Highly Selective CO2 Hydrogenation to Methanol over Complex In/Co Catalysts: Effect of Polymer Frame
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. CO2 Hydrogenation Reaction
2.3. Catalyst Characterization
3. Results and Discussion
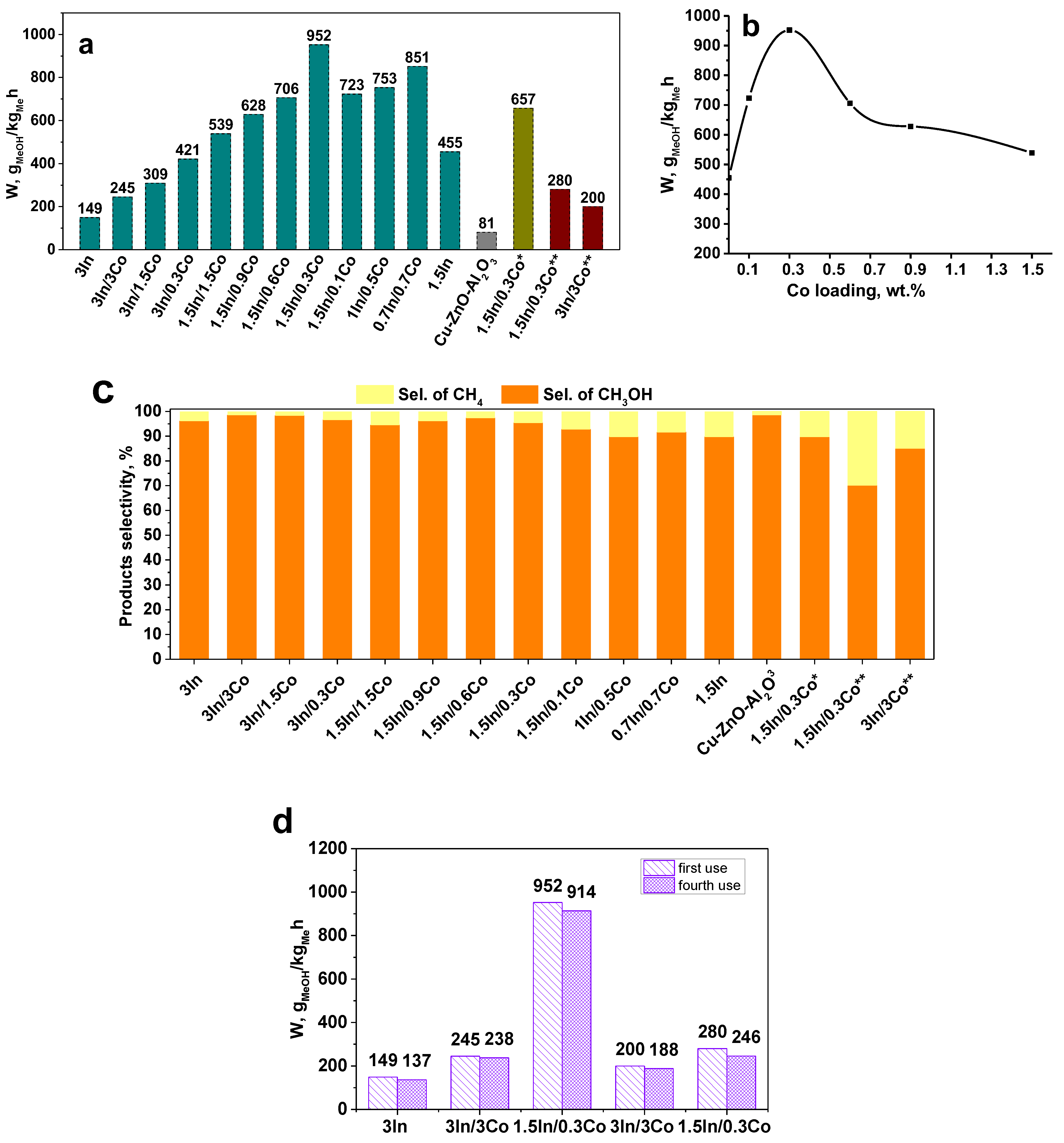
3.1. Catalytic Performance
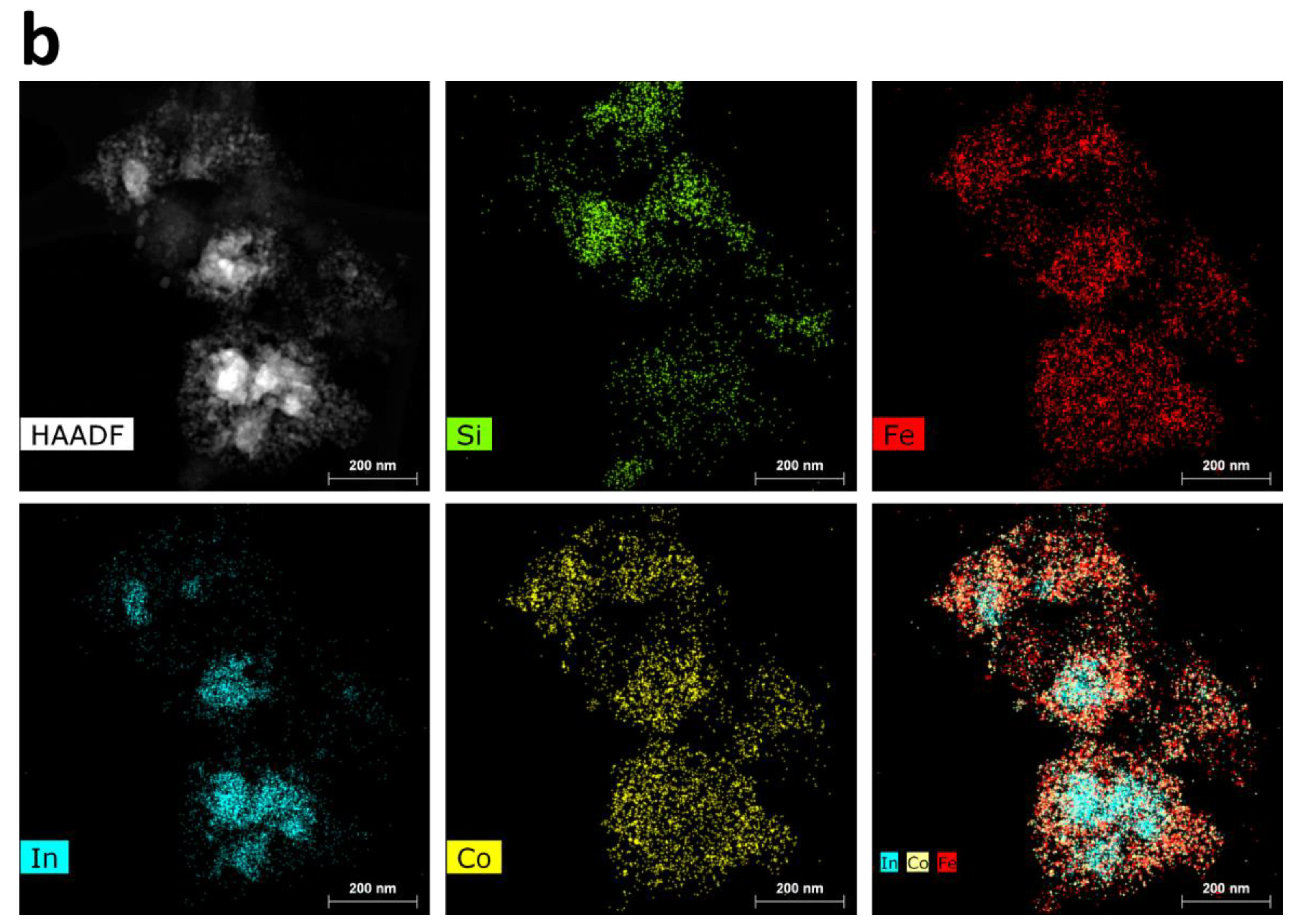
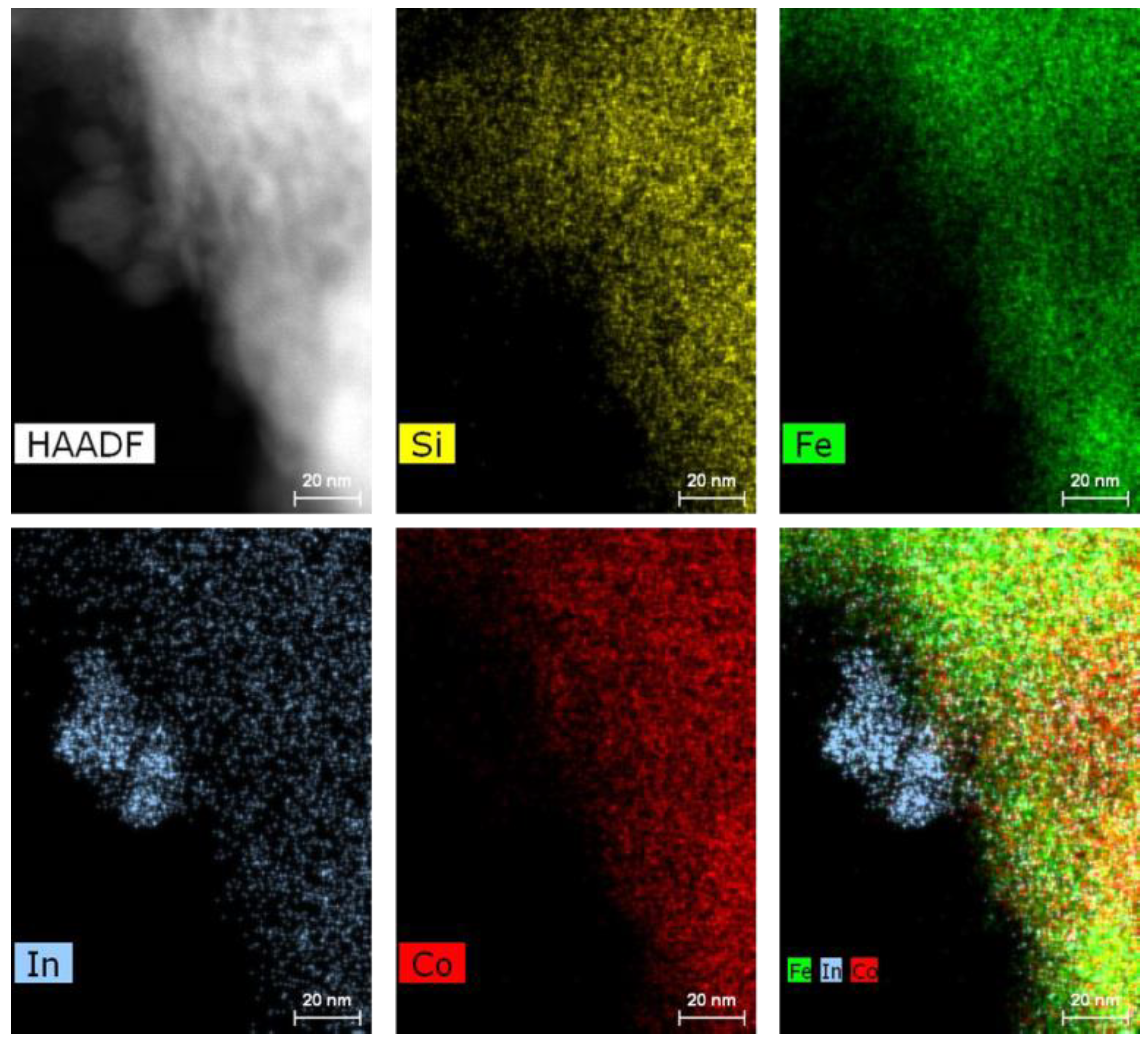
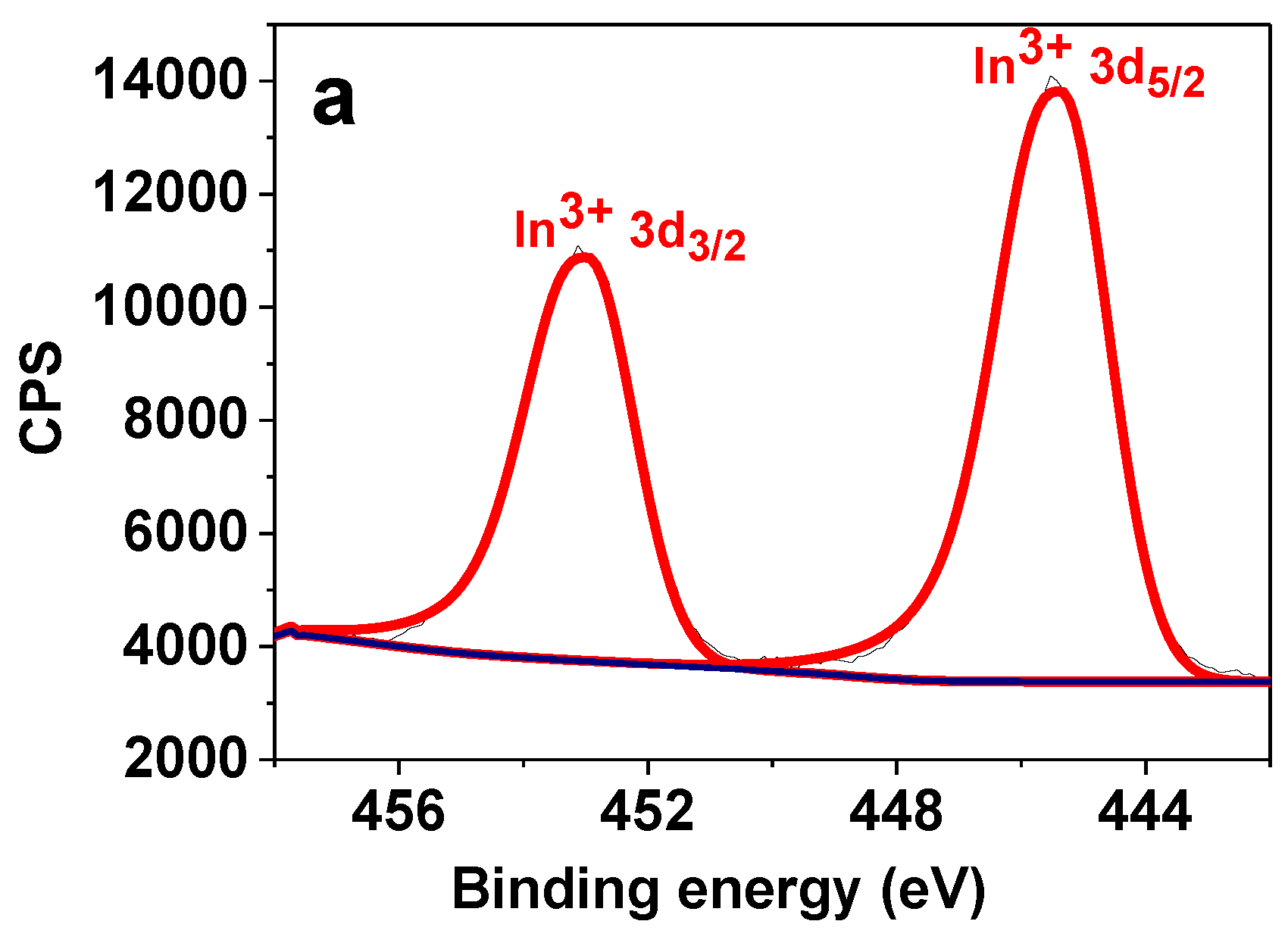
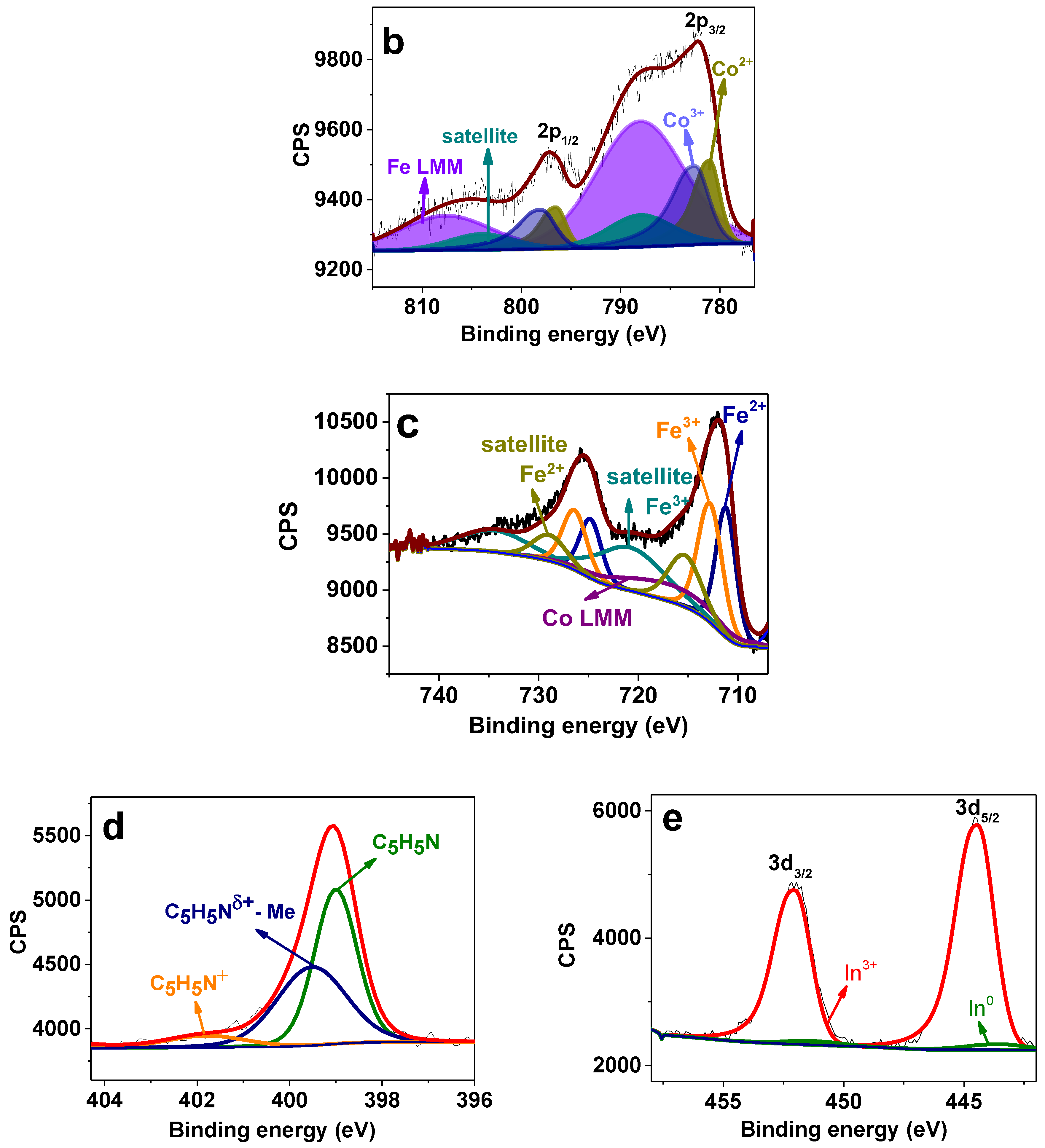
3.2. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 596. [Google Scholar] [CrossRef]
- Bowker, M. Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning Selectivity of CO2 Hydrogenation Reactions at the Metal/Oxide Interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Liang, B.; Ma, J.; Su, X.; Yang, C.; Duan, H.; Zhou, H.; Deng, S.; Li, L.; Huang, Y. Investigation on Deactivation of Cu/ZnO/Al2O3 Catalyst for CO2 Hydrogenation to Methanol. Ind. Eng. Chem. Res. 2019, 58, 9030–9037. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.; Mei, D.; Ge, Q. Active Oxygen Vacancy Site for Methanol Synthesis from CO2 Hydrogenation on In2O3(110): A DFT Study. ACS Catal. 2013, 3, 1296–1306. [Google Scholar] [CrossRef]
- Sun, K.; Fan, Z.; Ye, J.; Yan, J.; Ge, Q.; Li, Y.; He, W.; Yang, W.; Liu, C.-j. Hydrogenation of CO2 to methanol over In2O3 catalyst. J. CO2 Util. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Cao, A.; Wang, Z.; Li, H.; Nørskov, J.K. Relations between Surface Oxygen Vacancies and Activity of Methanol Formation from CO2 Hydrogenation over In2O3 Surfaces. ACS Catal. 2021, 11, 1780–1786. [Google Scholar] [CrossRef]
