Sensitive Immunochromatographic Determination of Salmonella typhimurium in Food Products Using Au@Pt Nanozyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Biotinylation of MAb
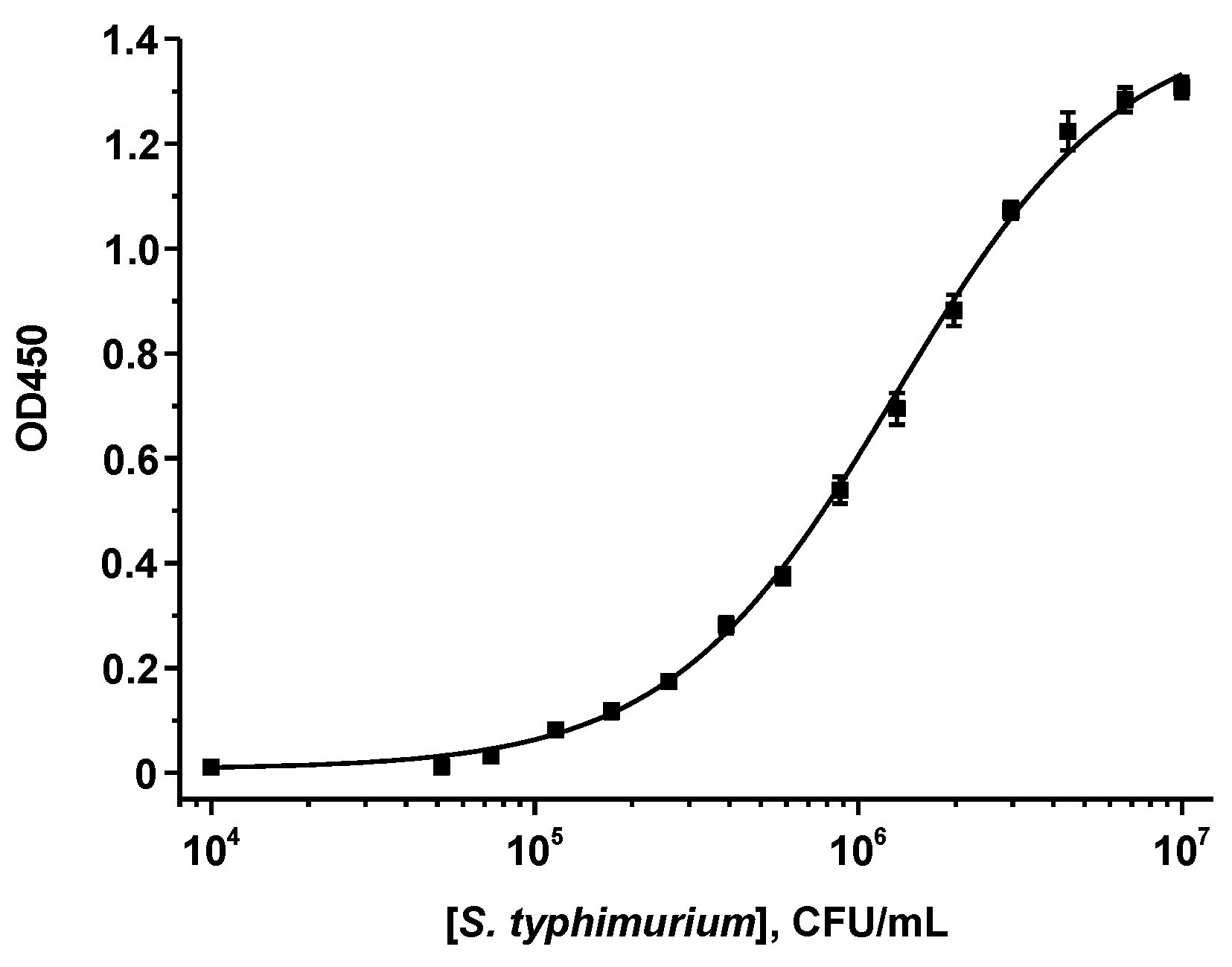
