From E-Waste to High-Value Materials: Sustainable Synthesis of Metal, Metal Oxide, and MOF Nanoparticles from Waste Printed Circuit Boards
Abstract
:1. Introduction
1.1. Significance of Waste Printed Circuit Boards (WPCBs)
1.2. Sustainable Recycling Methods
1.3. Nanoparticles from WPCBs
1.4. Review Objectives
2. Composition of WPCBs
3. Pretreatment of E-Waste
4. Recovery Systems
4.1. Froth Flotation
4.2. Hydrometallurgical Processes
4.3. Green Recovery Methods
4.3.1. Bioleaching
4.3.2. Biosorption
5. Nanoparticles from WPCBs: Synthesis, Concentration, and Purification
6. Green Synthesis of Nanoparticles from WPCBs
6.1. Green Organic Acids
6.2. Plants
6.3. Bacteria
6.4. Fungi
6.5. Algae
7. Industrial Applications of Nanoparticles Based on WPCBs
7.1. Degradation of Dyes
7.2. Photocatalysis
7.3. Coatings
7.4. Biomedical
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duman, G.M.; Kongar, E. ESG Modeling and Prediction Uncertainty of Electronic Waste. Sustainability 2023, 15, 11281. [Google Scholar] [CrossRef]
- Tatariants, M.; Yousef, S.; Skapas, M.; Juskenas, R.; Makarevicius, V.; Lukošiūtė, S.-I.; Denafas, G. Industrial Technology for Mass Production of SnO2 Nanoparticles and PbO2 Microcube/Microcross Structures from Electronic Waste. J. Clean. Prod. 2018, 203, 498–510. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Makarevičius, V.; Lukošiūtė, S.-I.; Bendikiene, R.; Denafas, G. A Strategy for Synthesis of Copper Nanoparticles from Recovered Metal of Waste Printed Circuit Boards. J. Clean. Prod. 2018, 185, 653–664. [Google Scholar] [CrossRef]
- Zielonka, A.; Klimek-Ochab, M. Fungal Synthesis of Size-Defined Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 043001. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal Bioleaching of WPCBs Using Aspergillus niger: Observation, Optimization and Kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices. Sustainability 2023, 15, 10222. [Google Scholar] [CrossRef]
- Caldas, M.P.K.; Martins, T.A.G.; de Moraes, V.T.; Tenório, J.A.S.; Espinosa, D.C.R. Synthesis of Ag Nanoparticles from Waste Printed Circuit Board. J. Environ. Chem. Eng. 2021, 9, 106845. [Google Scholar] [CrossRef]
- Xia, D.; Charpentier, N.M.; Maurice, A.A.; Brambilla, A.; Yan, Q.; Gabriel, J.C.P. Sustainable route for Nd recycling from waste electronic components featured with unique element-specific sorting enabling simplified hydrometallurgy. Chem. Eng. J. 2022, 441, 135886. [Google Scholar] [CrossRef]
- Hegde, M.; Naliyadhara, N.; Unnikrishnan, J.; Alqahtani, M.S.; Abbas, M.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Nanoparticles in the Diagnosis and Treatment of Cancer Metastases: Current and Future Perspectives. Cancer Lett. 2023, 556, 216066. [Google Scholar] [CrossRef]
- Manjusha, V.; Rajeev, M.R.; Anirudhan, T.S. Magnetic Nanoparticle Embedded Chitosan-Based Polymeric Network for the Hydrophobic Drug Delivery of Paclitaxel. Int. J. Biol. Macromol. 2023, 235, 123900. [Google Scholar] [CrossRef]
- Rajarao, R.; Ferreira, R.; Sadi, S.H.F.; Khanna, R.; Sahajwalla, V. Synthesis of Silicon Carbide Nanoparticles by Using Electronic Waste as a Carbon Source. Mater. Lett. 2014, 120, 65–68. [Google Scholar] [CrossRef]
- Kumar, S.; Karmacharya, M.; Joshi, S.R.; Gulenko, O.; Park, J.; Kim, G.-H.; Cho, Y.-K. Photoactive Antiviral Face Mask with Self-Sterilization and Reusability. Nano Lett. 2021, 21, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; de Camargo, E.R.; Barbosa, D.B. The Growing Importance of Materials That Prevent Microbial Adhesion: Antimicrobial Effect of Medical Devices Containing Silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Grimes, S.M.; Maguire, D. Assessment of Priorities in Critical Material Recovery from Waste Electrical and Electronic Equipment. Resour. Policy 2020, 68, 101658. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Priyanka, B.; Senthil Kumar, P.; Karishma, S.; Jeevanantham, S.; Indraganti, S. A Review on Recent Advancements in Recovery of Valuable and Toxic Metals from E-Waste Using Bioleaching Approach. Chemosphere 2022, 287, 132230. [Google Scholar] [CrossRef] [PubMed]
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Tatariants, M.; Yousef, S.; Sakalauskaitė, S.; Daugelavičius, R.; Denafas, G.; Bendikiene, R. Antimicrobial Copper Nanoparticles Synthesized from Waste Printed Circuit Boards Using Advanced Chemical Technology. Waste Manag. 2018, 78, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Hait, S. Extraction of Metals from High Grade Waste Printed Circuit Board by Conventional and Hybrid Bioleaching Using Acidithiobacillus Ferrooxidans. Hydrometallurgy 2018, 177, 132–139. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K. Sequential recovery of copper, nickel, gold, silver and zinc from WPCBs of obsolete mobile phones through hydrometallurgical recycling. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Huang, T.; Zhu, J.; Huang, X.; Ruan, J.; Xu, Z. Assessment of Precious Metals Positioning in Waste Printed Circuit Boards and the Economic Benefits of Recycling. Waste Manag. 2022, 139, 105–115. [Google Scholar] [CrossRef]