- Shen, C.; Sun, K.; Zhang, Z.; Rui, N.; Jia, X.; Mei, D.; Liu, C.-j. Highly Active Ir/In2O3 Catalysts for Selective Hydrogenation of CO2 to Methanol: Experimental and Theoretical Studies. ACS Catal. 2021, 11, 4036–4046. [Google Scholar] [CrossRef]
- Rui, N.; Zhang, F.; Sun, K.; Liu, Z.; Xu, W.; Stavitski, E.; Senanayake, S.D.; Rodriguez, J.A.; Liu, C.-J. Hydrogenation of CO2 to Methanol on a Auδ+–In2O3–x Catalyst. ACS Catal. 2020, 10, 11307–11317. [Google Scholar] [CrossRef]
- Jia, X.; Sun, K.; Wang, J.; Shen, C.; Liu, C.-j. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst. J. Energy Chem. 2020, 50, 409–415. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Kley, K.S.; Puértolas, B.; López, N.; Safonova, O.V.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 2019, 10, 3377. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-j. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Fang, T.; Liu, B.; Lian, Y.; Zhang, Z. Selective Methanol Synthesis from CO2 Hydrogenation over an In2O3/Co/C-N Catalyst. Ind. Eng. Chem. Res. 2020, 59, 19162–19167. [Google Scholar] [CrossRef]
- Pustovarenko, A.; Dikhtiarenko, A.; Bavykina, A.; Gevers, L.; Ramírez, A.; Russkikh, A.; Telalovic, S.; Aguilar, A.; Hazemann, J.-L.; Ould-Chikh, S.; et al. Metal–Organic Framework-Derived Synthesis of Cobalt Indium Catalysts for the Hydrogenation of CO2 to Methanol. ACS Catal. 2020, 10, 5064–5076. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Wang, J.; Li, L.; Li, C. Atomically dispersed Ptn+ species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 2021, 394, 236–244. [Google Scholar] [CrossRef]
