H.; Song, H.; Huang, J.; Shi, S. Cytocompatible Cerium Oxide-Mediated Antioxidative Stress in Inhibiting Ocular Inflammation-Associated Corneal Neovascularization. J. Mater. Chem. B 2019, 7, 6759–6769. [Google Scholar] [CrossRef]
- Badia, A.; Duarri, A.; Salas, A.; Rosell, J.; Ramis, J.; Gusta, M.F.; Casals, E.; Zapata, M.A.; Puntes, V.; García-Arumí, J. Repeated Topical Administration of 3 Nm Cerium Oxide Nanoparticles Reverts Disease Atrophic Phenotype and Arrests Neovascular Degeneration in AMD Mouse Models. ACS Nano 2022, 17, 910–926. [Google Scholar] [CrossRef] [PubMed]
- Serebrovska, Z.; Swanson, R.J.; Portnichenko, V.; Shysh, A.; Pavlovich, S.; Tumanovska, L.; Dorovskych, A.; Lysenko, V.; Tertykh, V.; Bolbukh, Y.; et al. Anti-Inflammatory and Antioxidant Effect of Cerium Dioxide Nanoparticles Immobilized on the Surface of Silica Nanoparticles in Rat Experimental Pneumonia. Biomed. Pharmacother. 2017, 92, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA—J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, U.; Wade, R.G.; Gourlay, T. Cytokines in the Systemic Inflammatory Response Syndrome: A Review. HSR Proc. Intensive Care Cardiovasc. Anesth. 2010, 2, 161–175. [Google Scholar] [PubMed]
- Selvaraj, V.; Manne, N.D.P.K.; Arvapalli, R.; Rice, K.M.; Nandyala, G.; Fankenhanel, E.; Blough, E.R. Effect of Cerium Oxide Nanoparticles on Sepsis Induced Mortality and NF-ΚB Signaling in Cultured Macrophages. Nanomedicine 2015, 10, 1275–1288. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.W.; Mozafari, M. Biomedical Applications of Nanoceria: New Roles for an Old Player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef]
- Wang, N.; Zhai, X.; Guan, F.; Zhang, R.; Hou, B.; Duan, J. N-Doped Carbon/CeO2 Composite as a Biomimetic Catalyst for Antibacterial Application. Int. J. Mol. Sci. 2023, 24, 2445. [Google Scholar] [CrossRef]
- Jegel, O.; Pfitzner, F.; Gazanis, A.; Oberländer, J.; Pütz, E.; Lange, M.; Von Der Au, M.; Meermann, B.; Mailänder, V.; Klasen, A.; et al. Transparent Polycarbonate Coated with CeO2nanozymes Repel: Pseudomonas Aeruginosa PA14 Biofilms. Nanoscale 2022, 14, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Opitz, P.; Jegel, O.; Nasir, J.; Rios-Studer, T.; Gazanis, A.; Pham, D.H.; Domke, K.; Heermann, R.; Schmedt auf der Günne, J.; Tremel, W. Defect-Controlled Halogenating Properties of Lanthanide-Doped Ceria Nanozymes. Nanoscale 2022, 14, 4740–4752. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Nepal, N.; Rogers, S.; Manne, N.D.P.K.; Arvapalli, R.; Rice, K.M.; Asano, S.; Fankhanel, E.; Ma, J.J.; Shokuhfar, T.; et al. Inhibition of MAP Kinase/NF-KB Mediated Signaling and Attenuation of Lipopolysaccharide Induced Severe Sepsis by Cerium Oxide Nanoparticles. Biomaterials 2015, 59, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Bandarupalli, V.V.K.; Manne, N.D.P.K.; Blough, E.R. Spleen Data: Cerium Oxide Nanoparticles Attenuate Polymicrobial Sepsis Induced Spenic Damage in Male Sprague Dawley Rats. Data Brief 2018, 18, 740–746. [Google Scholar] [CrossRef]
- Manne, N.D.P.K.; Arvapalli, R.; Nepal, N.; Thulluri, S.; Selvaraj, V.; Shokuhfar, T.; He, K.; Rice, K.M.; Asano, S.; Maheshwari, M.; et al. Therapeutic Potential of Cerium Oxide Nanoparticles for the Treatment of Peritonitis Induced by Polymicrobial Insult in Sprague-Dawley Rats. Crit. Care Med. 2015, 43, e477–e489. [Google Scholar] [CrossRef]
- Yang, M.; Wu, J.; Martin, C.M.; Kvietys, P.R.; Rui, T. Important Role of P38 MAP Kinase/NF-ΚB Signaling Pathway in the Sepsis-Induced Conversion of Cardiac Myocytes to a Proinflammatory Phenotype. Am. J. Physiol.—Heart Circ. Physiol. 2008, 294, H994–H1001. [Google Scholar] [CrossRef]
- Asano, S.; Arvapalli, R.; Manne, N.D.; Maheshwari, M.; Ma, B.; Rice, K.M.; Selvaraj, V.; Blough, E.R. Cerium Oxide Nanoparticle Treatment Ameliorates Peritonitis-Induced Diaphragm Dysfunction. Int. J. Nanomedicine 2015, 10, 6215–6226. [Google Scholar] [CrossRef]