- Mir, S.; Dhawan, N. A Comprehensive Review on the Recycling of Discarded Printed Circuit Boards for Resource Recovery. Resour. Conserv. Recycl. 2022, 178, 106027. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Han, Y.; Park, J. Apparatus for Electronic Component Disassembly from Printed Circuit Board Assembly in E-Wastes. Int. J. Miner. Process. 2015, 144, 11–15. [Google Scholar] [CrossRef]
- Oestreicher, V.; García, C.S.; Pontiggia, R.; Rossi, M.B.; Angelomé, P.C.; Soler-Illia, G.J.A.A. E-Waste Upcycling for the Synthesis of Plasmonic Responsive Gold Nanoparticles. Waste Manag. 2020, 117, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Palomero, J.; Cenci, M.P.; Scarazzato, T.; Bernardes, A.M. Electronic Waste in Brazil: Generation, Collection, Recycling and the COVID Pandemic. Clean. Waste Syst. 2022, 3, 100022. [Google Scholar] [CrossRef]
- Ravi, B.; Duraisamy, P.; Marimuthu, T. A Novel Integrated Circular Economy Approach in Green Synthesis of Copper Oxide Nanoparticles from Waste Printed Circuit Boards and Utilization of Its Residue for Preparation of Carbon Engulfed Nano Polymer Membrane. J. Clean. Prod. 2023, 383, 135457. [Google Scholar] [CrossRef]
- Gautam, P.; Behera, C.K.; Sinha, I.; Gicheva, G.; Singh, K.K. High Added-Value Materials Recovery Using Electronic Scrap-Transforming Waste to Valuable Products. J. Clean. Prod. 2022, 330, 129836. [Google Scholar] [CrossRef]
- Rozas, E.E.; Mendes, M.A.; Nascimento, C.A.O.; Espinosa, D.C.R.; Oliveira, R.; Oliveira, G.; Custodio, M.R. Bioleaching of Electronic Waste Using Bacteria Isolated from the Marine Sponge Hymeniacidon heliophila (Porifera). J. Hazard. Mater. 2017, 329, 120–130. [Google Scholar] [CrossRef]
- Najafi, A.; Khoeini, M.; Khalaj, G.; Sahebgharan, A. Synthesis of Silver Nanoparticles from Electronic Scrap by Chemical Reduction. Mater. Res. Express 2021, 8, 125009. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, E.Y.; Panda, S. A Novel Approach Based on Solvent Displacement Crystallisation for Iron Removal and Copper Recovery from Solutions of Semi-Pilot Scale Bioleaching of WPCBs. J. Clean. Prod. 2021, 294, 126346. [Google Scholar] [CrossRef]
- Cerchier, P.; Dabalà, M.; Brunelli, K. Synthesis of SnO2 and Ag Nanoparticles from Electronic Wastes with the Assistance of Ultrasound and Microwaves. JOM 2017, 69, 1583–1588. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Li, S.; Chen, M.; Chen, H.; Liu, B. Bioleaching Waste Printed Circuit Boards by Acidithiobacillus Ferrooxidans and Its Kinetics Aspect. J. Biotechnol. 2014, 173, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Xu, Z. Electrostatic Separation for Recovering Metals and Nonmetals from Waste Printed Circuit Board: Problems and Improvements. Environ. Sci. Technol. 2008, 42, 5272–5276. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Sheng, C.; Wu, L.; Zhao, Y.; He, J.; Zhou, E. Separation and Recovery of Fine Particles from Waste Circuit Boards Using an Inflatable Tapered Diameter Separation Bed. Sci. World J. 2014, 2014, 843579. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.P.M.; dos Santos, I.D.; Dutra, A.J.B. Copper and Metals Concentration from Printed Circuit Boards Using a Zig-Zag Classifier. J. Mater. Res. Technol. 2019, 8, 513–520. [Google Scholar] [CrossRef]
- Yao, Y.; Bai, Q.; He, J.; Zhu, L.; Zhou, K.; Zhao, Y. Reverse Flotation Efficiency and Mechanism of Various Collectors for Recycling Waste Printed Circuit Boards. Waste Manag. 2020, 103, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cang, D.; Zhang, L.; Guo, J. Mechanism of Recovery Processes for Rare Earth and Iron from Bayan Obo Tailings. Int. J. Chem. React. Eng. 2020, 18, 20200077. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Zhang, T.; Zhang, G.; Yang, X.; He, Y.; Wang, L.; Duan, C. Metals Recovery from Dust Derived from Recycling Line of Waste Printed Circuit Boards. J. Clean. Prod. 2017, 165, 452–457. [Google Scholar] [CrossRef]
- Lee, H.; Mishra, B. Selective Recovery and Separation of Copper and Iron from Fine Materials of Electronic Waste Processing. Miner. Eng. 2018, 123, 1–7. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, T.; Li, B.; Nie, C.; Zhang, H.; Lyu, X.; Tao, Y.; Qiu, J.; Li, L.; Zhang, G. Metal Recovery from Waste Printed Circuit Boards by Flotation Technology with Non-Ionic Renewable Collector. J. Clean. Prod. 2020, 255, 120289. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, X.; Wu, H.; Li, S.; Wang, G.; Liu, X.; Qiu, G.; Wang, D. A Novel Bio-Oxidation and Two-Step Thiourea Leaching Method Applied to a Refractory Gold Concentrate. Hydrometallurgy 2017, 171, 213–221. [Google Scholar] [CrossRef]
