Transition of Carbon Nanotube Sheets from Hydrophobicity to Hydrophilicity by Facile Electrochemical Wetting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the Hydrophobic CNT Sheets/PET (CP) Film
2.3. Characterization
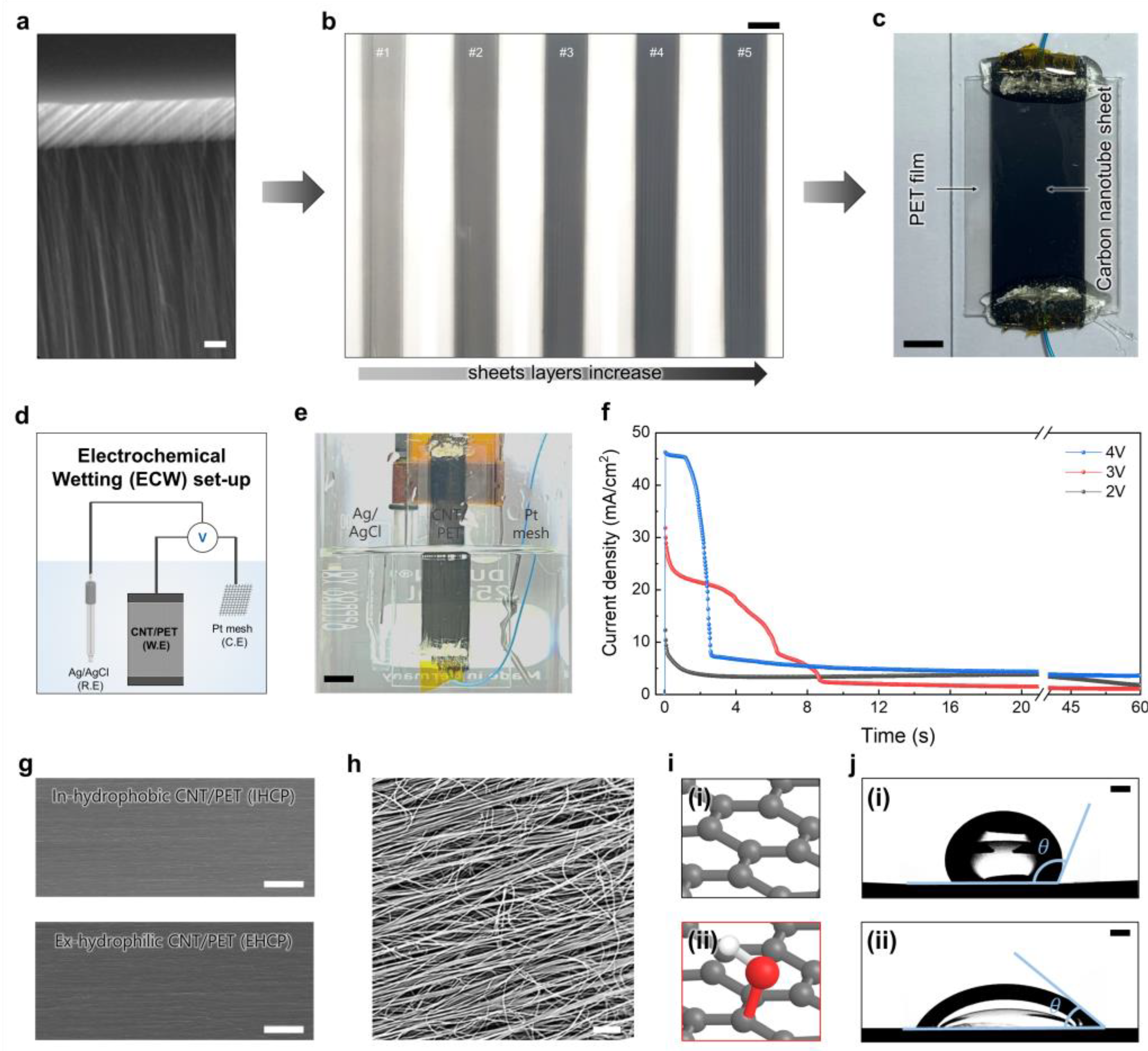
3. Results and Discussion
3.1. ECW-Treated Carbon Nanotube (CNT) Sheet on a Polyethylene Terephthalate (PET) Film
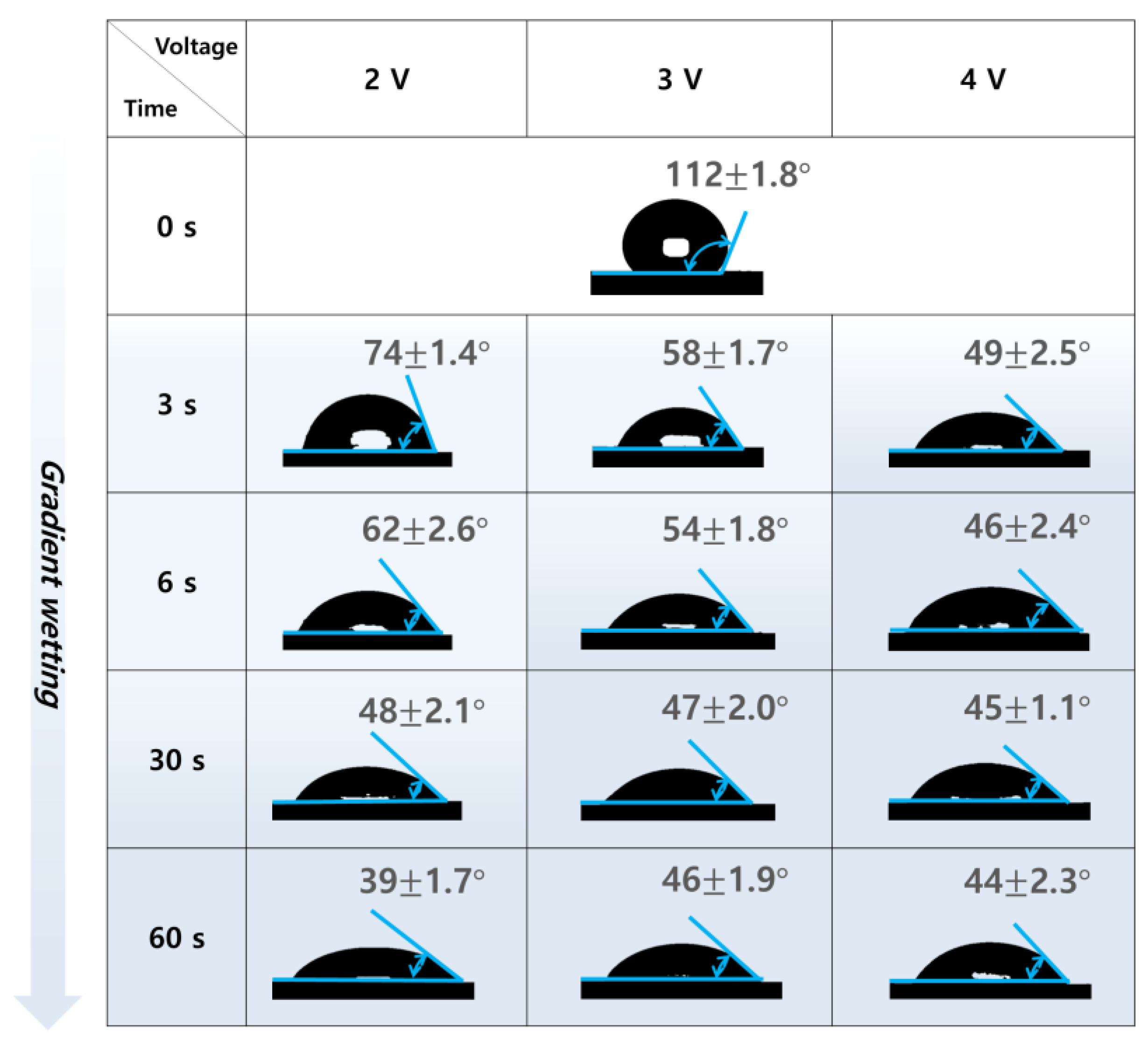
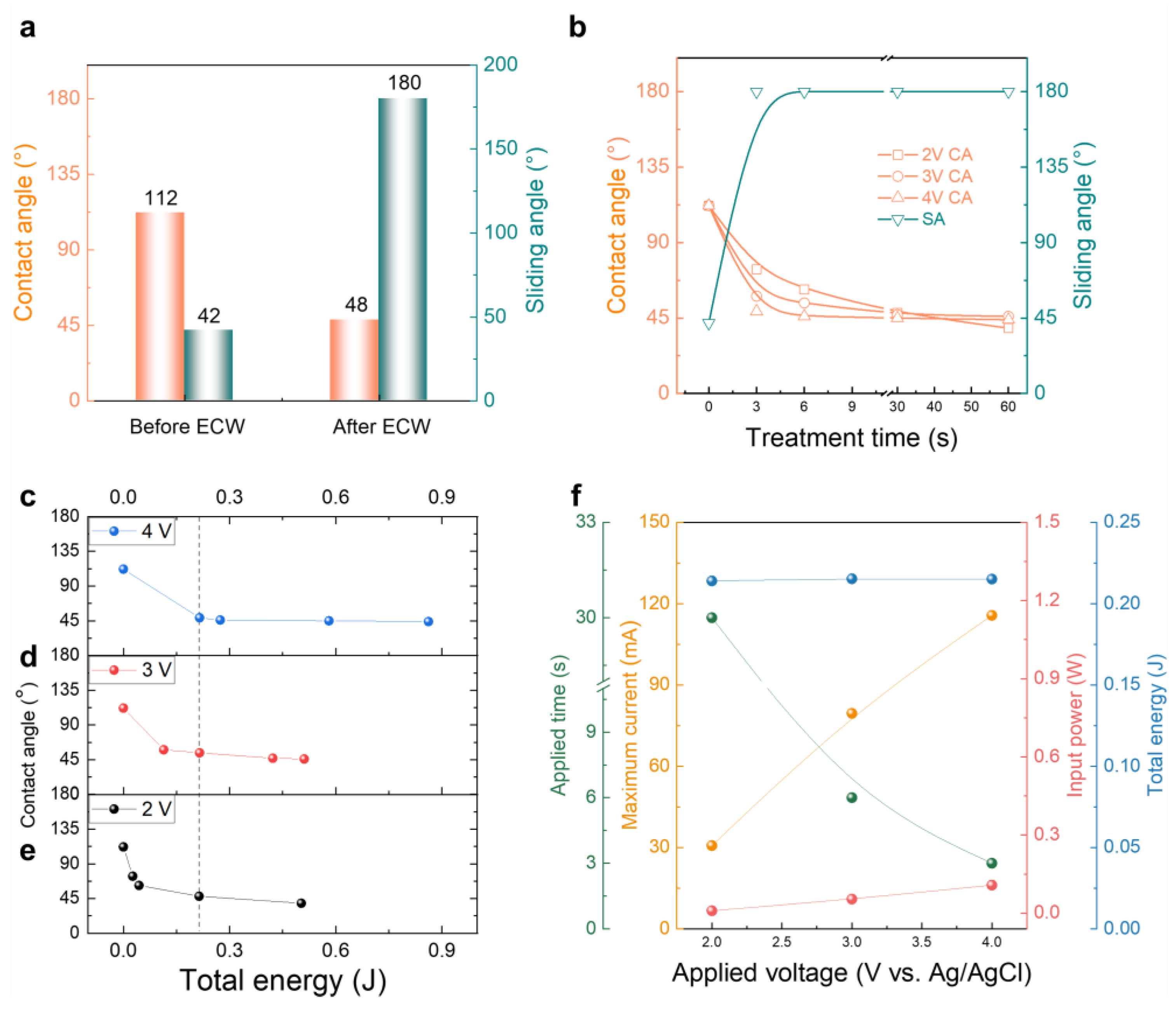
3.2. Changes in Contact Angle on the IHCP Surface
3.3. Sliding Angle Variation for IHCP and EHCP Film
3.4. Characterization as Applied Voltage and Time Correlation among Contact, Sliding Angle and ECW Treatment
3.5. Chemical and Electrochemical Characterization of the EHCP Surface after ECW Treatment Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Fang, S.; Zakhidov, A.A.; Lee, S.B.; Aliev, A.E.; Williams, C.D.; Atkinson, K.R.; Baughman, R.H. Strong, transparent, multifunctional, carbon nanotube sheets. Science 2005, 309, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Adusei, P.K.; Gbordzoe, S.; Kanakaraj, S.N.; Hsieh, Y.-Y.; Alvarez, N.T.; Fang, Y.; Johnson, K.; McConnell, C.; Shanov, V. Fabrication and study of supercapacitor electrodes based on oxygen plasma functionalized carbon nanotube fibers. J. Energy Chem. 2020, 40, 120–131. [Google Scholar] [CrossRef]
- Naqvi, S.T.R.; Rasheed, T.; Hussain, D.; Haq, M.N.; Majeed, S.; Shafi, S.; Ahmed, N.; Nawaz, R. Modification strategies for improving the solubility/dispersion of carbon nanotubes. J. Mol. Liq. 2020, 297, 111919. [Google Scholar] [CrossRef]