- Jeong, H.G.; Cha, B.G.; Kang, D.W.; Kim, D.Y.; Yang, W.; Ki, S.K.; Kim, S.I.; Han, J.; Kim, C.K.; Kim, J.; et al. Ceria Nanoparticles Fabricated with 6-Aminohexanoic Acid That Overcome Systemic Inflammatory Response Syndrome. Adv. Healthc. Mater. 2019, 8, 1801548. [Google Scholar] [CrossRef]
- The Potential Role of Regenerative Medicine in the Management of Traumatic Patients. J. Inj. Violence Res. 2014, 7, 27–35. [CrossRef]
- Crupi, A.; Costa, A.; Tarnok, A.; Melzer, S.; Teodori, L. Inflammation in Tissue Engineering: The Janus between Engraftment and Rejection. Eur. J. Immunol. 2015, 45, 3222–3236. [Google Scholar] [CrossRef]
- Corsi, F.; Carotenuto, F.; Di Nardo, P.; Teodori, L. Harnessing Inorganic Nanoparticles to Direct Macrophage Polarization for Skeletal Muscle Regeneration. Nanomaterials 2020, 10, 1963. [Google Scholar] [CrossRef]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Hoseini, S.J.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef] [PubMed]
- Schanen, B.C.; Das, S.; Reilly, C.M.; Warren, W.L.; Self, W.T.; Seal, S.; Drake, D.R. Immunomodulation and T Helper TH1/TH2 Response Polarization by CeO2 and TiO2 Nanoparticles. PLoS ONE 2013, 8, e62816. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Clin. Neurosurg. 2017, 80, S22–S90. [Google Scholar] [CrossRef]
- Schwab, M.E.; Bartholdi, D. Degeneration and Regeneration of Axons in the Lesioned Spinal Cord. Physiol. Rev. 1996, 76, 319–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Mahapatra, C.; Hong, J.Y.; Kim, M.S.; Leong, K.W.; Kim, H.W.; Hyun, J.K. Functional Recovery of Contused Spinal Cord in Rat with the Injection of Optimal-Dosed Cerium Oxide Nanoparticles. Adv. Sci. 2017, 4, 1700034. [Google Scholar] [CrossRef]
- Kumamaru, H.; Lu, P.; Rosenzweig, E.S.; Kadoya, K.; Tuszynski, M.H. Regenerating Corticospinal Axons Innervate Phenotypically Appropriate Neurons within Neural Stem Cell Grafts. Cell Rep. 2019, 26, 2329–2339.e4. [Google Scholar] [CrossRef]
- Liu, D.; Lu, G.; Shi, B.; Ni, H.; Wang, J.; Qiu, Y.; Yang, L.; Zhu, Z.; Yi, X.; Du, X.; et al. ROS-Scavenging Hydrogels Synergize with Neural Stem Cells to Enhance Spinal Cord Injury Repair via Regulating Microenvironment and Facilitating Nerve Regeneration. Adv. Healthc. Mater. 2023, 12, e2300123. [Google Scholar] [CrossRef]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Kean, T.; Seal, S.; Coathup, M.J. Multi-Functional Cerium Oxide Nanoparticles Regulate Inflammation and Enhance Osteogenesis. Mater. Sci. Eng. C 2021, 124, 112041. [Google Scholar] [CrossRef]
- Li, K.; Shen, Q.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Incorporation of Cerium Oxide into Hydroxyapatite Coating Regulates Osteogenic Activity of Mesenchymal Stem Cell and Macrophage Polarization. J. Biomater. Appl. 2017, 31, 1062–1076. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K.W.K.; Chu, P.K. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv. Sci. 2018, 5, 1700678. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T. Definition and Prevalence of Peri-Implant Diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Surface Treatments on Titanium Implants via Nanostructured Ceria for Antibacterial and Anti-Inflammatory Capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, S.; Gu, D.; Zhu, B.; Liu, H.; Wu, W.; Wu, J.; Wei, H.; Miao, L. Cerium Oxide Nanozyme Attenuates Periodontal Bone Destruction by Inhibiting the ROS-NFκB Pathway. Nanoscale 2022, 14, 2628–2637. [Google Scholar] [CrossRef]
- Mittal, S.; Pandey, A.K. Cerium Oxide Nanoparticles Induced Toxicity in Human Lung Cells: Role of ROS Mediated DNA Damage and Apoptosis. Biomed Res. Int. 2014, 2014, 891934. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Pulmonary Toxicity of Well-Dispersed Cerium Oxide Nanoparticles Following Intratracheal Instillation and Inhalation. J. Nanoparticle Res. 2015, 17, 442. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B.H. The Acute Pulmonary and Thrombotic Effects of Cerium Oxide Nanoparticles after Intratracheal Instillation in Mice. Int. J. Nanomedicine 2017, 12, 2913–2922. [Google Scholar] [CrossRef]