- Bilesan, M.R.; Makarova, I.; Wickman, B.; Repo, E. Efficient Separation of Precious Metals from Computer Waste Printed Circuit Boards by Hydrocyclone and Dilution-Gravity Methods. J. Clean. Prod. 2021, 286, 125505. [Google Scholar] [CrossRef]
- Zhang, S.; Forssberg, E. Optimization of Electrodynamic Separation for Metals Recovery from Electronic Scrap. Resour. Conserv. Recycl. 1998, 22, 143–162. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegliò, F. Study of Multi-Step Hydrometallurgical Methods to Extract the Valuable Content of Gold, Silver and Copper from Waste Printed Circuit Boards. J. Environ. Chem. Eng. 2016, 4, 20–29. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Chen, H.; Chen, S.; Sun, Q. Leaching Behavior of Copper from Waste Printed Circuit Boards with Brønsted Acidic Ionic Liquid. Waste Manag. 2014, 34, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of Hydrometallurgical Recovery of Zinc from Industrial Wastes. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Characterization of Particle Size-Based Deportment of Metals in Various Waste Printed Circuit Boards towards Metal Recovery. Clean. Mater. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Behmadi, R.; Mirzaei, M.; Afshar, M.R.; Najafi, H. Investigation of Chalcopyrite Removal from Low-Grade Molybdenite Using Response Surface Methodology and Its Effect on Molybdenum Trioxide Morphology by Roasting. RSC Adv. 2023, 13, 14899–14913. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Xing, B. Copper Recovery from Waste Printed Circuit Boards by the Flotation-Leaching Process Optimized Using Response Surface Methodology. J. Air Waste Manag. Assoc. 2021, 71, 1483–1491. [Google Scholar] [CrossRef]
- Mäkinen, J.; Bachér, J.; Kaartinen, T.; Wahlström, M.; Salminen, J. The Effect of Flotation and Parameters for Bioleaching of Printed Circuit Boards. Miner. Eng. 2015, 75, 26–31. [Google Scholar] [CrossRef]
- Hyder, M.K.M.; Ochiai, B. Selective Recovery of Au(III), Pd(II), and Ag(I) from Printed Circuit Boards Using Cellulose Filter Paper Grafted with Polymer Chains Bearing Thiocarbamate Moieties. Microsyst. Technol. 2018, 24, 683–690. [Google Scholar] [CrossRef]
- Elomaa, H.; Seisko, S.; Junnila, T.; Sirviö, T.; Wilson, B.P.; Aromaa, J.; Lundström, M. The Effect of the Redox Potential of Aqua Regia and Temperature on the Au, Cu, and Fe Dissolution from WPCBs. Recycling 2017, 2, 14. [Google Scholar] [CrossRef]
- Sheng, P.P.; Etsell, T.H. Recovery of Gold from Computer Circuit Board Scrap Using Aqua Regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef]
- Batnasan, A.; Haga, K.; Shibayama, A. Recovery of Precious and Base Metals from Waste Printed Circuit Boards Using a Sequential Leaching Procedure. JOM 2018, 70, 124–128. [Google Scholar] [CrossRef]
- Altansukh, B.; Haga, K.; Ariunbolor, N.; Kawamura, S.; Shibayama, A. Leaching and Adsorption of Gold from Waste Printed Circuit Boards Using Iodine-Iodide Solution and Activated Carbon. Eng. J. 2016, 20, 29–40. [Google Scholar] [CrossRef]
- Batnasan, A.; Haga, K.; Huang, H.-H.; Shibayama, A. High-Pressure Oxidative Leaching and Iodide Leaching Followed by Selective Precipitation for Recovery of Base and Precious Metals from Waste Printed Circuit Boards Ash. Metals 2019, 9, 363. [Google Scholar] [CrossRef]
- Molina, L. Identificación y Análisis de Agentes Lixiviantes No Convencionales Para La Recuperación de Elementos de Valor Desde Minerales, Concentrados y Escorias de Fundición. Bachelor’s Thesis, Universidad de Concepción, Chillán, Chile, 2018. [Google Scholar]
- Lombaba, L.; Saavedra, A.; Correa, F. Influential Variables in the Leaching Process for Metals Recovery Contained in Galvanic Sludges. Rev. Semilleros Form. Investig. 2018, 4, 93–104. [Google Scholar]
- Gámez, S.; Garcés, K.; de la Torre, E.; Guevara, A. Precious Metals Recovery from Waste Printed Circuit Boards Using Thiosulfate Leaching and Ion Exchange Resin. Hydrometallurgy 2019, 186, 1–11. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal Recovery from Waste Printed Circuit Boards: A Review for Current Status and Perspectives. Resour. Conserv. Recycl. 2020, 157, 104787. [Google Scholar] [CrossRef]
- Xavier, L.H.; Ottoni, M.; Abreu, L.P.P. A Comprehensive Review of Urban Mining and the Value Recovery from E-Waste Materials. Resour. Conserv. Recycl. 2023, 190, 106840. [Google Scholar] [CrossRef]
- Gahan, C.; Srichandan, D.; Kim, D.; Akcil, A. Biohydrometallurgy and Biomineral Processing Technology: A Review on Its Past, Present and Future. Res. J. Recent. Sci. 2012, 1, 85–99. [Google Scholar]