- Dong, C.; Mu, R.; Li, R.; Wang, J.; Song, T.; Qu, Z.; Fu, Q.; Bao, X. Disentangling Local Interfacial Confinement and Remote Spillover Effects in Oxide–Oxide Interactions. J. Am. Chem. Soc. 2023, 145, 17056–17065. [Google Scholar] [CrossRef]
- Shen, H.; Li, H.; Yang, Z.; Li, C. Magic of hydrogen spillover: Understanding and application. Green Energy Environ. 2022, 7, 1161–1198. [Google Scholar] [CrossRef]
- Parastaev, A.; Muravev, V.; Huertas Osta, E.; van Hoof, A.J.F.; Kimpel, T.F.; Kosinov, N.; Hensen, E.J.M. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 2020, 3, 526–533. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Bossola, F.; Roongcharoen, T.; Coduri, M.; Evangelisti, C.; Somodi, F.; Sementa, L.; Fortunelli, A.; Dal Santo, V. Discovering indium as hydrogen production booster for a Cu/SiO2 catalyst in steam reforming of methanol. Appl. Catal. B Environ. 2021, 297, 120398. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Morales-Vidal, J.; Philipp, M.; Safonova, O.V.; López, N.; Stewart, J.A.; Ferré, D.C.; Pérez-Ramírez, J. Nanostructure of nickel-promoted indium oxide catalysts drives selectivity in CO2 hydrogenation. Nat. Commun. 2021, 12, 1960. [Google Scholar] [CrossRef]
- Frei, M.S.; Capdevila-Cortada, M.; García-Muelas, R.; Mondelli, C.; López, N.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide. J. Catal. 2018, 361, 313–321. [Google Scholar] [CrossRef]