- Guo, C.; Robertson, S.; Weber, R.J.M.; Buckley, A.; Warren, J.; Hodgson, A.; Rappoport, J.Z.; Ignatyev, K.; Meldrum, K.; Römer, I.; et al. Pulmonary Toxicity of Inhaled Nano-Sized Cerium Oxide Aerosols in Sprague–Dawley Rats. Nanotoxicology 2019, 13, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B. Aortic Oxidative Stress, Inflammation and Dna Damage Following Pulmonary Exposure to Cerium Oxide Nanoparticles in a Rat Model of Vascular Injury. Biomolecules 2019, 9, 376. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Al Ansari, Z.; Alkharas, Z.A.; Al Ahbabi, R.M.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Impact of Pulmonary Exposure to Cerium Oxide Nanoparticles on Experimental Acute Kidney Injury. Cell. Physiol. Biochem. 2019, 52, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.M.; Mahmoud, S.S.; Abdelrahman, A.E.; Said, N.M.; Toam, M.; Samy, W.; Amer, M.A.E.M. Protective Effect of Cerium Oxide Nanoparticles on Cisplatin and Oxaliplatin Primary Toxicities in Male Albino Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2411–2425. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Kononenko, L.; Kyriienko, D.; Spivak, M. Neuropathic Diabetic Foot Ulcers Treated with Cerium Dioxide Nanoparticles: A Case Report. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Dhall, A.; Burns, A.; Dowding, J.; Das, S.; Seal, S.; Self, W. Characterizing the Phosphatase Mimetic Activity of Cerium Oxide Nanoparticles and Distinguishing Its Active Site from That for Catalase Mimetic Activity Using Anionic Inhibitors. Environ. Sci. Nano 2017, 4, 1742–1749. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, Z.; Zhai, W.; Qu, Y. Insights on Catalytic Mechanism of CeO2 as Multiple Nanozymes. Nano Res. 2022, 15, 10328–10342. [Google Scholar] [CrossRef]
- Yao, T.; Tian, Z.; Zhang, Y.; Qu, Y. Phosphatase-like Activity of Porous Nanorods of CeO2 for the Highly Stabilized Dephosphorylation under Interferences. ACS Appl. Mater. Interfaces 2019, 11, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Wu, T.S.; Soo, Y.L.; Peng, Y.K. Unravelling the True Active Site for CeO2-Catalyzed Dephosphorylation. Appl. Catal. B Environ. 2020, 264, 118508. [Google Scholar] [CrossRef]
- Manto, M.J.; Xie, P.; Wang, C. Catalytic Dephosphorylation Using Ceria Nanocrystals. ACS Catal. 2017, 7, 1931–1938. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IκB Kinase Complex: Master Regulator of NF-ΚB Signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef]
- Caputo, F.; Giovanetti, A.; Corsi, F.; Maresca, V.; Briganti, S.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium Oxide Nanoparticles Reestablish Cell Integrity Checkpoints and Apoptosis Competence in Irradiated HaCaT Cells via Novel Redox-Independent Activity. Front. Pharmacol. 2018, 9, 1183. [Google Scholar] [CrossRef]
- Corsi, F.; Caputo, F.; Traversa, E.; Ghibelli, L. Not Only Redox: The Multifaceted Activity of Cerium Oxide Nanoparticles in Cancer Prevention and Therapy. Front. Oncol. 2018, 8, 309. [Google Scholar] [CrossRef]

| Nanomaterial | Synthesis Method | Morphology | Size (nm) | Model | Dosage | Route of Administration | Markers | Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Hydrodynamic Radius | |||||||||
| CNPs | Precipitation | Cubic | 6–16 | ND | SH-SY5Y neuroblastoma cells treated with 12.5 μM Aβ 25–35 for 24 h | 100 μg/mL for 24 h | NA | ↑ β-TubIII, GAP43, NF-H 200 ↑ GPX1, catalase ↓ SOD1, SOD2 ↑ BDNF, TrkB ↓ p-ERK1,2 | ↑ cell viability ↓ apoptosis ↑ antioxidant response ↓ neurite atrophy ↑ neuronal differentiation Maintenance of cytoskeletal organization | [27] |
| Triphenyl phosphonium-conjugated CNPs (TPP-CNPs) | Hydrolytic sol-gel | ND | ND | 22 | SH-SY5Y neuroblastoma cells and U373 astrocytoma cells treated with 5 μM Aβ | 0.1 mM for 12 h | NA | ↓mitochondrial ROS | ↓ oxidative stress | [28] |