- Tay, S.B.; Natarajan, G.; Rahim, M.N.B.A.; Tan, H.T.; Chung, M.C.M.; Ting, Y.P.; Yew, W.S. Enhancing Gold Recovery from Electronic Waste via Lixiviant Metabolic Engineering in Chromobacterium Violaceum. Sci. Rep. 2013, 3, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in Bioleaching as a Sustainable Method for Metal Recovery from E-Waste: A Review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Borthakur, A.; Singh, P. Microbial Biotechnology for Sustainable Electronic Waste. In Proceedings of the Asia-Pacific Conference on Biotechnology for Waste Conversion, Hong Kong, China, 5 December 2016; pp. 314–317. [Google Scholar]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of Metals from E-Waste Using Microorganisms: A Review. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Desmarais, M.; Pirade, F.; Zhang, J.; Rene, E.R. Biohydrometallurgical Processes for the Recovery of Precious and Base Metals from Waste Electrical and Electronic Equipments: Current Trends and Perspectives. Bioresour. Technol. Rep. 2020, 11, 100526. [Google Scholar] [CrossRef]
- Narayanasamy, M.; Dhanasekaran, D.; Vinothini, G.; Thajuddin, N. Extraction and Recovery of Precious Metals from Electronic Waste Printed Circuit Boards by Bioleaching Acidophilic Fungi. Int. J. Environ. Sci. Technol. 2018, 15, 119–132. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A Comprehensive Review on Microbial Fuel Cell Technologies: Processes, Utilization, and Advanced Developments in Electrodes and Membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Guo, Y.; An, N.; Liang, C.; Ge, Z. Bioleaching of Gold from Waste Printed Circuit Boards by Alkali-Tolerant Pseudomonas Fluorescens. Hydrometallurgy 2020, 194, 105260. [Google Scholar] [CrossRef]
- Madrigal-Arias, J.E.; Argumedo-Delira, R.; Alarcón, A.; Mendoza-López, M.R.; García-Barradas, O.; Cruz-Sánchez, J.S.; Ferrera-Cerrato, R.; Jiménez-Fernández, M. Bioleaching of Gold, Copper and Nickel from Waste Cellular Phone PCBs and Computer Goldfinger Motherboards by Two Aspergillus nigerstrains. Braz. J. Microbiol. 2015, 46, 707–713. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Ge, Z. Review on Chromobacterium Violaceum for Gold Bioleaching from E-Waste. Procedia Environ. Sci. 2016, 31, 947–953. [Google Scholar] [CrossRef]
- Bindschedler, S.; Vu Bouquet, T.Q.T.; Job, D.; Joseph, E.; Junier, P. Chapter Two—Fungal Biorecovery of Gold from E-Waste; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: New York, NY, USA, 2017; Volume 99, pp. 53–81. ISBN 0065-2164. [Google Scholar]
- Kumar, R.R.; Lee, J.T.; Cho, J.Y. Toxic Cadmium Ions Removal by Isolated Fungal Strain from E-Waste Recycling Facility. J. Environ. Appl. Bioresearch 2012, 1, 1–4. [Google Scholar]
- Lakshmi, S.; Suvedha, K.; Sruthi, R.; Lavanya, J.; Varjani, S.; Nakkeeran, E. Hexavalent Chromium Sequestration from Electronic Waste by Biomass of Aspergillus carbonarius. Bioengineered 2020, 11, 708–717. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S.; Allosh Albarakaty, F.; Kalia, A.; Hassan, M.; Abd-Elsalam, K. Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera. Sustainability 2022, 14, 935. [Google Scholar] [CrossRef]
- Ramírez Carmona, M.E.; Pereira da Silva, M.A.; Ferreira Leite, S.G.; Vasco Echeverri, O.H.; Ocampo-López, C. Packed Bed Redistribution System for Cr(III) and Cr(VI) Biosorption by Saccharomyces Cerevisiae. J. Taiwan. Inst. Chem. Eng. 2012, 43, 428–432. [Google Scholar] [CrossRef]
- Gupta, N.K.; Gupta, A.; Ramteke, P.; Sahoo, H.; Sengupta, A. Biosorption-a Green Method for the Preconcentration of Rare Earth Elements (REEs) from Waste Solutions: A Review. J. Mol. Liq. 2019, 274, 148–164. [Google Scholar] [CrossRef]
- Sulaymon, A. Biosorption of Heavy Metals: A Review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Anaya-Garzon, J.; Hubau, A.; Joulian, C.; Guezennec, A.-G. Bioleaching of E-Waste: Influence of Printed Circuit Boards on the Activity of Acidophilic Iron-Oxidizing Bacteria. Front. Microbiol. 2021, 12, 669738. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.; Pant, D. Recovery of Gold from Electronic Waste Using Chemical Assisted Microbial Biosorption (Hybrid) Technique. Bioresour. Technol. 2018, 247, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Sharada, H.M.; Abdel-Halim, S.A.; Hafez, M.A.; Ibrahim, I.A.; Elbarbary, T.A.; Abdel-Fatah, Y. Bioleaching of Copper from Electronic Waste Using Aspergillus niger. Biointerface Res. Appl. Chem. 2022, 12, 8406–8425. [Google Scholar] [CrossRef]
- Di Piazza, S.; Cecchi, G.; Cardinale, A.M.; Carbone, C.; Mariotti, M.G.; Giovine, M.; Zotti, M. Penicillium Expansum Link Strain for a Biometallurgical Method to Recover REEs from WEEE. Waste Manag. 2017, 60, 596–600. [Google Scholar] [CrossRef]