- Ham, S.W.; Hong, H.P.; Kim, J.H.; Min, S.J.; Min, N.K. Effect of oxygen plasma treatment on carbon nanotube-based sensors. J. Nanosci. Nanotechnol. 2014, 14, 8476–8481. [Google Scholar] [CrossRef]
- Watanabe, H.; Kondo, H.; Sekine, M.; Hiramatsu, M.; Hori, M. Control of super hydrophobic and super hydrophilic surfaces of carbon nanowalls using atmospheric pressure plasma treatments. Jpn. J. Appl. Phys. 2012, 51, 01AJ07. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Marriam, I.; Liu, W.; Gao, Z.; Qiu, Y. Quasi–static and dynamic interfacial evaluations of plasma functionalized carbon nanotube fiber. Appl. Surf. Sci. 2019, 465, 795–801. [Google Scholar] [CrossRef]
- Park, O.-K.; Kim, W.Y.; Kim, S.M.; You, N.-H.; Jeong, Y.; Lee, H.S.; Ku, B.-C. Effect of oxygen plasma treatment on the mechanical properties of carbon nanotube fibers. Mater. Lett. 2015, 156, 17–20. [Google Scholar] [CrossRef]
- Dai, X.; Huang, X.; Yang, F.; Li, X.; Sightler, J.; Yang, Y.; Li, C. Enhanced nucleate boiling on horizontal hydrophobic-hydrophilic carbon nanotube coatings. Appl. Phys. Lett. 2013, 102, 161605. [Google Scholar] [CrossRef]
- Lai, C.-C.; Lo, C.-T. Plasma oxidation of electrospun carbon nanofibers as supercapacitor electrodes. RSC Adv. 2015, 5, 38868. [Google Scholar] [CrossRef]
- Son, W.; Chun, S.; Lee, J.M.; Jeon, G.; Sim, H.J.; Kim, H.W.; Cho, S.B.; Lee, D.; Park, J.; Jeon, J.; et al. Twist-stabilized, coiled carbon nanotube yarns with enhanced capacitance. ACS Nano 2022, 16, 2661–2671. [Google Scholar] [CrossRef]
- Komatsubara, K.; Suzuki, H.; Inoue, H.; Kishibuchi, M.; Takahashi, S.; Marui, T.; Umezawa, S.; Nakagawa, T.; Nasu, K.; Hayashi, Y. Highly Oriented Carbon Nanotube Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 1521–1532. [Google Scholar] [CrossRef]