| Transgenic 5XFAD mouse as in vivo model for Alzheimer’s disease (AD) | 3 μL solution of 1 mg/mL | Unilateral subicular injection | ↓ GFAP, Iba-1 ↓ 4-HNE | ↑ neuronal viability ↓ glial cell activation ↓ oxidative stress | ||||||
| CNPs modified with LXW7 peptide and polyacrylic acid (CeO2@PAA-LXW7) | EDC (1-ethyl-3-(3-dimethylaminopropyl carbodiimide) reaction | Spherical | ND | 2–5 | Murine BV2 microglial cells | 1 μM | NA | ↓ TNFα ↓ IL-1β ↓ ROS, NO ↓ p- FAK, p-STAT3 | ↓ inflammation | [29] |
| Europium doped CNPs (EuCNPs) | Solvothermal reactions | Spherical | ND | 159.06 | Murine BV2 microglial cells | 100 ng/mL | NA | ↑ CD36 ↑ co-localization of Aβ with Rab7 and LAMP1 ↓ IL-6, IL-1β | ↑ microglial phagocytosis of Aβ ↓ inflammation ↓ Aβ plaques ↓ oxidative stress ↓ Alzheimer’s disease (AD) symptoms | [30] |
| CNPs | NA (purchased) | Spherical | ND | ND | C57BL/6J male mice exposed to PM2.5 for 6 h/day, 5 times a week for 23 weeks as in vivo model for Alzheimer’s disease (AD) | 0.25 mg/kg or 0.5 mg/kg, 1 time a week for 10 weeks | Intravenous tail injection | ↓ IL-6, TNF-α, IL-1β ↓ NF-κBia and IκBκB ↓ GFAP ↓ ROS (O2, H2O2) ↑ SOD activity | ↓ inflammation ↓ glial cell activation ↓ oxidative stress ↑ antioxidant response | [32] |
| Mice-derived primary astrocytes treated with PM2.5 for 24 h | 5–200 ng/mL for 24 h | NA | ↓ GFAP ↓ IL-6, TNFα, IL-1β ↓ nuclear NF-κB ↑ cytoplasmatic NF-κB ↓ COX-2 ↓ ROS (O2−, H2O2), iNOS ↑ SOD1, SOD2, NQO1 | ↓ glial cell activation ↓ inflammation ↓ oxidative stress ↑ antioxidant response | ||||||
| CNPs | NA (purchased) | ND | ND | 3.3 | SOD1G93A transgenic mouse as in vivo model for amyotrophic lateral sclerosis (ALS) | 20 mg/kg, 2 times a week | Intravenous tail injection | ↑ muscle strength ↑ survival (+11 days ca.) ↓ clinical score ↓ body weight loss | [33] | |
| CNPs | NA (purchased) | Spherical | 3–5 | ND | Wistar rats exposed to unpredictable chronic mild stress (UCMS) as in vivo model for stress-induced depression | 10 nM | Intrahippocampal and intracerebroventricular injection | ↓ IL-6 ↓ MDA ↑ GAP-43+ neurons ↑ CA3 neurons number | ↓ depressive-like behavior ↓ inflammation ↓ oxidative stress ↑ neurogenesis ↑ neurite outgrow | [35] |
| CNPs | Reverse micelle | Spherical | 3.5 ± 0.5 | ND | VC57BL/6J male mice exposed to open head injury using a stereotaxic impactor as in vivo model for traumatic brain injury (TBI) | 11.6 mM | Retro-orbital injection | ↓ FJB+ and TUNEL+ cells ↑ SOD1 and SOD2 mRNA ↓ COX-2 | ↓ neuronal cell death ↓ oxidative stress ↓ inflammation ↑ cognitive functions ↓ cerebral edema | [36] |
| Hydrothermal | Rods | 9.4 ± 2.1 Length: 130.1 ± 42.1 | ND | |||||||
| Nanomaterial | Synthesis Method | Morphology | Size (nm) | Model | Dosage | Route of Administration | Markers | Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Hydrodynamic Radius | |||||||||
| Albumin-conjugated CNPs (A-CNP) | Biomineralization | Spherical | 30 ± 8.9 | ND | DBA/1J mice exposed to collagen-induced arthritis (CIA) mouse as in vivo model for rheumatoid arthritis | 1 mg/kg, 2 times a week | Intra-articular injection | ↓ iNOS ↑ Arg-1 | M2 polarization ↓ clinical score | [39] |
| THP-1 human monocytes and RAW 264.7 murine macrophages | 0.5 μg/mL for 24 h | NA | ↓ iNOS, IL-1β ↑ Arg-1 ↓ HIF-1α | M2 polarization ↑ antioxidant response ↓ hypoxia | ||||||
| Citric-acid-coated CNPs (CA-CNPs) | Alkaline-based precipitation | Spherical | 2.8 ± 0.4 | 3.4 ± 1.1 | HepG2 hepatocyte cells, RAW264.7 murine macrophages, Renca epithelial kidney cells and SVEC4-10EHR1 endothelial cells exposed to LPS or H2O2 | 0.1–1 mg/mL for 24 h | NA | ↑ SOD, CAT and HORAC activity ↓ ROS (OH•) ↓ TNF-α, IL-1β | ↑ cell viability ↓ oxidative stress ↓ inflammation | [40] |
| C57BL/6J mice treated with complete Freund’s adjuvant (CFA) as in vivo model for peripheral Inflammation | 100 mg/kg | Intravenous tail injection | ↓ TNF-α, IL-1β ↑ IL-10 | ↓ paw inflammation ↓ edema formation ↓ immune cell infiltration | ||||||
| CNPs + Hyaluronic acid | hydrothermal | Cubic | 10–60 | ND | Chondrocytes treated with H2O2 for 30 min | 0.02 μg/mL | NA | ↑ ACAN, COL1A1, COL2A1 | ↓ cell apoptosis ↓ oxidative stress ↓ glycosaminoglycan synthesis | [42] |