- Vauthier, C.; Cabane, B.; Labarre, D. How to Concentrate Nanoparticles and Avoid Aggregation? Eur. J. Pharm. Biopharm. 2008, 69, 466–475. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Hammache, Z.; Bensaadi, S.; Berbar, Y.; Audebrand, N.; Szymczyk, A.; Amara, M. Recovery of Rare Earth Elements from Electronic Waste by Diffusion Dialysis. Sep. Purif. Technol. 2021, 254, 117641. [Google Scholar] [CrossRef]
- Dalwadi, G.; Benson, H.A.E.; Chen, Y. Comparison of Diafiltration and Tangential Flow Filtration for Purification of Nanoparticle Suspensions. Pharm. Res. 2005, 22, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, L. Metallurgical Recovery of Metals from Electronic Waste: A Review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Yamada, K.; Usami, H. Electrochemical Extraction of Gold from Wastes as Nanoparticles Stabilized by Phospholipids. Waste Manag. 2017, 60, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Kotze, M.; Green, B.; Mackenzie, J.; Virnig, M. Chapter 32—Resin-in-Pulp and Resin-in-Solution, 2nd ed.; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherland, 2016; pp. 561–583. ISBN 978-0-444-63658-4. [Google Scholar]
- Escorcia-Díaz, D.; García-Mora, S.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review. Nanomaterials 2023, 13, 2586. [Google Scholar] [CrossRef]
- Punjabi, K.; Mehta, S.; Yedurkar, S.; Jain, R.; Mukherjee, S.; Kale, A.; Deshpande, S. Extracellular Synthesis of Silver Nanoparticle by Pseudomonas Hibiscicola—Mechanistic Approach. Adv. Nano Res. 2018, 6, 81–92. [Google Scholar] [CrossRef]
- Shokri, A.; Pahlevani, F.; Levick, K.; Cole, I.; Sahajwalla, V. Synthesis of Copper-Tin Nanoparticles from Old Computer Printed Circuit Boards. J. Clean. Prod. 2017, 142, 2586–2592. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. Kona 2020, 37, 224–232. [Google Scholar] [CrossRef]
- Martins, T.A.G.; Falconi, I.B.A.; Pavoski, G.; de Moraes, V.T.; Galluzzi Baltazar, M.d.P.; Espinosa, D.C.R. Green Synthesis, Characterization, and Application of Copper Nanoparticles Obtained from Printed Circuit Boards to Degrade Mining Surfactant by Fenton Process. J. Environ. Chem. Eng. 2021, 9, 106576. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Rayan, D.A.; Ismail, M.M. Synthesis of Cu and CuO Nanoparticles from E-Waste and Evaluation of Their Antibacterial and Photocatalytic Properties. Res. Sq. 2023, 30, 89690–89704. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Mishra, P.; Wahid, Z.; Thakur, S.; Pant, D.; Singh, L. Microbe-Mediated Sustainable Bio-Recovery of Gold from Low-Grade Precious Solid Waste: A Microbiological Overview. J. Environ. Sci. 2020, 89, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Nie, C.; Ni, Y.; Zhang, T.; Li, B.; Wang, D.; Qu, S.; Qiao, F.; Lyu, X.; Qiu, J.; et al. Advanced Utilization of Copper in Waste Printed Circuit Boards: Synthesis of Nano-Copper Assisted by Physical Enrichment. J. Hazard. Mater. 2021, 401, 123294. [Google Scholar] [CrossRef] [PubMed]
- Alexandre-Franco, M.F.; Fernández-González, C.; Reguero-Padilla, G.; Cuerda-Correa, E.M. Olive-Tree Polyphenols and Urban Mining. A Greener Alternative for the Recovery of Valuable Metals from Scrap Printed Circuit Boards. Environ. Res. 2022, 214, 114112. [Google Scholar] [CrossRef] [PubMed]
- Antunes Filho, S.; dos Santos, M.S.; dos Santos, O.A.L.; Backx, B.P.; Soran, M.-L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef]
- Ettadili, F.E.; Aghris, S.; Laghrib, F.; Farahi, A.; Saqrane, S.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Recent Advances in the Nanoparticles Synthesis Using Plant Extract: Applications and Future Recommendations. J. Mol. Struct. 2022, 1248, 131538. [Google Scholar] [CrossRef]
- Nithya, R.; Sivasankari, C.; Thirunavukkarasu, A.; Selvasembian, R. Novel Adsorbent Prepared from Bio-Hydrometallurgical Leachate from Waste Printed Circuit Board Used for the Removal of Methylene Blue from Aqueous Solution. Microchem. J. 2018, 142, 321–328. [Google Scholar] [CrossRef]
- Priya, B.; Sobiya, S.; Kshayalakshmi, S.; Umamaheshwari, S.; Priyadharshini, M.; Rathi Devi, P. Phyto-Mediated Synthesis of Copper Nanoparticles by Cassia Auriculata and Its Characterization with Reference to E-Waste Management. Int. J. Environ. Agric. Biotechnol. 2017, 2, 808–811. [Google Scholar] [CrossRef]
- Pawlowska, A.; Sadowski, Z. Biosynthesis of Copper Nanoparticles Using Aqueous Extracts of Aloe Vera and Geranium and Bioleaching Solutions. Solid State Phenom. 2017, 262, 193–196. [Google Scholar] [CrossRef]
