Dewatered Sludge Decorated with Nanoparticles for Alum Sludge Conditioning towards the Concept of “End-of-Waste”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TAS-cake@Fe-(5-1) Conditioner
2.3. Morphological and Structural Characterization
2.4. Co-Conditioning Procedure
3. Results and Discussion
3.1. TAS-cake@Fe-(5-1) Composite Conditioner Characterization
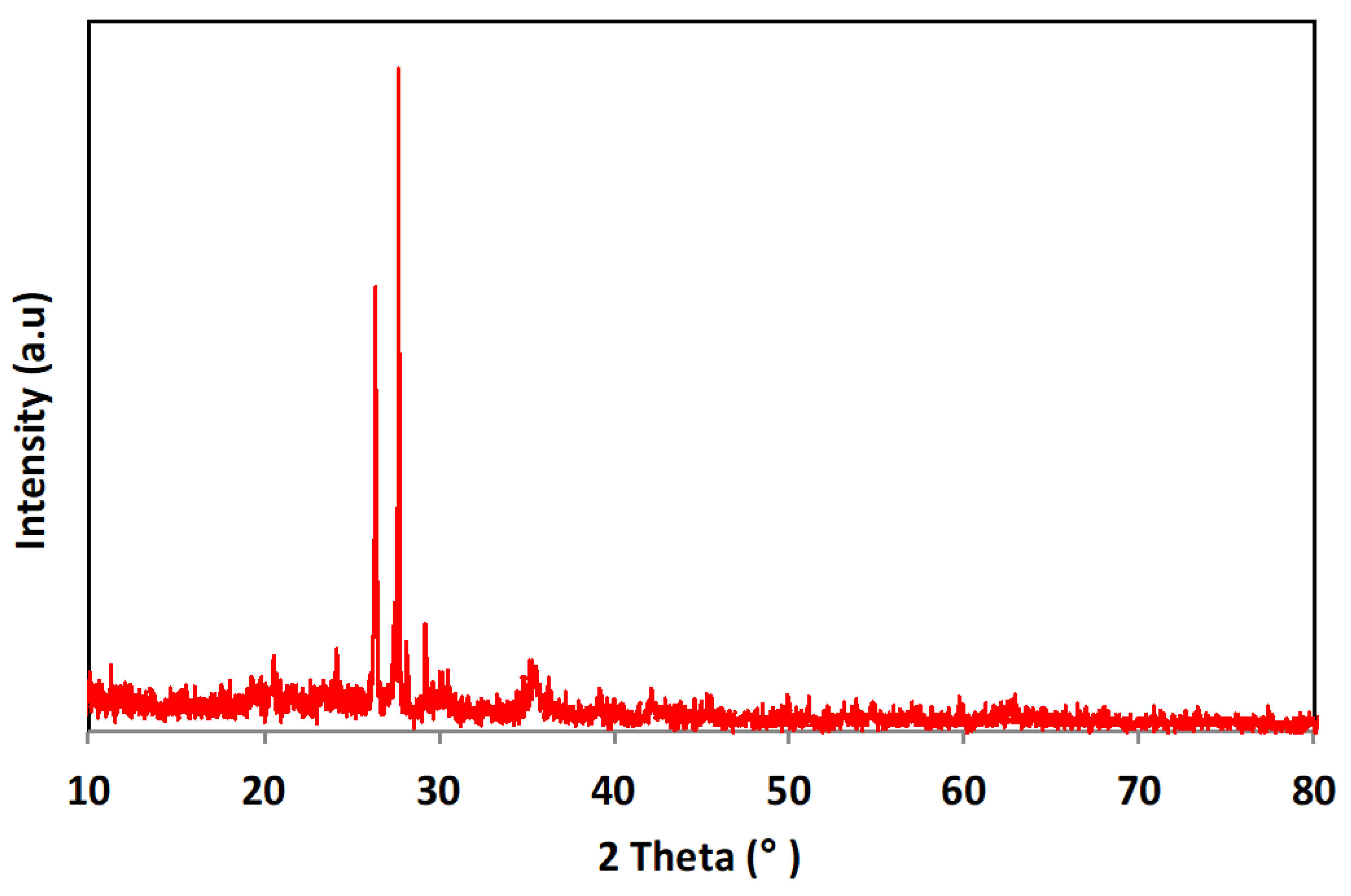
3.1.1. X-ray Diffraction Pattern
3.1.2. Fourier-Transform Infrared Spectroscopy
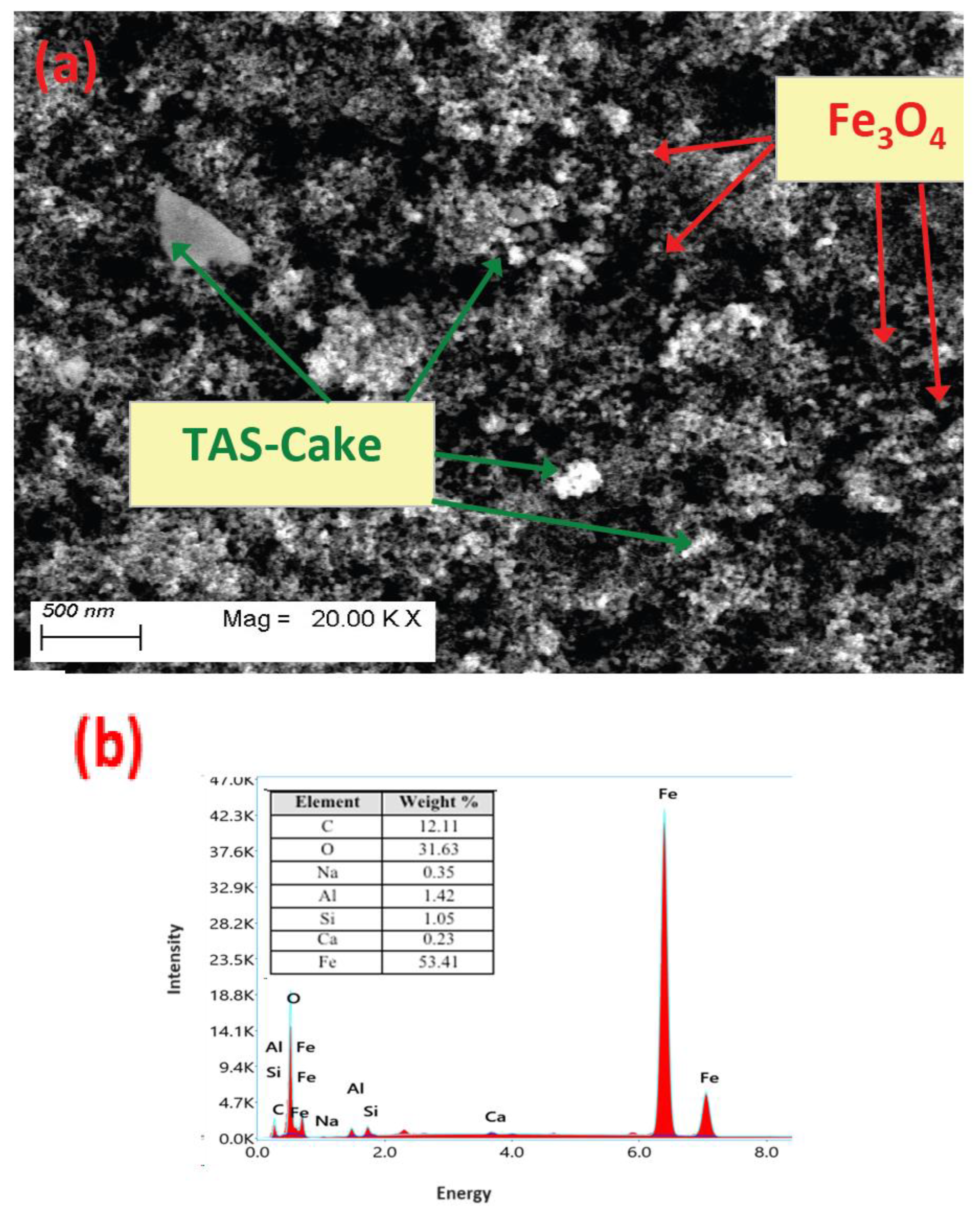
3.1.3. Scanning Electron Microscope Image
3.1.4. Transmission Electron Microscope Image
3.2. Aluminum-Based Sludge Dewatering Performances
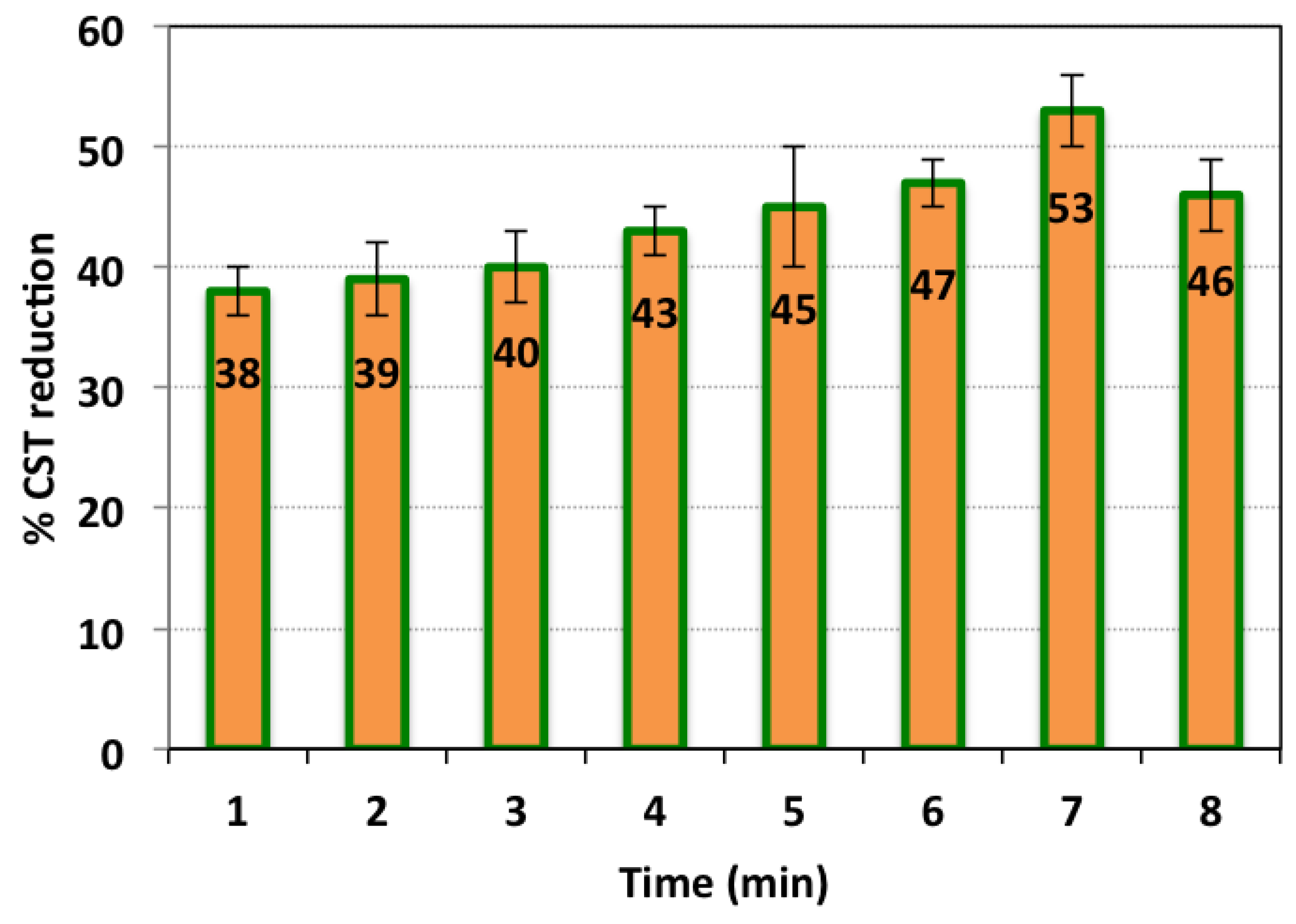
3.2.1. Conditioning Time Based on Hybrid Conditioner
3.2.2. Effect of TAS-cake@Fe-(5-1) Dose
3.2.3. Effect of Hydrogen Peroxide Dose
3.2.4. Effect of pH Value
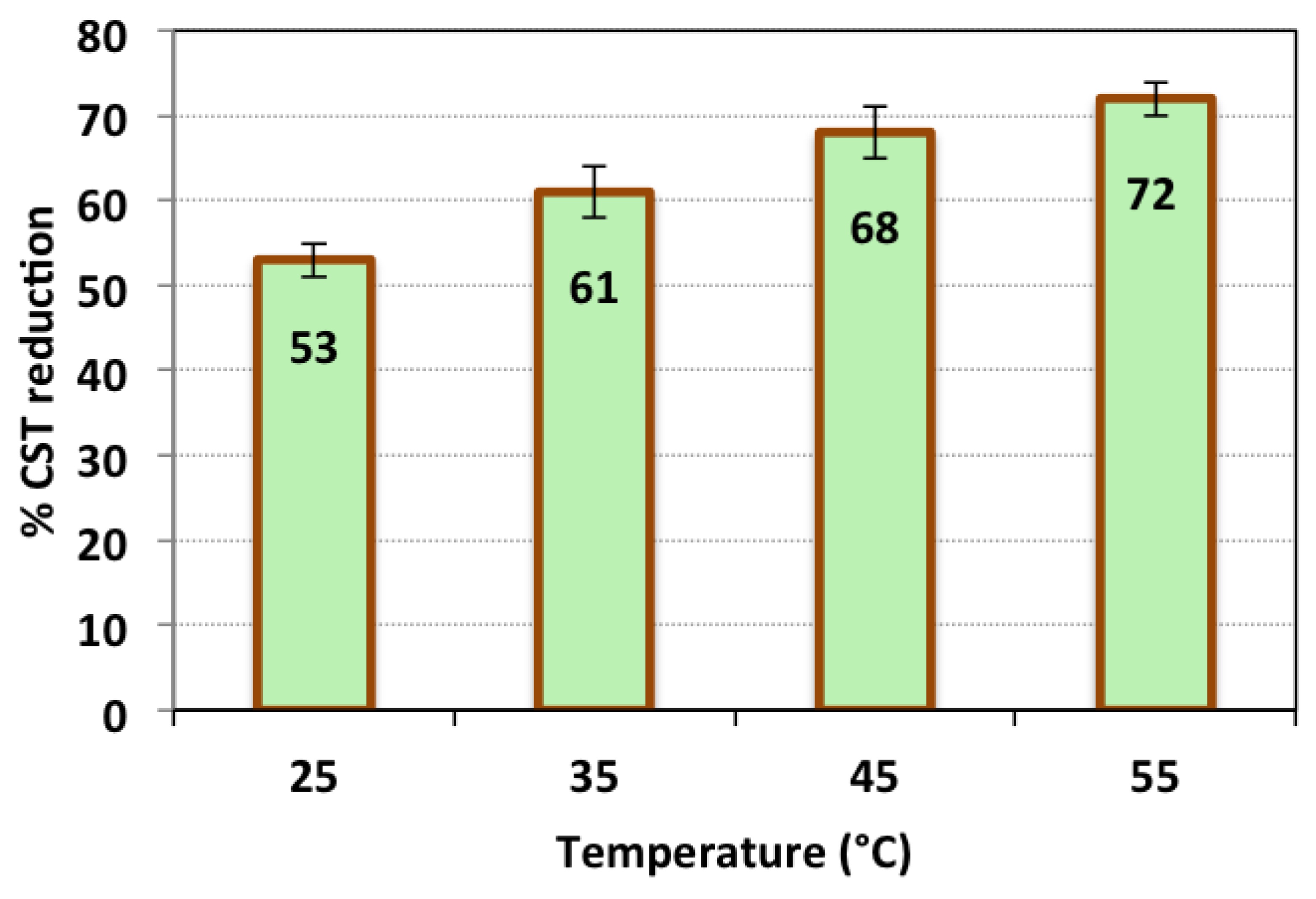
3.2.5. Effect of Temperature
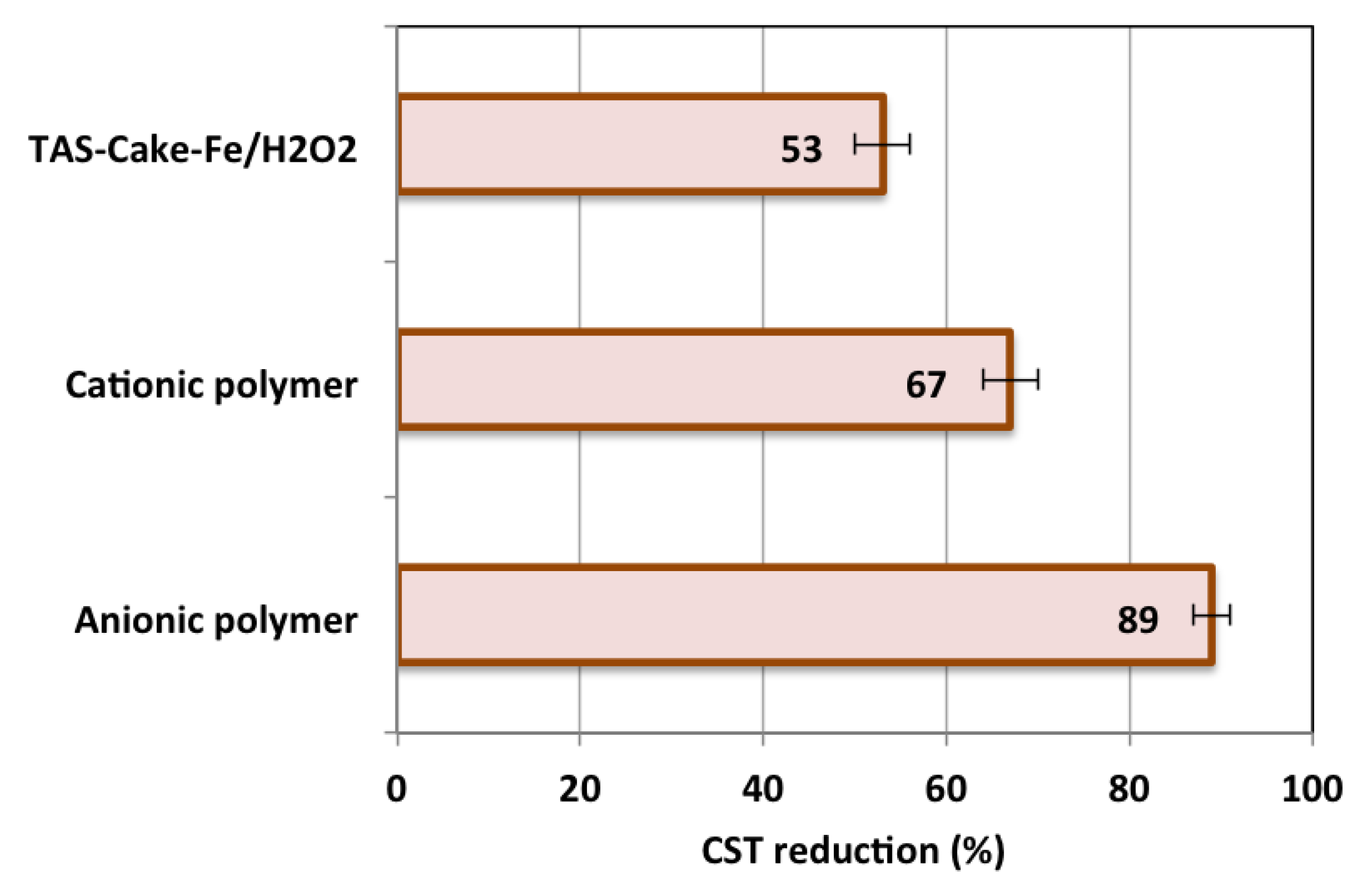
3.2.6. Comparison of Fenton with Commercial Conditioners
3.2.7. Comparative Investigation
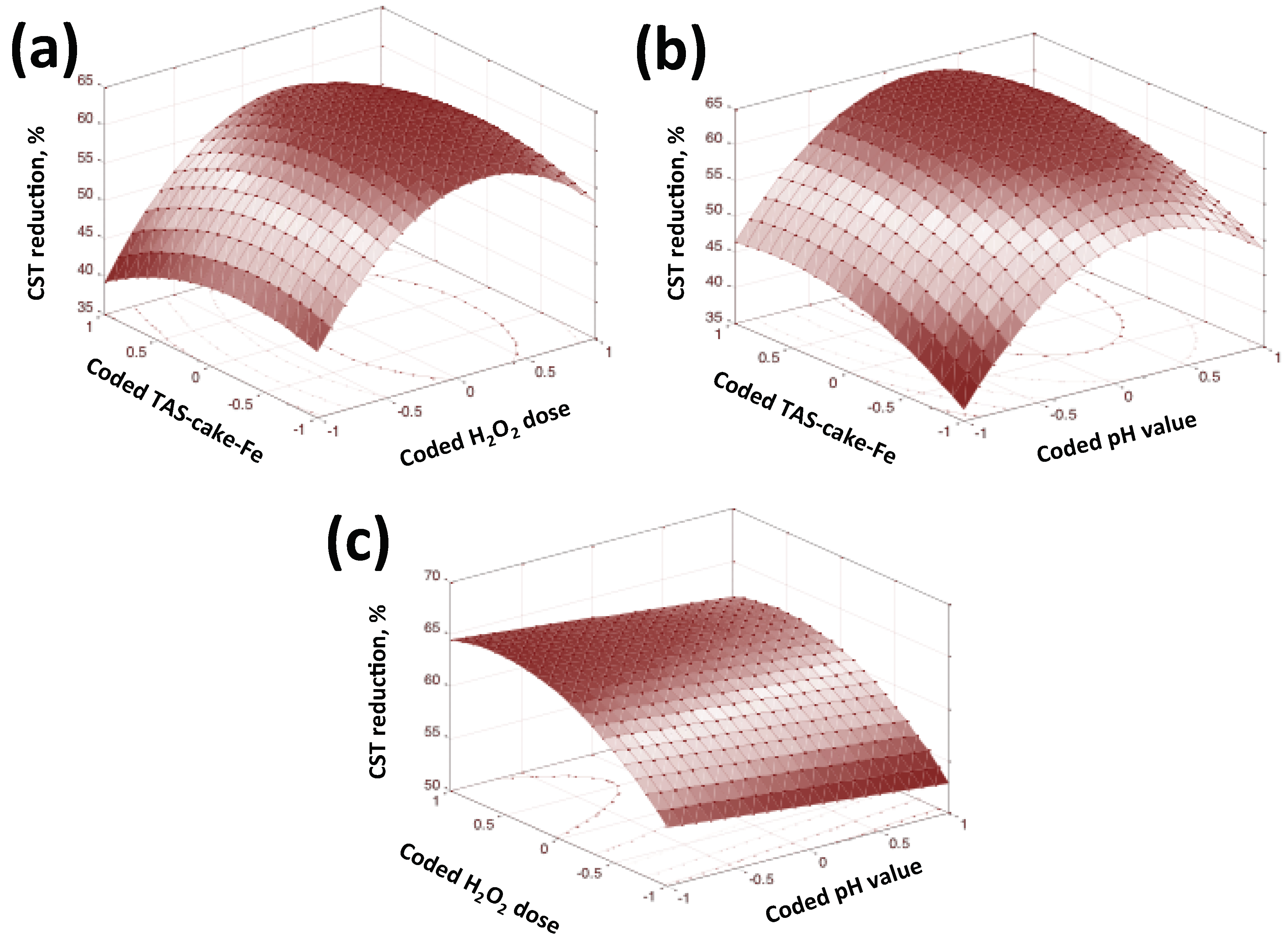
3.2.8. Statistical Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Babatunde, A.O.; Zhao, Y.Q.; Yang, Y.; Kearney, P. From “fills” to filter: Insights into the reuse of dewatered alum sludge as a filter media in a constructed wetland. J. Residuals Sci. Technol. 2007, 4, 147–152. [Google Scholar]
- Dassanayake, K.B.; Jayasinghe, G.Y.; Surapaneni, A.; Hetherington, C. A review on alum sludge reuse with special reference to agricultural applications and future challenges. Waste Manag. 2015, 38, 321–335. [Google Scholar] [CrossRef]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
- Zhijun, R.; Pengfei, W.; Jiayu, T.; Zhiliu, Z. Effects of a low-strength magnetic field on the characteristics of activated sludge for membrane fouling mitigation. RSC Adv. 2019, 9, 9180–9186. [Google Scholar] [CrossRef]
- Bi, D.S.; Guo, X.P.; Cai, Z.H.; Yu, Z.; Wang, D.M.; Wang, Y.Q. Enhanced dewaterability of waste-activated sludge by combined cationic polyacrylamide and magnetic field pretreatment. Environ. Technol. 2015, 36, 455–462. [Google Scholar] [CrossRef]