| CNPs | NA | Spherical | 5 | 10 | Sprague-Dawley-rat-derived chondrocytes treated with IL-1β | 160 μg/mL | NA | ↓ ROS (O2−) ↓ NO ↑ Nrf2, HO-1, SOD, CAT, GPX ↑ ACAN, COL1A1, COL2A1 ↓ MMP13, ADAMTS4 ↓iNOS, COX-2, IL-6 | ↓ oxidative stress ↑ antioxidant response ↓ cell apoptosis ↓ ECM degradation ↓ inflammation | [43] |
| Sprague-Dawley-rat-derived condylar cartilage explants treated with IL-1β | ↓ ROS (O2−) ↓ NO | ↓ apoptosis ↓oxidative stress | ||||||||
| CNPs + Lenalidomide | Precipitation | Spherical | 3–5 | 34 ± 6.8 | C57BL/6 mice treated with MOG 35–55 peptide and pertussis toxin (experimental autoimmune encephalomyelitis) as in vivo model for multiple sclerosis (MS) | 1 mg/kg, every fourth day | Intravenous injection | ↑ MBP ↓ TNF-α ↓ IL-17, INF-γ, TNF-α (mRNA) ↓ GFAP, Iba-1 ↓ CD86+ dendritic cells | ↓ clinical score ↑ body weight ↓ ventricular volume ↓ myelin loss ↓ inflammation ↓ glial cell activation ↓ peripheral immune reaction | [45] |
| Nanomaterial | Synthesis Method | Morphology | Size (nm) | Model | Dosage | Route of Administration | Markers | Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Hydrodynamic Radius | |||||||||
| CNPs | ND | ND | ND | ND | Rats treated with monosodium glutamate (MSG) as in vivo model for non-alcoholic fatty liver disease (NAFLD) | 1 mg/kg 1 time for month | Oral gavage | ↓ total lipids, triglycerides ↓ IL-1β, IL-12Bp40 ↑ TGF-β, IL-4 ↓ IL-10 | ↓ liver damage ↓ NAFLD activity score ↓ inflammation ↓ obesity | [48] |
| CNPs | NA (purchased) | Spherical | 10–30 | ND | Male Wistar rats treated with carbon tetrachloride (CCl4) as in vivo model for non-alcoholic fatty liver disease (NAFLD) | 0.1 mg/kg 2 times a week for 2/4 weeks | Intravenous injection | ↑ TAC ↑ GSH ↓ TNF-α Normalization of ALP, ALT, and AST levels ↓ MDA | ↑ antioxidant capacity ↓ inflammation ↓ CCl4-induced liver injury | [49] |
| CNPs | NA (purchased) | Spherical and cuboidal | 10–25 | ND | Female Wistar rats subjected to ovariectomy operation as in vivo model for postmenopausal obesity | 0.1 mg/kg 2 times a week for 2 weeks | Intraperitoneal injection | ↓ LXR, AST, ALT ↓ FFA, TG, TC, LDL-C ↑ HDL-C ↓ MDA, TAC ↓TNF-α, TGF-1β | ↓ obesity ↓ steatosis ↓ lipogenesis ↓ oxidative stress ↓ inflammation | [50] |
| CNPs | Chemical precipitation | Spherical | 4–20 | ND | Carbon tetrachloride (CCl4)-treated rats as in vivo model for liver fibrosis | 0.1 mg/kg 2 times a week for 2 weeks | Intravenous tail injection | ↓ AST, ALT, α-SMA ↓ CD68+ cells ↓ TUNEL+ cells ↓ activated- caspase 3 ↓ IL-1β, TNF-α, COX-2, iNOS ↓ Ncf1, Ncf2, Epx | ↓ steatosis ↓ fibrogenesis ↓ apoptotic cell death ↓ inflammation ↓ oxidative stress | [52] |
| HepG2 hepatocyte cells treated with H2O2 | 100 μg/mL | NA | ↓ ROS | ↓ oxidative stress | ||||||
| CNPs | NA (purchased) | Cubical | 120 ± 7.5 | ND | Male C57BL/6J mice subjected to bile duct ligation (BDL) as in vivo model for liver fibrosis | 0.5 mg/kg or 2 mg/kg for 12 days | Intraperitoneal injection | ↓ SGOT, SGPT, ALP, bilirubin ↓ MDA ↓ nitrite level ↑ SOD, CAT, GSH ↓ IL-1β, IL-6, IL-17, TNF-α, TGF-β ↓ p65-NF-κB, COX-2, iNOS ↓ Snail, TIMP-1, α-SMA, LOXL-2, N-Cad, fibronectin | ↓ liver fibrosis ↓ oxidative stress ↓ nitrative stress ↑ antioxidant response ↓ liver fibrosis ↓ inflammation | [53] |
| CNPs | NA (purchased) | Spherical | 10–30 | 70 | Male Sprague-Dawley rats as in vivo model for hepatic ischemia reperfusion (IR) | 0.5 mg/kg 20 min before IR | IV tail injection | ↓ ALT, LDH ↓ KC/GRO, MDC, MIP-2, myoglobin, leptin, insulin, PAI-1, vWF (inflammatory mediators) ↑ growth hormone | ↓ liver damage ↓ hepatocyte necrosis ↓ inflammation | [54] |
| CNPs | ND | ND | ND | ND | Male mice as in vivo model for hepatic ischemia reperfusion (IR) | 300 μg/kg 24 h before IR | Intraperitoneal injection or oral gavage | ↓ LDH, ALT, AST ↓ MDA ↑ GSH ↓ lipid peroxidation ↑ SOD, CAT, GPx ↓ p65-NF-κB, MPO activity ↓ TNF-α, IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, IL-17, ICAM-1 ↑ IL-10 ↓ MMP-2, MMP-9, TIMP-1 | ↓ liver edema ↓ liver injury ↓ oxidative stress ↑ antioxidant response ↓ inflammation ↓ MPP activation | [56] |