- Cai, D.; Cai, Y.; Tan, K.B.; Zhan, G. Recent Advances of Indium Oxide-Based Catalysts for CO2 Hydrogenation to Methanol: Experimental and Theoretical. Materials 2023, 16, 2803. [Google Scholar] [CrossRef]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Abdala, P.M.; Stoian, D.; Huang, X.; Willinger, M.-G.; Fedorov, A.; Müller, C.R. Structural Evolution and Dynamics of an In2O3 Catalyst for CO2 Hydrogenation to Methanol: An Operando XAS-XRD and In Situ TEM Study. J. Am. Chem. Soc. 2019, 141, 13497–13505. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; Cesarini, A.; Krumeich, F.; Hauert, R.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Role of Zirconia in Indium Oxide-Catalyzed CO2 Hydrogenation to Methanol. ACS Catal. 2020, 10, 1133–1145. [Google Scholar] [CrossRef]
- Kuchkina, N.V.; Haskell, A.K.; Sorokina, S.A.; Torozova, A.S.; Nikoshvili, L.Z.; Sulman, E.M.; Stein, B.D.; Morgan, D.G.; Bronstein, L.M.; Shifrina, Z.B. Pd Catalyst Based on Hyperbranched Polypyridylphenylene Formed In Situ on Magnetic Silica Allows for Excellent Performance in Suzuki–Miyaura Reaction. ACS Appl. Mater. Interfaces 2020, 12, 22170–22178. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Kuchkina, N.V.; Grigoriev, M.E.; Bykov, A.V.; Ratnikov, A.K.; Doluda, V.Y.; Sulman, M.G.; Shifrina, Z.B. Cr-Zn/Ni-Containing Nanocomposites as Effective Magnetically Recoverable Catalysts for CO2 Hydrogenation to Methanol: The Role of Metal Doping and Polymer Co-Support. Catalysts 2023, 13, 1. [Google Scholar] [CrossRef]