- Pambou, Y.B.; Fraikin, L.; Salmon, T.; Crine, M.; Leonard, A. Enhanced sludge dewatering and drying comparison of two linear polyelectrolytes co-conditioning with polyaluminum chloride. Desalination Water Treat. 2016, 57, 27989–28006. [Google Scholar] [CrossRef]
- Guo, J.; Chen, C.; Jiang, S.; Zhou, Y. Feasibility and mechanism of combined conditioning with coagulant and flocculant to enhance sludge dewatering. ACS Sustain. Chem. Eng. 2018, 6, 10758–10765. [Google Scholar] [CrossRef]
- Xu, Z.X.; Song, H.; Deng, X.Q.; Zhang, Y.Y.; Xue-Qin, M.; Tong, S.Q.; He, Z.X.; Wang, Q.; Shao, Y.W.; Hu, X. Dewatering of sewage sludge via thermal hydrolysis with ammonia-treated Fenton iron sludge as skeleton material. J. Hazard. Mater. 2019, 379, 120810. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, P.; Wang, H.; Ye, J. Conditioning of sewage sludge via combined ultrasonication-flocculation-skeleton building to improve sludge dewaterability. Ultrason. Sonochem. 2018, 40, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Lyczko, N.; Zhao, Y.Q.; Nzihou, A. Integrating alum sludge with waste-activated sludge in co-conditioning and dewatering: A case study of a city in south France. Environ. Sci. Pollut. Res. 2020, 27, 14863–14871. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, M.; Jenkins, D.; Parker, D.S. A unified theory of filamentous activated sludge bulking. J. Water Pollut. Control. Fed. 1978, 50, 362–381. [Google Scholar]
- Zhou, X.; Jiang, G.; Wang, Q.; Yuan, Z. A review on sludge conditioning by sludge pre-treatment with a focus on advanced oxidation. RSC Adv. 2014, 4, 50644–50652. [Google Scholar] [CrossRef]
- Rebosura, M., Jr.; Salehin, S.; Pikaar, I.; Kulandaivelu, J.; Jiang, G.; Keller, J.; Sharma, K.; Yuan, Z. Effects of in-sewer dosing of iron-rich drinking water sludge on wastewater collection and treatment systems. Water Res. 2020, 171, 115396. [Google Scholar] [CrossRef] [PubMed]