- Subbaiyan, R.; Ganesan, A.; Sasikumar, B.; Rajendran, S.; Ramasubramanian, B. Synthesis and Characterization of Ferrous and Copper Nanoparticles from E-Waste Using Biological Reduction by Lichen-Associated Bacteria and Their Application in Antifouling Activity. Appl. Biochem. Biotechnol. 2023, 195, 3142–3155. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 59361. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dong, B.; Ting, Y.P. Gold Biodissolution from Electronic Scrap and Biomineralization of Bacterial Gold Nanoparticles. Adv. Mat. Res. 2015, 1130, 668–672. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.d.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 660582. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus Interaction: Review on Cellular Processes Underlying Heavy Metal Detoxification and Synthesis of Metal Nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef]
- Pineda-Vásquez, T.G.; Casas-Botero, A.E.; Ramírez-Carmona, M.E.; Torres-Taborda, M.M.; Soares, C.H.L.; Hotza, D. Biogeneration of Silica Nanoparticles from Rice Husk Ash Using Fusarium Oxysporum in Two Different Growth Media. Ind. Eng. Chem. Res. 2014, 53, 6959–6965. [Google Scholar] [CrossRef]
- Ponduru, H.K.; Gurubilli, C.S.R.; Mudunuru, S.; Banisetti, D.K. Green Synthesis of Nano Particles from Biodegradable Waste Extracts and Their Applications. Int. J. Pharmacogn. Chem. 2023, 4, 46–54. [Google Scholar] [CrossRef]
- Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolenčík, M. Mycosynthesis of Metal-Containing Nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and Their Application. Int. J. Mol. Sci. 2023, 24, 304. [Google Scholar] [CrossRef]
- Pekkoh, J.; Ruangrit, K.; Kaewkod, T.; Tragoolpua, Y.; Hoijang, S.; Srisombat, L.; Wichapein, A.; Pathom-aree, W.; Kato, Y.; Wang, G.; et al. Innovative Eco-Friendly Microwave-Assisted Rapid Biosynthesis of Ag/AgCl-NPs Coated with Algae Bloom Extract as Multi-Functional Biomaterials with Non-Toxic Effects on Normal Human Cells. Nanomaterials 2023, 13, 2141. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of Gold Using Cyanobacteria and an Eukaryotic Alga with Special Reference to Nanogold Formation—A Novel Phenomenon. J. Appl. Phycol. 2009, 21, 145–152. [Google Scholar] [CrossRef]
- Soleimani, M.; Habibi-Pirkoohi, M. Biosynthesis of Silver Nanoparticles Using Chlorella Vulgaris and Evaluation of the Antibacterial Efficacy Against Staphylococcus Aureus. Avicenna J. Med. Biotechnol. 2017, 9, 120–125. [Google Scholar] [PubMed]
- Babu, S.; Jojo, L.; James, A.; Melethil, K.; Thomas, B. Metal-Based Catalysis for Biomass and Renewables Valorization- Current Status. Tetrahedron Green. Chem. 2023, 2, 100018. [Google Scholar] [CrossRef]
- Hublikar, L.V.; Ganachari, S.V.; Raghavendra, N.; Banapurmath, N.R.; Patil, V.B.; Yunus Khan, T.M.; Badruddin, I.A. Biogenesis of Silver Nanoparticles and Its Multifunctional Anti-Corrosion and Anticancer Studies. Coatings 2021, 11, 1215. [Google Scholar] [CrossRef]
- Gola, D.; Kriti, A.; Bhatt, N.; Bajpai, M.; Singh, A.; Arya, A.; Chauhan, N.; Srivastava, S.K.; Tyagi, P.K.; Agrawal, Y. Silver Nanoparticles for Enhanced Dye Degradation. Curr. Res. Green Sustain. Chem. 2021, 4, 100132. [Google Scholar] [CrossRef]
- Wardhani, S.; Rahman, M.F.; Purwonugroho, D.; Tjahjanto, R.T.; Damayanti, C.A.; Wulandari, I.O. Photocatalytic Degradation of Methylene Blue Using TiO2-Natural Zeolite as A Photocatalyst. J. Pure Appl. Chem. Res. 2016, 5, 19–27. [Google Scholar] [CrossRef]
- Nascimento, M.A.; Castro Cruz, J.; dos Reis, M.F.; de Carvalho Damasceno, O.I.; Lázaro Reis, E.; Reis, C.; de Oliveira, A.F.; Pereira Lopes, R. Synthesis of Polymetallic Nanoparticles from Printed Circuit Board Waste and Application in Textile Dye Remediation. J. Environ. Chem. Eng. 2018, 6, 5580–5586. [Google Scholar] [CrossRef]
- Krishna, P.G.; Chandra Mishra, P.; Naika, M.M.; Gadewar, M.; Ananthaswamy, P.P.; Rao, S.; Boselin Prabhu, S.R.; Yatish, K.V.; Nagendra, H.G.; Moustafa, M.; et al. Photocatalytic Activity Induced by Metal Nanoparticles Synthesized by Sustainable Approaches: A Comprehensive Review. Front. Chem. 2022, 10, 917831. [Google Scholar] [CrossRef]
- Leong, C.Y.; Wahab, R.A.; Lee, S.L.; Ponnusamy, V.K.; Chen, Y.-H. Current Perspectives of Metal-Based Nanomaterials as Photocatalytic Antimicrobial Agents and Their Therapeutic Modes of Action: A Review. Environ. Res. 2023, 227, 115578. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and Photocatalysis of Methylene Blue Dye Using Various Nanocatalytic Assemblies—A Critical Review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Awais, M.; Khursheed, S.; Tehreem, R.; Sirajuddin; Mok, Y.S.; Siddiqui, G.U. PH Regulated Rapid Photocatalytic Degradation of Methylene Blue Dye via Niobium-Nitrogen Co-Doped Titanium Dioxide Nanostructures under Sunlight. Appl. Catal. A Gen. 2022, 643, 118764. [Google Scholar] [CrossRef]
- Sundararajan, M.; John Kennedy, L.; Nithya, P.; Judith Vijaya, J.; Bououdina, M. Visible Light Driven Photocatalytic Degradation of Rhodamine B Using Mg Doped Cobalt Ferrite Spinel Nanoparticles Synthesized by Microwave Combustion Method. J. Phys. Chem. Solids 2017, 108, 61–75. [Google Scholar] [CrossRef]