| Nanomaterial | Synthesis Method | Morphology | Size (nm) | Model | Dosage | Route of Administration | Markers | Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Hydrodynamic Radius | |||||||||
| CNPs | Precipitation | ND | 160 | ND | Female Sprague-Dawley rats treated with 90% ethanol as in vivo model for gastric ulcers | 1 mg/kg | Oral gavage | ↑ SOD and CAT activity | ↓ ulcerative lesions ↑ antioxidant response | [59] |
| Citrate-coated CNPs | Sol-gel | ND | 3–7 | 4.9 | Male albino nonlinear rats exposed to ulcerogenic factor as in vivo model for gastric ulcers | 1 mg/kg 24 h before exposure to ulcerogenic factor | Oral gavage | ↓ lipid peroxidation ↓ ROS (H2O2) ↑ SOD activity ↓ CAT activity ↓ IL-1β, IL-12Bp40, INF-γ ↑ IL-4, IL-10, TGF-β | ↓ ulcerative lesions ↑ protective protein of gastric mucosa (GM) ↓ inflammation ↓ oxidative stress ↑ antioxidant response | [60] |
| Sulfasalazine-linked NH2-CNPs (SSZ@NH2-CNPs) | UiO-66 (Ce) synthetic | Semi-spherical | 64.9 ± 15.6 | ND | C57BL/6 male mice treated with 1.5% (w/v) dextran sodium sulphate (DSS) for 7 day as in vivo model for ulcerative colitis | 258 mg/kg/day for 7 days | Oral gavage | ↓ MDA ↓ collagen deposition ↓ lipid peroxidation ↑ SOD and CAT activity ↑ total thiols level | ↓ disease activity index ↑ body weight ↓ colon shortening and spleen weight ↓ inflammation ↓ oxidative stress ↑ antioxidant response ↓ fibrosis | [61] |
| Nanomaterial | Synthesis Method | Morphology | Size (nm) | Model | Dosage | Route of Administration | Markers | Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Hydrodynamic Radius | |||||||||
| CNPs | ND | ND | ND | ND | Rat-derived primary retinal neurons exposed to 1 mM H2O2 for 30 min | 1–20 nM | NA | ↓ ROS | ↓ oxidative stress | [71] |
| Sprague-Dawley albino rats exposed to 2700 lux of light for 6 h as in vivo model for light-induced photoreceptor degeneration | 1–20 nM pre- and post-light damage induction | Intravitreal injection | ↑ Thickness of ONL ↓ TUNEL+ cells | Protection of retina photoreceptor ↓ retinal degeneration ↓ photoreceptor cell apoptosis ↑ retinal function | ||||||
| CNPs | ND | ND | 3–5 | ND | Vldlr−/− mutant mice exposed to 80 lux of light as in vivo model for age-related macular degeneration (AMD) | 1 mL of 1 mM (172 ng) of CNPs | Intravitreal injection | ↓ ROS ↓ VEGF-A ↓ p-ERK1/2, p-JNK1/2, p-p38, p-Akt | ↓ vascular lesion ↓ angiogenesis ↓ inflammation | [72] |
| CNPs | NA (purchased) | ND | 10–100 | ND | Human corneal epithelial cells (HCECs) and RAW264.7 murine macrophages treated with H2O2 | 2–80 μg/mL | NA | ↓ ROS, NO ↓ TNF-α, IL-6 | ↓ oxidative stress ↓ inflammation | [73] |
| Sprague-Dawley albino rats and adult Japanese white rabbits exposed to ocular alkali burns as in vivo models for corneal neovascularization | 20 μL of 200 mg/mL of CNPs | Dropping into the lower conjunctival sac | ↓ TNF-α | ↓ corneal opacification ↓ corneal neovascularization ↓ inflammation | ||||||
| CNPs | Hydrolysis and condensation of Ce(Cit)2−3+ at high pH | Spherical | 3 | 4.3 | ARPE19 retinal cells and HUVEC human vascular endothelial cells | 5–500 nM | NA | ↓ ROS ↑ SOD expression ↓ VEGFA ↓ microvessels | ↑ antioxidant response ↓ cell migration and neovascularization | [74] |
| DKOrd8 mouse model as in vivo model of dry AMD-like pathology and laser-induced choroidal neovascularisation (LI-CNV) mouse model | 2 mg/mL 2-months daily treatment | Intravitreal and topical administration | ↓ number of lesions in photoreceptors layers ↑ Nrf2 ↓ microglia cell number ↓ IL-18 | ↓ oxidative stress ↑ retinal function ↑ DNA repair ↑ senescence | ||||||
| Nanomaterial | Synthesis Method | Morphology | Size (nm) | Model | Dosage | Route of Administration | Markers | Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Hydrodynamic Radius | |||||||||