- Maamoun, I.; Falyouna, O.; Eljamal, R.; Bensaida, K.; Tanaka, K.; Tosco, T.; Sugihara, Y.; Eljamal, O. Multi-functional magnesium hydroxide coating for iron nanoparticles towards prolonged reactivity in Cr (VI) removal from aqueous solutions. J. Environ. Chem. Eng. 2022, 10, 107431. [Google Scholar] [CrossRef]
- Falyouna, O.; Bensaida, K.; Maamoun, I.; Ashik, U.P.M.; Tahara, A.; Tanaka, K.; Aoyagi, N.; Sugihara, Y.; Eljamal, O. Synthesis of hybrid magnesium hydroxide/magnesium oxide nanorods [Mg(OH)2/MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutions. J. Clean. Prod. 2022, 342, 130949. [Google Scholar] [CrossRef]
- Eljamal, O.; Jinno, K.; Hosokawa, T. Modeling of solute transport with bioremediation processes using sawdust as a matrix. Water Air Soil Pollut. 2008, 195, 115–127. [Google Scholar] [CrossRef]
- Falyouna, O.; Idham, M.F.; Maamoun, I.; Bensaida, K.; Ashik, U.P.M.; Sugihara, Y.; nd Eljamal, O. Promotion of ciprofloxacin adsorption from contaminated solutions by oxalate modified nanoscale zerovalent iron particles. J. Mol. Liq. 2022, 359, 119323. [Google Scholar] [CrossRef]
- Eljamal, O.; Maamoun, I.; Alkhudhayri, S.; Eljamal, R.; Falyouna, O.; Tanaka, K.; Kozai, N.; Sugihara, Y. Insights into boron removal from water using Mg-Al-LDH: Reaction parameters optimization & 3D-RSM modeling. J. Water Process Eng. 2022, 46, 102608. [Google Scholar]
- Zhou, X.; Jin, W.; Wang, L.; Che, L.; Chen, C.; Li, S.F.; Wang, X.T.; Tu, R.; Han, S.F.; Feng, X.; et al. Alum sludge conditioning with ferrous iron/peroxymonosulfate oxidation: Characterization and mechanism. Korean J. Chem. Eng. 2020, 37, 663–669. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; Ali, I.; El Sherbiney, S.A.; Tony, M.A. Magnetite-based nanoparticles as an efficient hybrid heterogeneous adsorption/oxidation process for reactive textile dye removal from wastewater matrix. Int. J. Environ. Anal. Chem. 2023, 103, 2636–2658. [Google Scholar] [CrossRef]
- Falyouna, O.; Maamoun, I.; Bensaida, K.; Tahara, A.; Sugihara, Y.; Eljamal, O. Encapsulation of iron nanoparticles with magnesium hydroxide shell for remarkable removal of ciprofloxacin from contaminated water. J. Colloid Interface Sci. 2022, 605, 813–827.–827. [Google Scholar] [CrossRef]