- Avasthi, P.; Kumar, A.; Balakrishnan, V. Aligned CNT Forests on Stainless Steel Mesh for Flexible Supercapacitor Electrode with High Capacitance and Power Density. ACS Appl. Nano Mater. 2019, 2, 1484–1495. [Google Scholar] [CrossRef]
- Senokos, E.; Rana, M.; Santos, C.; Marcilla, R.; Vilatela, J.J. Controlled electrochemical functionalization of CNT fibers: Structure–chemistry relations and application in current collector-free all-solid supercapacitors. Carbon 2019, 142, 599–609. [Google Scholar] [CrossRef]
- Liu, C.-M.; Cao, H.-B.; Li, Y.-P.; Xu, H.-B.; Zhang, Y. The effect of electrolytic oxidation on the electrochemical properties of multi-walled carbon nanotubes. Carbon 2006, 44, 2919–2924. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Lima, M.D.; Li, N.; Andrade, M.J.; Fang, S.; Oh, J.; Spinks, G.M.; Kozlov, M.E.; Haines, C.S.; Suh, D.; Foroughi, J.; et al. Electrically, chemically, and photonically powered torsional and tensile actuation of hybrid carbon nanotube yarn muscles. Science 2012, 338, 928–932. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G.M.; Wallace, G.G.; Oh, J.; Kozlov, M.E.; Fang, S.; Mirfakhrai, T.; Madden, J.D.W.; Shin, M.K.; Kim, S.J.; et al. Torsional carbon nanotube artificial muscles. Science 2011, 334, 494–497. [Google Scholar] [CrossRef]
- Gao, G.; Pan, M.; Vecitis, C.D. Effect of the oxidation approach on carbon nanotube surface functional groups and electrooxidative filtration performance. J. Mater. Chem. 2015, 3, 7575. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, D.; Williams, T.S.; Severino, J.; Rosa, I.M.D.; Carlson, L.; Hicks, R.F. Rapid oxidative activation of carbon nanotube yarn and sheet by a radio frequency, atmospheric pressure, helium and oxygen plasma. Carbon 2013, 57, 11–21. [Google Scholar] [CrossRef]