| CNPs immobilized on the surface of silica NPs (S-CNPs) | Precipitation | ND | 220 ± 5 | ND | Wistar male rats treated with 1 mg/kg LPS as in vivo model for experimental pneumonia | 0.6 mg/kg at 0, 1, 3 and 24 h after LPS injection | Orogastric catheter | ↓ ROS (O2•- •OH, H2O2) ↓ TNF-α, IL-6 ↓ CXCL2 | ↓ lung injuries ↓ oxidative stress ↓ inflammation ↑ oxygen consumption | [75] |
| CNPs | NA (purchased) | Spherical | ND | 140 ± 53 | Sprague-Dawley male rats subjected to intraperitoneal injection of cecal material (400 mg/kg) as in vivo model for polymicrobial sepsis | 3.5 mg/kg | Intravenous injection | ↓ IL-6 ↓ blood urea nitrogen | ↑ animal survival ↓ inflammation ↓ liver and renal dysfunction | [78] |
| RAW264.7 murine macrophages treated with LPS (2 μg/mL) for 24 h | 0.72–8.6 μg/mL for 24 h | NA | ↓ ROS ↓ TNF-α, IL-1β, IL-6 ↓ iNOS, COX-2 ↓ nuclear NF-κB ↑ cytoplasmic NF-κB | ↑ cell survival ↓ oxidative damage ↓ inflammation | ||||||
| Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus | 1–100 mg/mL | NA | ↓ bacterial growth (only for E. coli) | |||||||
| CNPs | NA (purchased) | Spherical | 200–400 | 53.36 ± 7.04 | Sprague-Dawley rats subjected to intraperitoneal injection of LPS as in vivo model of hepatic dysfunction | 0.5 mg/kg | Intravenous tail injection | ↓ TNF-α, IL-1β, IL-1α ↓ bilirubin, ALT, GST-Mu, GST-α ↓ MyD88, p-p38-MAPK, p-p44/42-MAPK, p-ERK1/2 ↓ iNOS, HMBG-1 ↓ cleaved caspase 3 ↓ Bax/Bcl-2 ratio ↓ ROS | ↑ animal survival ↑ blood pressure ↓ immune cell infiltration ↓ inflammation ↓ hepatic damage ↓ apoptosis ↓ oxidative stress | [83] |
| RAW264.7 murine macrophages treated with LPS | 0.1–1000 ng/mL | NA | ↓ ROS ↓ TNF-α, IL-1β, IL-6 ↓ iNOS, HMBG-1 ↓ COX-2 ↓ nuclear NF-κB | ↓ cell apoptosis ↓ oxidative stress ↓ inflammation | ||||||
| CNPs | NA (purchased) | ND | ND | ND | Sprague-Dawley male rats subjected to intravenous tail injection of LPS (40 mg/kg) as in vivo model for severe sepsis | 0.5 mg/kg | Intravenous tail injection | ↓ HMGB1 | ↓ splenic damage ↓ inflammation ↓ bacterial load in blood and peritoneal fluid | [84] |
| CNPs | NA (purchased) | Cubic | 10–30 | 90 | Male Sprague-Dawley rats subjected to intraperitoneal injection of cecal material (600 mg/kg) as in vivo model for polymicrobial sepsis | 0.5 mg/kg | intravenous tail injection | ↓ ROS (superoxide levels) ↓ iNOS, nitrotyrosine ↓ TNF-α, INF-γ, IL-6, GST-α, GST-μ ↓ p-ERK1/2, p-Stat-3 ↓ P-selectin, VCAM-1 | ↑ animal survival ↓ animal hypothermia ↓ oxidative stress ↓ hepatic damage ↓ nitrosative stress ↓ inflammation ↓ immune cell infiltration | [85] |
| RAW264.7 murine macrophages | 1–100 ug/mL for 48 h | NA | Non-toxic effect | |||||||
| CNPs | NA (purchased) | Spherical | 15–20 | ND | Male Sprague-Dawley rats subjected to intraperitoneal injection of cecal material (600 mg/kg) as in vivo model for polymicrobial sepsis | 0.5 mg/kg | Intravenous tail injection | ↓ p-Stat-3 ↓ iNOS ↑ p-Akt, p-FOXO-1, p-4EBP1 ↓ caspase-8 cleavage | ↑ diaphragmatic function ↓ cell infiltration ↓ inflammation ↓ oxidative stress ↓ protein degradation | [87] |
| 6-aminohexanoic acid (6-AHA)-conjugated CNPs (6-AHA-CNPs) | Sol–gel | Spherical and square | 25 | 35 | Male C57BL/6 mice subjected to cecal ligation puncture peritonitis as in vivo model for systemic inflammatory response syndrome (SIRS) | 0.5 mg/kg | Intravenous tail injection | ↓ MPO, CD68+ cells | ↓ lung and liver injuries ↓ hepatocellular necrosis ↓ immune cell infiltration ↓ inflammation ↑ animal survival | [88] |
| RAW 264.7 murine macrophages and U937 human leukemic monocytes treated with H2O2 or LPS | 0.1 mM | NA | ↓ ROS (O2−, H2O2) ↓ IL-1β, LDH ↓ iNOS | ↓ oxidative stress ↓ inflammation | ||||||
| Nanomaterial | Synthesis Method | Morphology | Size (nm) | Model | Dosage | Route of Administration | Markers | Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | Hydrodynamic Radius | |||||||||