- Ramadan, H.; El Sayed, A.E.A. Optimization of alum recovery from water treatment sludge-case study: Samannoud water treatment plant, Egypt. Water Environ. J. 2020, 34, 464–473. [Google Scholar] [CrossRef]
- Rashad, M.M.; El-Sayed, I.E.; Galhoum, A.A.; Abdeen, M.M.; Mira, H.I.; Elshehy, E.A.; Zhang, S.; Lu, X.; Xin, J.; Guibal, E. Synthesis of α-aminophosphonate based sorbents–Influence of inserted groups (carboxylic vs. amine) on uranyl sorption. Chem. Eng. J. 2021, 421, 127830. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, T.P.; Pham, T.N.; Tran, T.T.N.; Tran, T.V.T. Synthesis and characterization of chitosan-coated magnetite nanoparticles and their application in curcumin drug delivery. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045010. [Google Scholar] [CrossRef]
- Kamizela, T.; Kowalczyk, M. Impact of conditioning substances and filtration pressure on dewatering efficiency of sewage sludge. Energies 2021, 14, 361. [Google Scholar] [CrossRef]
- Pati, S.; Mahendran, V.; Philip, J. A simple approach to produce stable ferrofluids without surfactants and with high temperature stability. J. Nanofluids 2013, 2, 94–103. [Google Scholar] [CrossRef]
- Repo, E.; Mäkinen, M.; Rengaraj, S.; Natarajan, G.; Bhatnagar, A.; Sillanpää, M. Lepidocrocite and its heat-treated forms as effective arsenic adsorbents in aqueous medium. Chem. Eng. J. 2012, 180, 159–169. [Google Scholar] [CrossRef]
- Singh, C.; Chaudhary, R.; sGandhi, K. Preliminary study on optimization of pH, oxidant and catalyst dose for high COD content: Solar parabolic trough collector. Iran. J. Environ. Health Sci. Eng. 2013, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, M.; Siminiceanu, I.; Yediler, A.; Kettrup, A. Kinetics of decolorization and mineralization of reactive azo dyes in aqueous solution by the UV/H2O2 oxidation. Dye. Pigment. 2002, 53, 93–99. [Google Scholar] [CrossRef]
- Rezgui, S.; Díez, A.M.; Monser, L.; Adhoum, N.; Pazos, M.; Sanromán, M.A. ZnFe2O4-chitosan magnetic beads for the removal of chlordimeform by photo-Fenton process under UVC irradiation. J. Environ. Manag. 2021, 283, 111987. [Google Scholar] [CrossRef] [PubMed]