- Abdo, D.M.; Abdelbasir, S.M.; El-Sheltawy, S.T.; Ibrahim, I.A. Recovery of Tin as Tin Oxide Nanoparticles from Waste Printed Circuit Boards for Photocatalytic Dye Degradation. Korean J. Chem. Eng. 2021, 38, 1934–1945. [Google Scholar] [CrossRef]
- Yeganeh, M.; Farzadkia, M.; Jonidi Jafari, A.; Sobhi, H.R.; Esrafili, A.; Gholami, M. Utilization of the Copper Recovered from Waste Printed Circuit Boards as a Metal Precursor for the Synthesis of TiO2/Magnetic-MOF(Cu) Nanocomposite: Application in Photocatalytic Degradation of Pesticides in Aquatic Solutions. J. Environ. Manag. 2023, 345, 118755. [Google Scholar] [CrossRef]
- Mondal, M.S.; Paul, A.; Rhaman, M. Recycling of Silver Nanoparticles from Electronic Waste via Green Synthesis and Application of AgNPs-Chitosan Based Nanocomposite on Textile Material. Sci. Rep. 2023, 13, 13798. [Google Scholar] [CrossRef]
- Mathew, A.A.; Parthasarathy, P.; Vivekanandan, S. Development of Copper Nanoparticles from E-Waste for Biomedical Applications BT—Advances in Automation, Signal Processing, Instrumentation, and Control; Komanapalli, V.L.N., Sivakumaran, N., Hampannavar, S., Eds.; Springer Nature: Singapore, 2021; pp. 703–715. [Google Scholar]
- Kalirajan, J. Antifungal Activity of Biogenic Selenium Nanoparticles Synthesized from Electronic Waste Big Data for Toxicity Prediction View Project Identification of Source for MTGase and Media Optimization for Their Production View Project Antifungal Activity of Biogenic Selenium Nanoparticles Synthesized from Electronic Waste. Int. J. PharmTech Res. 2015, 8, 383–386. [Google Scholar]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An Overview on Nanoparticles Used in Biomedicine and Their Cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 102316. [Google Scholar] [CrossRef]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef]
- Adeleke, O.; Idowu, I.; Chidinma, M.; Olaitan, B.; Busola, A.; Oyinloye, B.E.; Ramalingam, M. Nanoparticles and Their Biomedical Applications. Biointerface Res. Appl. Chem. 2020, 11, 8431–8445. [Google Scholar] [CrossRef]

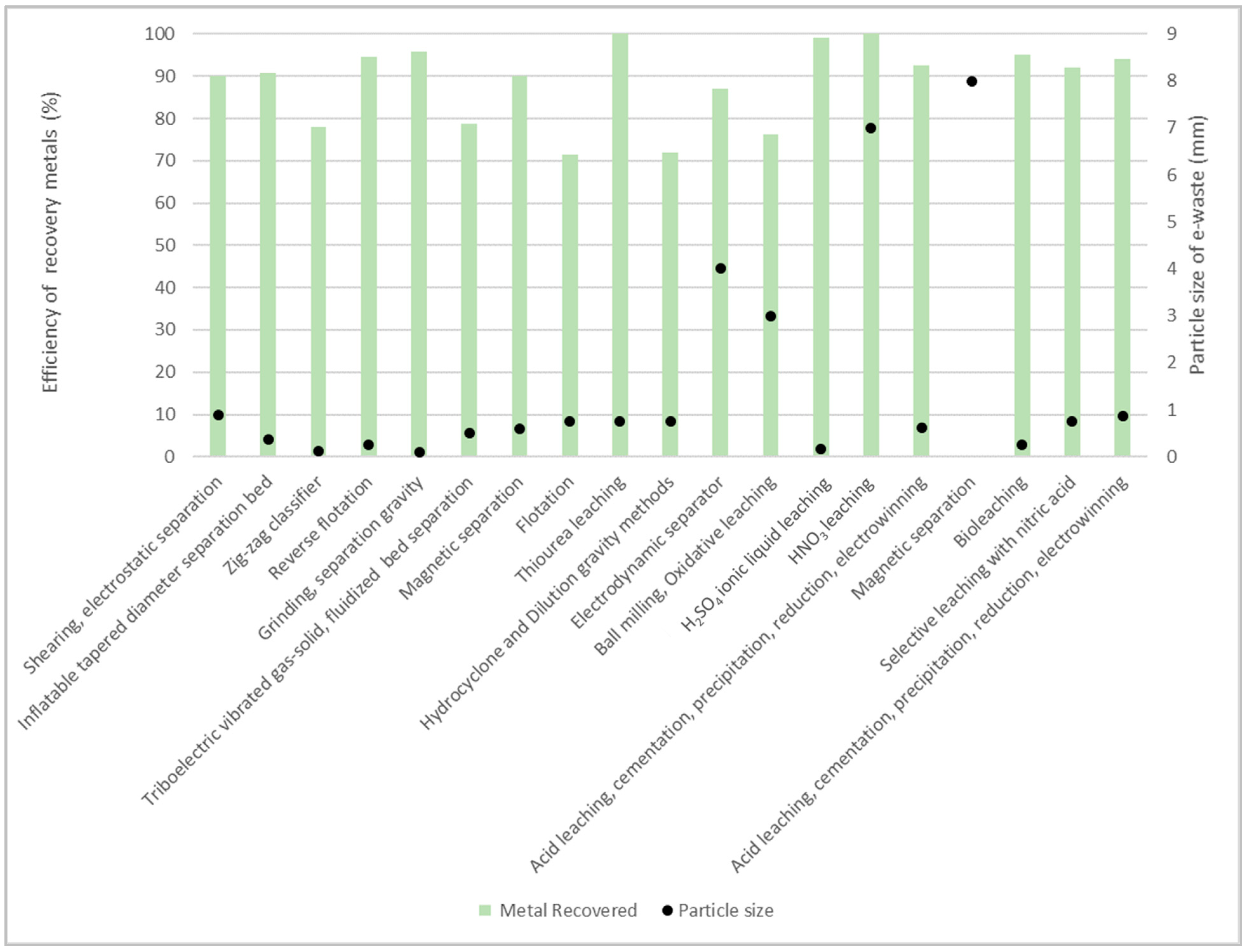

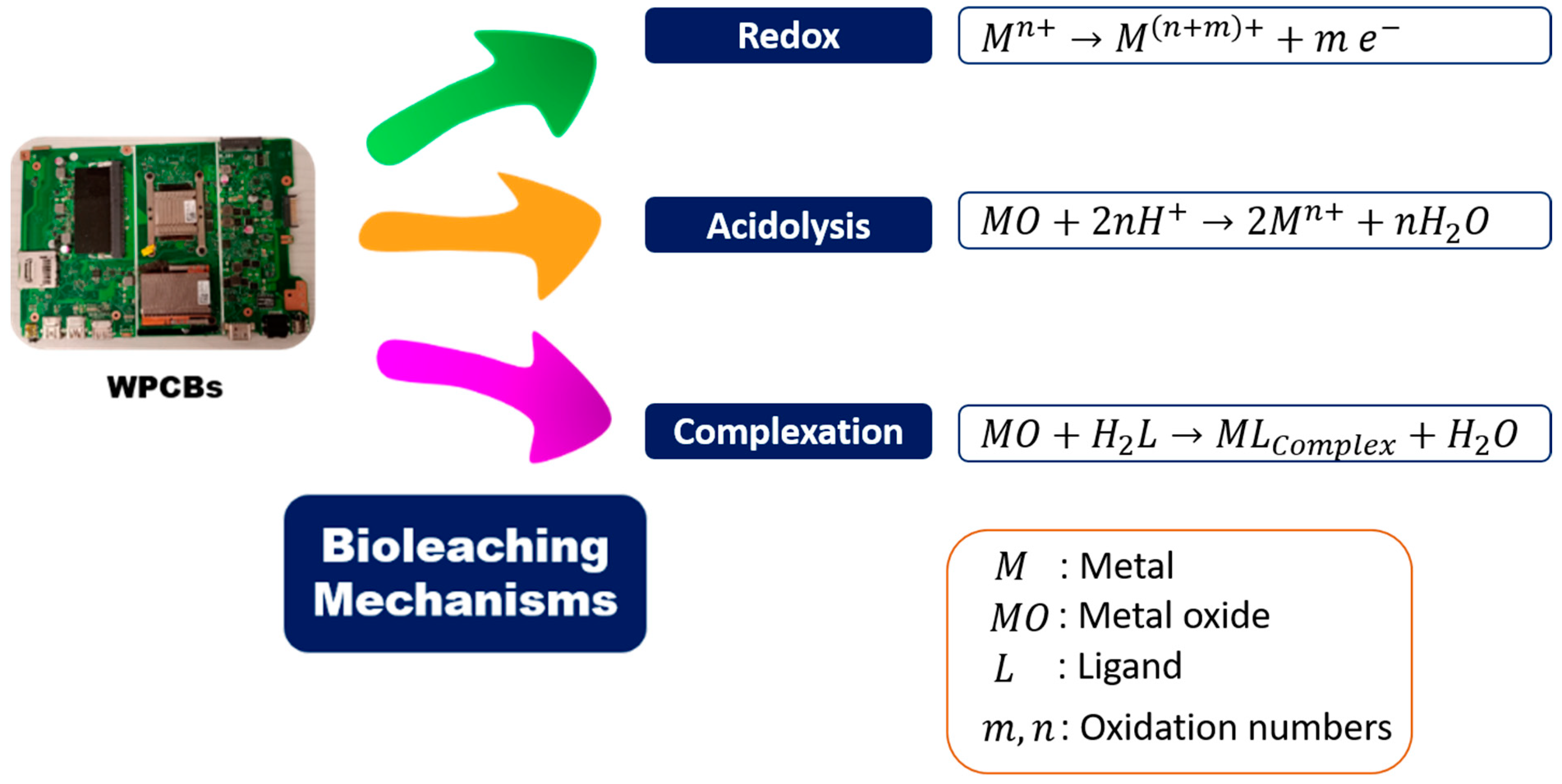


| Classification | Percent (%) | Element |
|---|---|---|
| Major elements | 5–20 | Fe, Cu, Al |
| Intermediate elements | 0.1–10 | Zn, Sn, Ni, Pb, Cr |
| Minor elements | <0.1 | Ag, Au, Ba, Bi, Cd, Ga, Ge, In, Mn, Pb, Pd, Ta, Ti |
| Type of Pretreatment of E-Waste | Recovery Method | Metal | Nanoparticles | Reference |
|---|---|---|---|---|
| Autoclave treatment: polymer separation Ball mill–hammer mill | Selective leaching: ammonia | Cu | CuO: 5 nm | [25] |
| Crushing in a crystal agate mortar, 200 mesh | Selective leaching with nitric acid | Ag | 8–30 nm | [28] |
| Cutting mill: 0.075–1 mm | Acidithiobacillus ferrooxidans, Leptospirillum ferrooxidans, and Acidithiobacillus thiooxidans | Cu | — | [29] |
| Shredding in a cutting mill equipped with 8 mm mesh | Magnetic separation concentrated by magnetic separation Hydrometallurgical HCl (ranging 1 M to 6 M) and H2O2 (ranging 4 M to 8.8 M) | Au | AuNP: 5–20 nm | [23] |
| Cutting: surface areas 100 to 1600 mm2 | Floating in vibrated fluid ascorbic acid, ß-cyclodextrin, and 80 °C and 700 rpm | Cu | Cu: 7 nm | [17] |
| Hammer and cutting mill: 0.075–1 mm | Bioleaching: pure culture of A. ferrooxidans and citric acid | Cu Zn Pb Ni | — | [18] |
| Grinding | Thermal concentrated precipitation: conventional, microwave, and ultrasound SnO2: metastannic acid AgNp: HCl-ammonia | Sn Ag | SnO2: 15 nm AgNp: 0.7 nm and 200 nm | [30] |
| Griding in a knife mill and crushing in a hammer mill with a 2 mm grid | Bacteria obtained from the marine sponge Hymeniacidon heliophila used in selective bioleaching | Cu | Cu: 1 nm | [27] |
| Cutting mill 0.8–0.4 mm | Bioleaching: pure culture of A. ferrooxidans and citric acid | Cu Zn Al | — | [31] |
| Leaching Agent | Toxicity | Advantages | Disadvantages |
|---|---|---|---|
| Cyanide | Very High |
|
|
| Aqua regia | High |
|
|
| Bromide and Iodide | Low |
|
|
| Thiosulfate | Medium |
|
|
| Thiourea | High |
|
|
| H2SO4 | Medium |
|
|
| HCl | Medium |
|
|
| HNO3 | Medium |
|
|
| Leaching Agent | Metal | Concentration of Metal from Leaching (mg/L or Leaching Percentage) | Reference |
|---|---|---|---|
| Aqua regia | Au | 125 | [50] |
| Pd | 13 | ||
| Ag | 163 | ||
| Aqua regia | Au | 57% | [51] |
| Cu | 44% | ||
| Aqua regia | Au | 550 | [52] |
| Thiourea | Au | 100% | [53] |
| Ag | 81% | ||
| Pd | 13% | ||
| Halogens (Iodine) | Au | 99.8% | [53] |
| Ag | 81.7% | ||
| Pd | 74% | ||
| Iodine-iodide | Au | 98% | [54] |
| KI | Au | 99.2% | [55] |
| Thiosulfate | Au | 81% | [58] |
| Ag | 88% | ||
| Cu | 32% |