2.3. ELISA of S. typhimurium
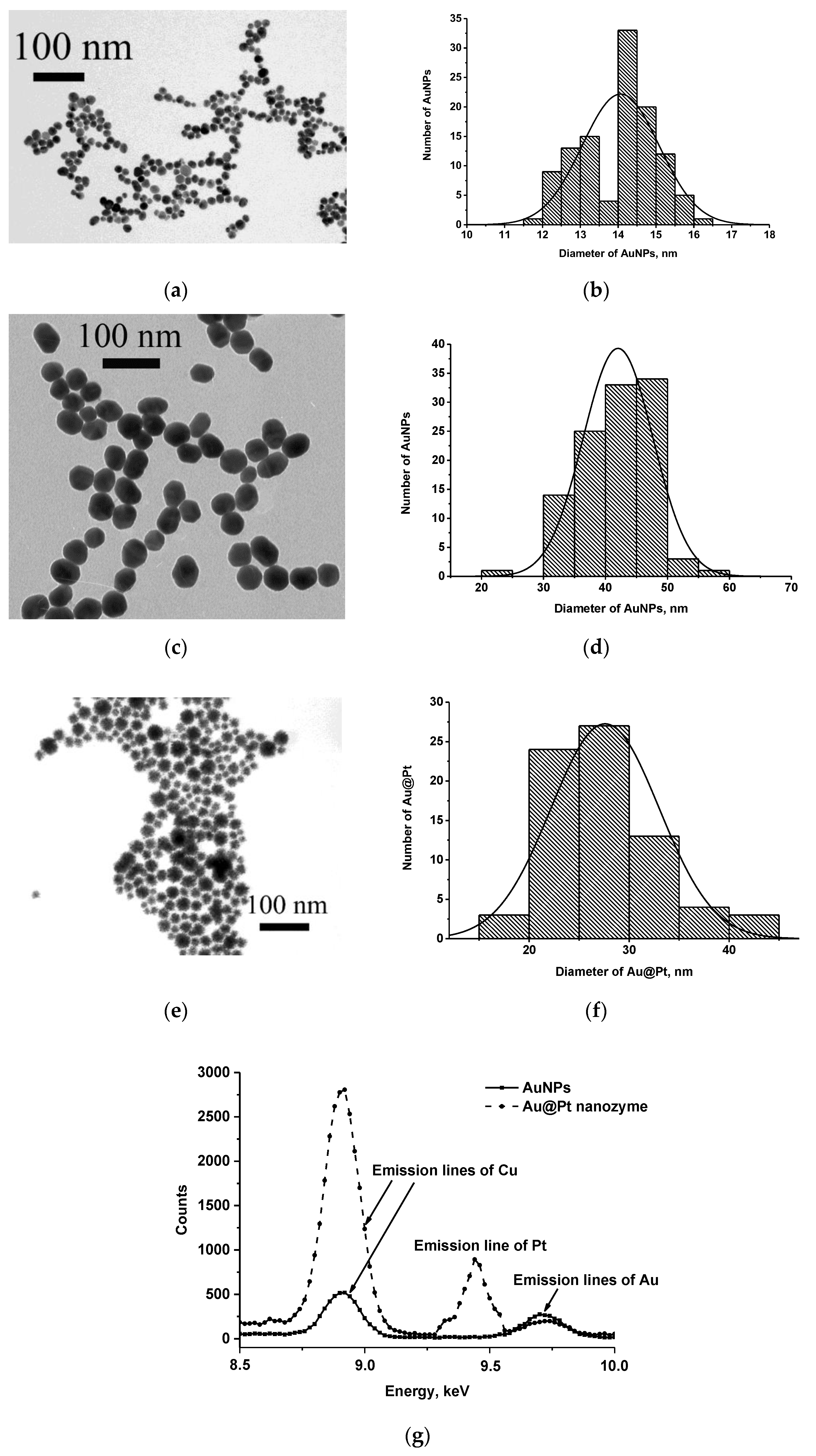
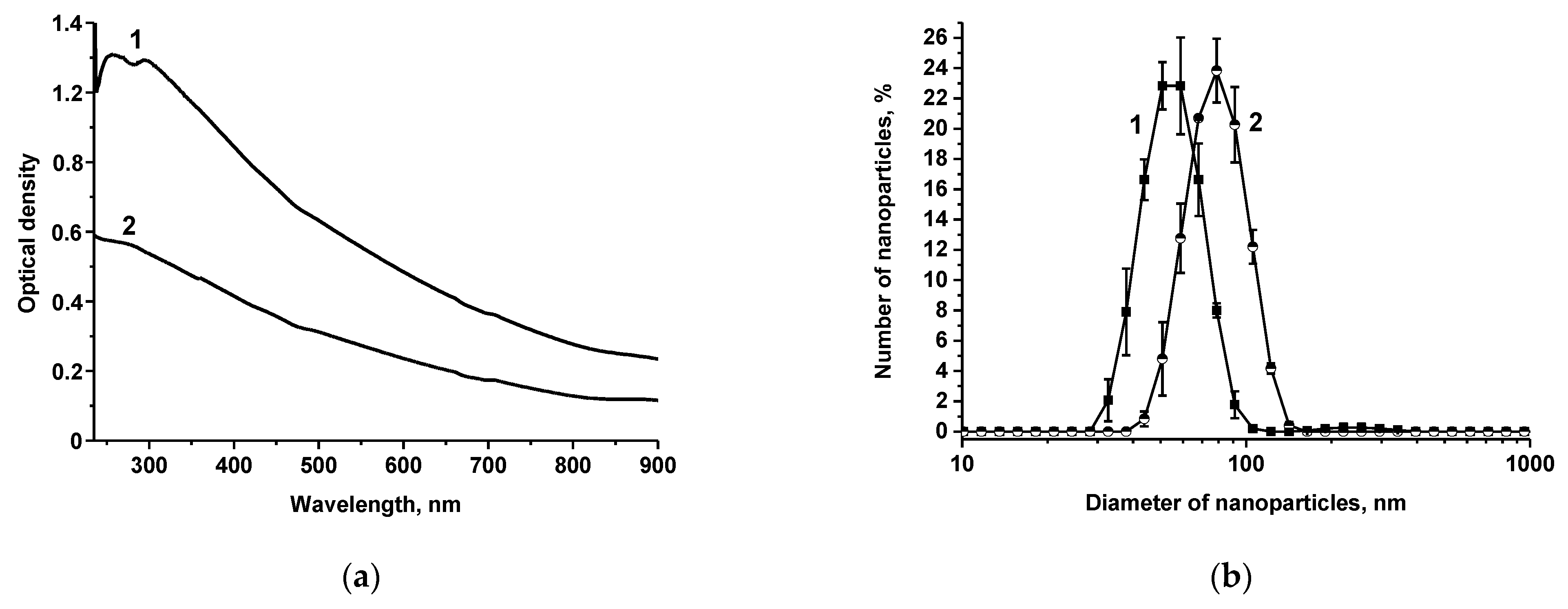
2.4. Synthesis and Characterization of AuNPs and Au@Pt Nanozyme
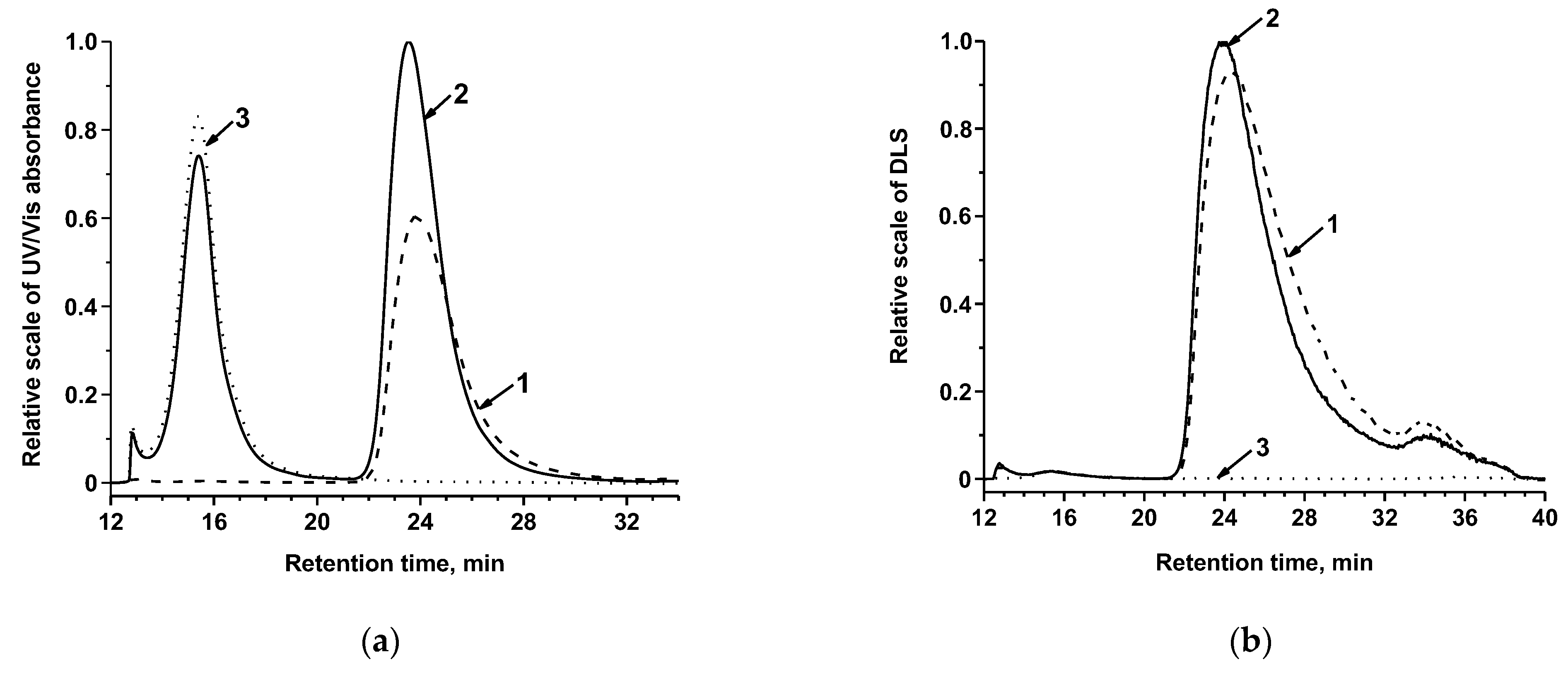