- Pourali, P.; Behzad, M.; Arfaeinia, H.; Ahmadfazeli, A.; Afshin, S.; Poureshgh, Y.; Rashtbari, Y. Removal of acid blue 113 from aqueous solutions using low-cost adsorbent: Adsorption isotherms, thermodynamics, kinetics and regeneration studies. Sep. Sci. Technol. 2021, 56, 3079–3091. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; El Sherbiney, S.A.; Tony, M.A. Solar assisted green photocatalysis for deducing carbamate insecticide from agriculture stream into water reclaiming opportunity. Int. J. Environ. Anal. Chem. 2022, 1–23. [Google Scholar] [CrossRef]
- Tony, M.A. Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 2485–2498. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; Sherbiny, S.A.E.; Tony, M.A. Zero-Waste Approach: Assessment of Aluminum-Based Waste as a Photocatalyst for Industrial Wastewater Treatment Ecology. Int. J. Environ. Res. 2022, 16, 36. [Google Scholar] [CrossRef]
- Tantawy, M.A. Characterization and pozzolanic properties of calcined alum sludge. Mater. Res. Bull. 2015, 61, 415–421. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Mafhouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Atia, A.A.; Vincent, T.; Guibal, E. Cysteine-Functionalized Chitosan Magnetic Nano-Based Particles for the Recovery of Uranium(VI): Uptake Kinetics and Sorption Isotherms. Sep. Sci. Technol. 2015, 50, 2776–2789. [Google Scholar] [CrossRef]
- Duman, O.; Özcan, C.; Polat, T.G.; Tunç, S. Carbon nanotube-based magnetic andnon-magnetic adsorbents for the high-efficiency removal of diquat dibromide herbicide from water: OMWCNT, OMWCNT-Fe3O4 and OMWCNT-κ-carrageenan-Fe3O4 nanocomposites. Environ. Pollut. 2019, 244, 723–732. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Amani, M.; Shakeri, A. Synthesis and Characterization of Water-Based Epoxy-Acrylate/Graphene Oxide Decorated with Fe3O4 Nanoparticles Coatings and Its Enhanced Anticorrosion Properties. Polym. Plast. Technol. Mater. 2020, 59, 1910–1931. [Google Scholar]