| Green Recovery Method | Microorganism | Metal | Concentration of Metal in Leaching (% or mg/L) | Operation | Reference |
|---|---|---|---|---|---|
| Biohydrometallurgy | Acidithiobacillus ferrooxidans and Acidiphilium acidophilus | Cu | 96% | Concentrated by fractional chemical precipitation | [19] |
| Zn | 94.5% | ||||
| Ni | 75% | ||||
| Pb | 74.5% | ||||
| Bioleaching | Acidithiobacillus ferrooxidans, Leptospirillum ferrooxidans, and Acidithiobacillus thiooxidans | Cu | 95% | Concentrated by electrowinning | [29] |
| Bioleaching | Leptospirillum ferriphilum | Fe | 8000 mg/L | pH 1.2 T: 35 °C | [79] |
| Bioleaching | Pseudomonas fluorescens | Au | 54% | — | [69] |
| Bioleaching | Chromobacterium violaceum | Au | 11–30% | — | [62] |
| Hybrid combination: Bioleaching-Biosorption | Lactobacillus acidophilus | Au | 85% | 5 mL of microbial inoculum and 1 g of contact material 90 days | [80] |
| Bioleaching | Aspergillus niger | Cu | 100% | pH 7.0, 5 days, T: 30 °C, 200 rpm Pulp density: 0.5% | [81] |
| Bioleaching | Aspergillus niger | Ag | 67% | — | [67] |
| Cu | 50% | ||||
| Bioleaching | Aspergillus niger consortium | Au | 87% | — | [70] |
| Cu | 81.7% | ||||
| Ni | 74% | ||||
| Hybrid combination: Bioleaching-Biosorption | Pleurotus florida and Pseudomonas spp. | Cu | 18% | 1 g biomass pH 7.2 T: 27 °C Lacase production | [75] |
| Fe | 12.4% | ||||
| Biosorption | Aspergillus carbonarius | Cr6+ | 92.43% | pH 2.0 during 12 h at 37 °C | [74] |
| Bioaccumulation | Penicillium expansum | La | 390 mg/L | — | [82] |
| Tb | 1520 mg/L | ||||
| Biosorption | Aspergillus spp. | Cd | 88% | pH 4 T: 30 °C | [73] |
| Method | Conditions | Element | Reference |
|---|---|---|---|
| Fractional chemical precipitation, solvent extraction, and electrowinning | A two-stage process for the separation of Cu using a phenolic oxime dissolved in kerosene as an organic liquid phase for the recovery of copper and nickel from the leach liquor. The availed residue is dissolved in the halide salts in the presence of acidic conditions, showing the quantitative dissolution of gold and silver. Solvent extraction with amide-based reagents recovered gold by leaving the silver-rich raffinate. A precipitation or cementation technique using copper powder is implemented for the precipitation of silver. | Cu, Ni, Ag, Au | [19] |
| Solvent extraction (SX) | Solvents: organophosphorus derivatives, guanidine derivations, and a mixture of amines–organophosphorus derivatives prior to chemical reduction. | Au | [87] |
| Electrowinning (EW) | Solutions: 8.1 g/L Cu and 9.1 g/L Fe Cathode: copper Anode: Ti-metal oxide Current density of 300 A/m2 | Cu (66%) | [29] |
| Electrowinning (EW) | Cathode and anode: Au, 100 AC voltage for 5 Solution: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution containing 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) | Au | [88] |
| Dialysis | Polyethyleneimine (PEI) membrane | Nd Pr | [85] |
| Algae | Highlights | Element | Nanoparticle Size | Reference |
|---|---|---|---|---|
| Sargassum spp. | The microwave-assisted synthe-sis (MAS) of AgNPs using Sar-gassum spp. biomass involves the reduction of Ag+ ions by diverse organic compounds present in the macroalgae extract, including polysaccharides, proteins, poly-phenols, and flavonoids, through redox reactions. | Ag | 10 to 175 nm. Average size of 36.43 nm | [115] |
| Rhizoclonium hieroglyphicum, Lyngbya majuscule and Spirulina subsalsa | The reduction of gold particles is attributed to the presence of cellular reductases, with proteins such as cysteine serving to stabilize the nanoparticles. | Au | <20 nm | [116] |
| Chlorella vulgaris | Carboxylate groups on the surface of Chlorella vulgaris cells capture metal ions, which are subsequently reduced to silver nanoparticles by reductase enzymes. | Ag | Average size 10.95 nm | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Vásquez, T.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. From E-Waste to High-Value Materials: Sustainable Synthesis of Metal, Metal Oxide, and MOF Nanoparticles from Waste Printed Circuit Boards. Nanomaterials 2024, 14, 69. https://doi.org/10.3390/nano14010069
Pineda-Vásquez T, Rendón-Castrillón L, Ramírez-Carmona M, Ocampo-López C. From E-Waste to High-Value Materials: Sustainable Synthesis of Metal, Metal Oxide, and MOF Nanoparticles from Waste Printed Circuit Boards. Nanomaterials. 2024; 14(1):69. https://doi.org/10.3390/nano14010069
Chicago/Turabian StylePineda-Vásquez, Tatiana, Leidy Rendón-Castrillón, Margarita Ramírez-Carmona, and Carlos Ocampo-López. 2024. "From E-Waste to High-Value Materials: Sustainable Synthesis of Metal, Metal Oxide, and MOF Nanoparticles from Waste Printed Circuit Boards" Nanomaterials 14, no. 1: 69. https://doi.org/10.3390/nano14010069