- Frackowiak, E.; Be’guin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Silva, T.D.; Damery, C.; Alkhaldi, R.; Karunanithy, R.; Gallaba, D.H.; Patil, P.D.; Wasala, M.; Sivakumar, P.; Migone, A.; Talapatra, S. Carbon nanotube based robust and flexible solid-state supercapacitor. ACS Appl. Mater. 2021, 13, 56004–56013. [Google Scholar] [CrossRef]
- Ye, J.-S.; Liu, X.; Cui, H.F.; Zhang, W.-D.; Sheu, F.-S.; Lim, T.M. Electrochemical oxidation of multi-walled carbon nanotubes and its application to electrochemical double layer capacitors. Electrochem. Commun. 2005, 7, 249–255. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Acauan, L.; Kalfon-Cohen, E.; Ni, X.; Stein, Y.; Gleason, K.K.; Wardle, B.L. Ultrahigh-areal-capacitance flexible supercapacitor electrodes enabled by conformal P3MT on horizontally aligned carbon-nanotube arrays. Adv. Mater. 2019, 31, 1901916. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Y.; Wang, S.; Wang, Q.; Li, S.; Wang, W.; Dong, X. Stretchable supercapacitor based on a hierarchical PPy/CNT electrode and hybrid hydrogel electrolyte with a wide operating temperature. Carbon Energy 2022, 4, 527–538. [Google Scholar] [CrossRef]
- Choi, C.; Kim, J.H.; Sim, H.J.; Di, J.; Baughman, R.H.; Kim, S.J. Microscopically buckled and macroscopically coiled fibers for ultra-stretchable supercapacitors. Adv. Energy Mater. 2017, 7, 1602021. [Google Scholar] [CrossRef]