- Wang, J.; Meng, X.; Chen, Y.; Zheng, G.; Zhou, L. Simultaneously attenuating antibiotic resistance genes and improving the dewaterability of sewage sludge by conditioning with Fenton’s reagent: The pivotal role of sludge pre-acidification. Environ. Sci. Pollut. Res. 2021, 28, 13300–13311. [Google Scholar] [CrossRef]
- Rozhkovskaya, A.; Rajapakse, J.; Millar, G.J. Synthesis of high-quality zeolite LTA from alum sludge generated in drinking water treatment plants. J. Environ. Chem. Eng. 2021, 9, 104751. [Google Scholar] [CrossRef]
- Mo, R.; Huang, S.; Dai, W.; Liang, J.; Sun, S. A rapid Fenton treatment technique for sewage sludge dewatering. Chem. Eng. J. 2015, 269, 391–398. [Google Scholar] [CrossRef]
- Dawood, A.S.; Li, Y. Modeling and optimization of new flocculant dosage and pH for flocculation: Removal of pollutants from wastewater. Water 2013, 5, 342–355. [Google Scholar] [CrossRef]
- Kwon, J.H.; Park, K.Y.; Park, J.H.; Lee, S.H.; Ahn, K.H. Acidic and hydrogen peroxide treatment of polyaluminum chloride (PACL) sludge from water treatment. Water Sci. Technol. 2004, 50, 99–105. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Bao, B.; Sun, M.; Chen, J.; Luo, H.; Li, J. Enhanced dewatering of activated sludge by acid assisted Heat–CaO2 treatment: Simultaneously removing heavy metals and mitigating antibiotic resistance genes. J. Hazard. Mater. 2021, 418, 126248. [Google Scholar] [CrossRef] [PubMed]
- Tony, M.A.; Tayeb, A.A. Response surface regression model in optimization of alum sludge drying facility: Solar-fenton’s reagent dewatering. Int. J. Chem. Eng. Appl. 2016, 7, 331. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Babatunde, A.; Wang, L.; Ren, Y.; Han, Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Wang, B.; Zhao, Y.; Chai, X.; Niu, D.; Zhao, A.; Li, Y.; Song, Y.; Cao, X. Synergetic pretreatment of waste activated sludge by Fe (II)–activated persulfate oxidation under mild temperature for enhanced dewaterability. Bioresour. Technol. 2012, 124, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mustranta, A.; Viikari, L. Dewatering of activated sludge by an oxidative treatment. Water Sci. Technol. 