- Baird, N.; Dittmar, J.W.; Losovyj, Y.B.; Morgan, D.G.; Stein, B.D.; Pink, M.; Kuchkina, N.V.; Serkova, E.S.; Lependina, O.L.; Grigoriev, M.E.; et al. Enhancing the Catalytic Activity of Zn-Containing Magnetic Oxides in a Methanol Synthesis: Identifying the Key Factors. ACS Appl. Mater. Interfaces 2017, 9, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.; Dittmar, J.W.; Losovyj, Y.B.; Pink, M.; Morgan, D.G.; Stein, B.D.; Torozova, A.S.; Krasnova, I.Y.; Grigoriev, M.E.; Sidorov, A.I.; et al. Cr–Containing Magnetic Oxides in a Methanol Synthesis: Does Cr Ion Distribution Matter? ChemistrySelect 2017, 2, 6269–6276. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Kuchkina, N.V.; Ezernitskaya, M.G.; Bykov, A.V.; Vasiliev, A.L.; Efimov, N.N.; Shifrina, Z.B. Ni Nanoparticles Stabilized by Hyperbranched Polymer: Does the Architecture of the Polymer Affect the Nanoparticle Characteristics and Their Performance in Catalysis? Int. J. Mol. Sci. 2022, 23, 13874. [Google Scholar] [CrossRef]
- Sorokina, S.A.; Mikhailov, S.P.; Kuchkina, N.V.; Bykov, A.V.; Vasiliev, A.L.; Ezernitskaya, M.G.; Golovin, A.L.; Nikoshvili, L.Z.; Sulman, M.G.; Shifrina, Z.B. Ru@hyperbranched Polymer for Hydrogenation of Levulinic Acid to Gamma-Valerolactone: The Role of the Catalyst Support. Int. J. Mol. Sci. 2022, 23, 799. [Google Scholar] [CrossRef]