- Choi, C.; Kim, K.M.; Kim, K.J.; Lepro’, X.; Spinks, G.M.; Baughman, R.H.; Kim, S.J. Improvement of system capacitance via weavable superelastic biscrolled yarn supercapacitors. Nat. Commun. 2016, 7, 13811. [Google Scholar] [CrossRef]
- Choi, C.; Lee, J.M.; Kim, S.H.; Kim, S.J. Twistable and stretchable sandwich structured fiber for wearable sensors and supercapacitors. Nano Lett. 2016, 16, 7677–7684. [Google Scholar] [CrossRef]
- Choi, C.; Sim, H.J.; Spinks, G.M.; Lepró, X.; Baughman, R.H.; Kim, S.J. Elastomeric and dynamic MnO2/CNT core-shell structure coiled yarn supercapacitor. Adv. Energy Mater. 2016, 6, 1502119. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.H.; Sim, H.J.; Lee, J.A.; Choi, A.Y.; Kim, Y.T.; Lepró, X.; Spinks, G.M.; Baughman, R.H.; Kim, S.J. Stretchable, weavable coiled carbon nanotube/MnO2/polymer fiber solid-state supercapacitors. Sci. Rep. 2015, 5, 9387. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lu, W.; Smith, J.P.; Booksh, K.S.; Meng, L.; Huang, Y.; Li, Q.; Byun, J.-H.; Oh, Y.; Yan, Y.; et al. A high performance stretchable asymmetric fiber-shaped supercapacitor with a core-sheath helical structure. Adv. Energy Mater. 2017, 7, 1600976. [Google Scholar] [CrossRef]
- Dawoud, H.D.; Tahtamouni, T.A.; Bensalah, N. Sputtered manganese oxide thin film on carbon nanotubes sheet as a flexible and binder-ree electrode for supercapacitor. Int. J. Energy Res. 2019, 43, 1245–1254. [Google Scholar] [CrossRef]
- Son, W.; Lee, J.M.; Kim, S.H.; Kim, H.W.; Cho, S.B.; Suh, D.; Chun, S.; Choi, C. High-power hydro-actuators fabricated from biomimetic carbon nanotube coiled yarns with fast electrothermal recovery. Nano Lett. 2022, 22, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.G.; Cheng, H.K.F.; Cai, J.; Li, L.; Chan, S.H.; Zhao, J.; Yu, S. Improvement of Mechanical and Thermal Properties of Carbon Nanotube Composites through Nanotube Functionalization and Processing Methods. Mater. Chem. Phys. 2009, 117, 313–320. [Google Scholar] [CrossRef]
- Pei, X.W.; Liu, W.M.; Hao, J. Functionalization of multiwalled carbon nanotube via surface reversible addition fragmentation chain transfer polymerization and as lubricant additives. J. Polym. Sci. Polym. Chem. 2008, 46, 3014–3023. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Hu, Z.; Huang, Y.-C.; Wu, X.; Dai, J.; Dong, C.-L.; Xu, X.; Lin, H.-J.; Che, C.-T.; et al. Exceptional lattice-oxygen participation on artificially controllable electrochemistry-induced crystalline-amorphous phase to boost oxygen-evolving performance. Appl. Catal. 2021, 297, 120484. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, M.; Seo, H.; Choi, J.; Noh, J.H.; Kim, J.; Jeon, J.; Choi, C. Transition of Carbon Nanotube Sheets from Hydrophobicity to Hydrophilicity by Facile Electrochemical Wetting. Nanomaterials 2023, 13, 2834. https://doi.org/10.3390/nano13212834
Oh M, Seo H, Choi J, Noh JH, Kim J, Jeon J, Choi C. Transition of Carbon Nanotube Sheets from Hydrophobicity to Hydrophilicity by Facile Electrochemical Wetting. Nanomaterials. 2023; 13(21):2834. https://doi.org/10.3390/nano13212834
Chicago/Turabian StyleOh, Myoungeun, Hyunji Seo, Jimin Choi, Jun Ho Noh, Juwan Kim, Joonhyeon Jeon, and Changsoon Choi. 2023. "Transition of Carbon Nanotube Sheets from Hydrophobicity to Hydrophilicity by Facile Electrochemical Wetting" Nanomaterials 13, no. 21: 2834. https://doi.org/10.3390/nano13212834