1993, 28, 213–221. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.-S.; Gu, G. Influence of pretreating activated sludge with acid and surfactant prior to conventional conditioning on filtration dewatering. Chem. Eng. J. 2004, 99, 137–143. [Google Scholar] [CrossRef]
- Ding, N.; Peng, C.; Ren, Y.; Liu, Y.; Wang, P.; Dong, L.; Liu, H.; Wang, D. Improving the dewaterability of citric acid wastewater sludge by Fenton treatment. J. Clean. Prod. 2018, 196, 739–746. [Google Scholar] [CrossRef]
- Fontmorin, J.-M.; Sillanpää, M. Dewatering and removal of metals from urban anaerobically digested sludge by Fenton’s oxidation. Environ. Technol. 2017, 38, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, R.; Merzari, F.; Andreottola, G. Optimisation of Fe2+/H2O2 ratio in Fenton process to increase dewaterability and solubilisation of sludge. Environ. Technol. 2020, 41, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Masihi, H.; Gholikandi, G.B. Employing Electrochemical-Fenton process for conditioning and dewatering of anaerobically digested sludge: A novel approach. Water Res. 2018, 144, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, S.; Dai, Y.; Li, L.; Sun, S. Dewaterability of five sewage sludges in Guangzhou conditioned with Fenton’s reagent/lime and pilot-scale experiments using ultrahigh pressure filtration system. Water Res. 2015, 84, 243–254. [Google Scholar] [CrossRef]






| Peak | Wavenumber (cm−1) |
|---|---|
| Fe–O bond | 420 and 556 |
| O–Si–O bending vibration | 467.65 |
| Al–Mg–OH bending vibration | 1201.5 |
| C=O bending | 1420.32 |
| C=C | 1629 |
| O–H stretching vibration | 3440.38 |
| Fenton Conditioner | Sludge | Time Required, min | Operational Circumstances * | Performance, % | Ref. |
|---|---|---|---|---|---|
| TAS-cake@Fe-(5-1)/H2O2 | Aluminum-based sludge | 7 | TAS-cake@Fe-(5-1) (101 mg/g-DS); H2O2 (62 mg/g-DS); pH 3.0 | 53% | Current study |
| FeSO4/H2O2 | Wastewater sludge | 63 | FeSO4.7H2O (80 mg/g-DS); H2O2 (20 mg/g-DS); pH 5.0 | 85% | [52] |