- Parastaev, A.; Muravev, V.; Osta, E.H.; Kimpel, T.F.; Simons, J.F.M.; van Hoof, A.J.F.; Uslamin, E.; Zhang, L.; Struijs, J.J.C.; Burueva, D.B.; et al. Breaking structure sensitivity in CO2 hydrogenation by tuning metal–oxide interfaces in supported cobalt nanoparticles. Nat. Catal. 2022, 5, 1051–1060. [Google Scholar] [CrossRef]
- Bavykina, A.; Yarulina, I.; Al Abdulghani, A.J.; Gevers, L.; Hedhili, M.N.; Miao, X.; Galilea, A.R.; Pustovarenko, A.; Dikhtiarenko, A.; Cadiau, A.; et al. Turning a Methanation Co Catalyst into an In–Co Methanol Producer. ACS Catal. 2019, 9, 6910–6918. [Google Scholar] [CrossRef]
- Lian, Y.; Fang, T.; Zhang, Y.; Liu, B.; Li, j. Hydrogenation of CO2 to alcohol species over Co@Co3O4/C-N catalysts. J. Catal. 2019, 379, 46–51. [Google Scholar] [CrossRef]
- Singh, R.; Tripathi, K.; Pant, K.K. Investigating the role of oxygen vacancies and basic site density in tuning methanol selectivity over Cu/CeO2 catalyst during CO2 hydrogenation. Fuel 2021, 303, 121289. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Tian, C.; Pan, Y.; Sun, X.; Zhang, J.; Wu, D. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: Effect of reduction temperature. J. Catal. 2019, 379, 78–89. [Google Scholar] [CrossRef]
- Shen, C.; Sun, K.; Zou, R.; Wu, Q.; Mei, D.; Liu, C.-j. CO2 Hydrogenation to Methanol on Indium Oxide-Supported Rhenium Catalysts: The Effects of Size. ACS Catal. 2022, 12, 12658–12669. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Wang, H.; Cao, L.; Huang, C.; Li, S.; Zhang, X.; Guan, Q.; Shao, X.; Lu, J. Size-dependent strong metal–support interaction in Pd/ZnO catalysts for hydrogenation of CO2 to methanol. Catal. Sci. Technol. 2021, 11, 4398–4405. [Google Scholar] [CrossRef]