| CNPs | Hydrothermal | Cubic | 19.5 | 33.7 | Sprague-Dawley-rat-derived primary cortical neurons treated with H2O2 | 1–4000 μg/mL for 24 h | NA | ↓ iNOS | ↑ neuronal cell survival ↓ oxidative stress | [96] |
| Sprague-Dawley rats exposed to contusion injury at T9 level as in vivo model for spinal cord injury (SCI) | 0.5–4 mg/mL | Lesion cavity injection | ↓ ED1+ cells ↓ iNOS ↓ Nr-f2, COX-2 ↓ TNF-α, IL-1β, IL-6 ↑ IL-10 ↓ p53, caspase 3 | ↓ lesion cavity ↓ inflammation ↓ oxidative stress ↑ locomotor functions ↓ apoptosis ↑ axonal regeneration | ||||||
| BSA-incubated-CNP-based hydrogel (CNPs-Gel) | BSA-incubation method | Spherical | ND | 4.97 ± 1.39 | Sprague-Dawley rats exposed to injury at T10 level as in vivo model for spinal cord injury (SCI) | 3–6 mM | Implantation of NSC-loaded CNP gel into the lesion | ↓ ROS, 4-HNE ↓ CD68+ cells ↓ iNOS ↑ Arg-1 ↑ p-FAK, p-PI3K, p-AKT1 ↓ GFAP ↑ MAP-2 ↑ NF+ and MBP+ cells | ↑ motor function ↓ cavity area ↓ oxidative stress ↓ inflammation M2 polarization ↓ glial cell activation ↑ neuron and axonal regeneration | [98] |
| BV2 microglial cell and neuronal stem cells (NSCs) treated with H2O2 | ND | NA | ↓ ROS ↓ TNF-α, iNOS, IL-6 ↑ IL-10, TGF-β, Arg-1 ↑ p-FAK, p-PI3K, p-AKT1 | ↑ cell survival ↓ oxidative stress ↓ inflammation M2 polarization | ||||||
| CNPs | Wet chemical | Spherical | 5 | 35 | RAW 264.7 murine macrophages and human bone mesenchymal stem cells (BMSC) treated with LPS | 1, 10, or 20 μg/mL | NA | ↓ iNOS ↓ IL-6, IL-1β, TNF-α ↑ IL-10, TGF-β | ↓ inflammation ↑ osteogenic differentiation | [99] |
| CNPs incorporated hydroxyapatite coating | Plasma spraying | ND | ND | ND | Rat bone mesenchymal stem cells and RAW264.7 macrophages | 10–30 wt% | NA | ↑ ALP, OCN, Runx-2 ↑ BMP2, BMPR1, BMPR2, Smad1, Smad5, and Smad8 ↓ TNF-α, IL-6 ↓ CCR7 and CD11c ↑ CD163, CD206 ↑ IL-1rα, IL-10, TGF-β | ↑ cell proliferation M2 polarization ↑ osteogenic differentiation ↓ inflammation | [100] |
| Immobilized CNPs on titanium-based biomaterial | Deposition by magnetron sputtering and vacuum annealing | ND | ND | ND | Rat bone marrow mesenchymal stem cells (BMSCs), RAW264.7 murine macrophages | Elemental concentration: 3.57–7.58% | NA | ↑ ALP, Col-I, OCN, OPN, Runx-2 ↑ IL-10 ↓ TNF-α | ↑ cell proliferation ↑ osteogenic differentiation M2 polarization | [101] |
| Sprague-Dawley rats | Femoral bone implantation | Femoral bone implantation | ||||||||
| CNP-modified titanium disks (CNPs@TiO2) | Hydrothermal | Nanorods | 9.6 ± 1.2 Length: 50–600 | ND | Gram-positive bacteria S. sanguinis and Gram-negative bacteria F. nucleatum | 0.1 M | NA | ↓ bacterial growth | [103] | |
| Cubic | 57.2 ± 17.5 | ND | RAW 264.7 murine macrophages treated with LPS | NA | ↓ ROS ↓ TNF-α, IL-1β, IL-6 ↓ nuclear NF-κB (NF-κB/p65) | ↓ oxidative stress ↓ inflammation | ||||
| Octahedral | 29.1 ± 11.7 | ND | Wistar rats | Subcutaneous implantation | ↓ TNF-α, IL-1β, IL-6 | ↓ immune cell infiltration ↓ inflammation | ||||
| CNPs | NA | spherical | 5 | 10 | RAW 264.7 cells | 2–50 μg/mL | NA | ↓ MAPK NFkB pathway ↓ IL-1β ↓ TNF-α ↓ iNOS ↓ Nrf2, HO-1 ↓ ROS | ↓ oxidative stress ↓ inflammation | [104] |
| Male Sprague-Dawley rats treated with LPS as in vivo model of gingival inflammation | 10 μL; 2 mg/mL | Gingival injection | ↓ ROS | ↓ destruction of periodontal tissues | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsi, F.; Deidda Tarquini, G.; Urbani, M.; Bejarano, I.; Traversa, E.; Ghibelli, L. The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More than Redox? Nanomaterials 2023, 13, 2803. https://doi.org/10.3390/nano13202803
Corsi F, Deidda Tarquini G, Urbani M, Bejarano I, Traversa E, Ghibelli L. The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More than Redox? Nanomaterials. 2023; 13(20):2803. https://doi.org/10.3390/nano13202803
Chicago/Turabian StyleCorsi, Francesca, Greta Deidda Tarquini, Marta Urbani, Ignacio Bejarano, Enrico Traversa, and Lina Ghibelli. 2023. "The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More than Redox?" Nanomaterials 13, no. 20: 2803. https://doi.org/10.3390/nano13202803