| Fe2+/H2O2 | Wastewater sludge | 120 | Fe2+ (36 mM); H2O2 (360mM) | 98% | [53] |
| Fe2+/H2O2 | Wastewater sludge | 30 | Fe2+ (0.1 g/g-DS), pH 5.8, | 79% | [20] |
| Fe2+/H2O2 | Wastewater sludge | 60 | Fe2+ (750 mg/g-DS); H2O2 (825 mg/g-DS); pH 2.0 | 76% | [54] |
| Fe2+/H2O2 | Municipal sludge | 60 | Fe2+ (32 mg/g-DS); H2O2 (40 mg/g-DS); pH 3.0 | 70% | [46] |
| Electro-Fenton | Industrial sludge | 60 | Fe2+ (15 mg/g-DS); H2O2 (25 mg/g DS) | 94% | [55] |
| Fenton/Lime | Sewage sludge | 90 | Fe2+ (50 mg/g-DS); H2O2 (30 mg/g DS); pH 3.0; lime (50 mg/g DS) | 96% | [43] |
| Fenton/Lime | Municipal sludge | 120 | Fe2+ (50 mg/g DS), H2O2 (30 mg/g DS); pH 3.0; lime (50 mg/g DS) | 95% | [56] |
| Exp. no. | Variables | |||||
|---|---|---|---|---|---|---|
| TAS-cake@Fe-(5-1) Dos | H2O2 Dose | pH-Value | ||||
| Coded | Un-Coded | Coded | Un-Coded | Coded | Un-Coded | |
| 1 | −1 | 0.5 | −1 | 700 | 0 | 3.0 |
| 2 | −1 | 0.5 | 1 | 900 | 0 | 3.0 |
| 3 | 1 | 1.5 | −1 | 700 | 0 | 3.0 |
| 4 | 1 | 1.5 | 1 | 900 | 0 | 3.0 |
| 5 | 0 | 1.0 | −1 | 700 | −1 | 2.5 |
| 6 | 0 | 1.0 | −1 | 700 | 1 | 3.5 |
| 7 | 0 | 1.0 | 1 | 900 | −1 | 2.5 |
| 8 | 0 | 1.0 | 1 | 900 | 1 | 3.5 |
| 9 | −1 | 0.5 | 0 | 800 | −1 | 2.5 |
| 10 | 1 | 1.5 | 0 | 800 | −1 | 2.5 |
| 11 | −1 | 0.5 | 0 | 800 | 1 | 3.5 |
| 12 | 1 | 1.5 | 0 | 800 | 1 | 3.5 |
| 13 | 0 | 1.0 | 0 | 800 | 0 | 3.0 |
| 14 | 0 | 1.0 | 0 | 800 | 0 | 3.0 |
| 15 | 0 | 1.0 | 0 | 800 | 0 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabwey, H.A.; Tony, M.A. Dewatered Sludge Decorated with Nanoparticles for Alum Sludge Conditioning towards the Concept of “End-of-Waste”. Nanomaterials 2023, 13, 2903. https://doi.org/10.3390/nano13212903
Nabwey HA, Tony MA. Dewatered Sludge Decorated with Nanoparticles for Alum Sludge Conditioning towards the Concept of “End-of-Waste”. Nanomaterials. 2023; 13(21):2903. https://doi.org/10.3390/nano13212903
Chicago/Turabian StyleNabwey, Hossam A., and Maha A. Tony. 2023. "Dewatered Sludge Decorated with Nanoparticles for Alum Sludge Conditioning towards the Concept of “End-of-Waste”" Nanomaterials 13, no. 21: 2903. https://doi.org/10.3390/nano13212903