- Elnabawy, A.O.; Schimmenti, R.; Cao, A.; Nørskov, J.K. Why ZnO is the Support for Cu in Methanol Synthesis? A Systematic Study of the Strong Metal Support Interactions. ACS Sustain. Chem. Eng. 2022, 10, 1722–1730. [Google Scholar] [CrossRef]
- Zhu, J.; Ciolca, D.; Liu, L.; Parastaev, A.; Kosinov, N.; Hensen, E.J.M. Flame Synthesis of Cu/ZnO–CeO2 Catalysts: Synergistic Metal–Support Interactions Promote CH3OH Selectivity in CO2 Hydrogenation. ACS Catal. 2021, 11, 4880–4892. [Google Scholar] [CrossRef]
- Detweiler, Z.M.; Wulfsberg, S.M.; Frith, M.G.; Bocarsly, A.B.; Bernasek, S.L. The oxidation and surface speciation of indium and indium oxides exposed to atmospheric oxidants. Surf. Sci. 2016, 648, 188–195. [Google Scholar] [CrossRef]
- Cole, K.M.; Kirk, D.W.; Thorpe, S.J. Co3O4 nanoparticles characterized by XPS and UPS. Surf. Sci. Spectra 2021, 28, 014001. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Huygh, S.; Bogaerts, A.; Neyts, E.C. How Oxygen Vacancies Activate CO2 Dissociation on TiO2 Anatase (001). J. Phys. Chem. C 2016, 120, 21659–21669. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Chen, J.G.; Liu, P. CO2 hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of synergy between Pt and oxide support. J. Catal. 2016, 343, 115–126. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Bi, F.; Chen, Y.; Wu, M.; Zhang, X.; Wang, G. Oxygen vacancies in the catalyst: Efficient degradation of gaseous pollutants. Chem. Eng. J. 2023, 454, 140376. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Gong, Y.; Moran, C.M.; Wang, J.; Zhu, J.; Chang, H.; Guo, X.; Walton, K.S.; Song, C. A combined experimental and DFT study of H2O effect on In2O3/ZrO2 catalyst for CO2 hydrogenation to methanol. J. Catal. 2020, 383, 283–296. [Google Scholar] [CrossRef]
- Nomura, N.; Tagawa, T.; Goto, S. Effect of acid-base properties on copper catalysts for hydrogenation of carbon dioxide. React. Kinet. Catal. Lett. 1998, 63, 21–25. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokina, S.A.; Kuchkina, N.V.; Mikhailov, S.P.; Mikhalchenko, A.V.; Bykov, A.V.; Doluda, V.Y.; Bronstein, L.M.; Shifrina, Z.B. Highly Selective CO2 Hydrogenation to Methanol over Complex In/Co Catalysts: Effect of Polymer Frame. Nanomaterials 2023, 13, 2996. https://doi.org/10.3390/nano13232996
Sorokina SA, Kuchkina NV, Mikhailov SP, Mikhalchenko AV, Bykov AV, Doluda VY, Bronstein LM, Shifrina ZB. Highly Selective CO2 Hydrogenation to Methanol over Complex In/Co Catalysts: Effect of Polymer Frame. Nanomaterials. 2023; 13(23):2996. https://doi.org/10.3390/nano13232996
Chicago/Turabian StyleSorokina, Svetlana A., Nina V. Kuchkina, Stepan P. Mikhailov, Alexander V. Mikhalchenko, Alexey V. Bykov, Valentin Yu. Doluda, Lyudmila M. Bronstein, and Zinaida B. Shifrina. 2023. "Highly Selective CO2 Hydrogenation to Methanol over Complex In/Co Catalysts: Effect of Polymer Frame" Nanomaterials 13, no. 23: 2996. https://doi.org/10.3390/nano13232996