2.5. Conjugation of MAb with AuNPs and Au@Pt
2.6. Production of Immunochromatographic Tests
2.7. Sample Preparation before the ICA and ELISA
2.8. ICA of S. typhimurium Using AuNPs
2.9. Common and Enhanced ICAs of S. typhimurium Using Au@Pt Nanozyme
2.10. Evaluation of the ELISA and ICA Results and Statistical Analysis
3. Results and Discussion
3.1. Obtaining and Characterization of the Reagents
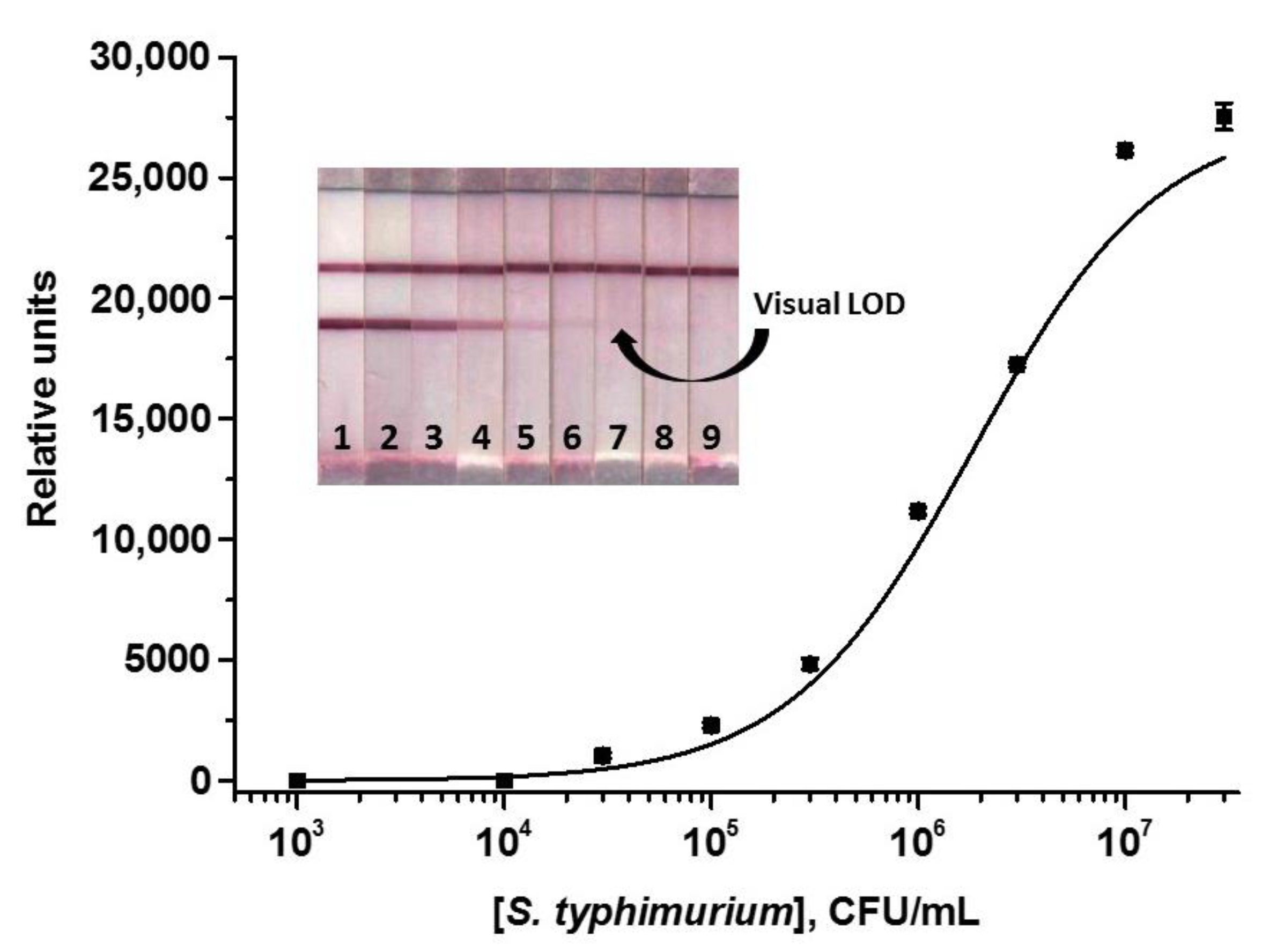
3.2. ICA of S. typhimurium Using AuNPs
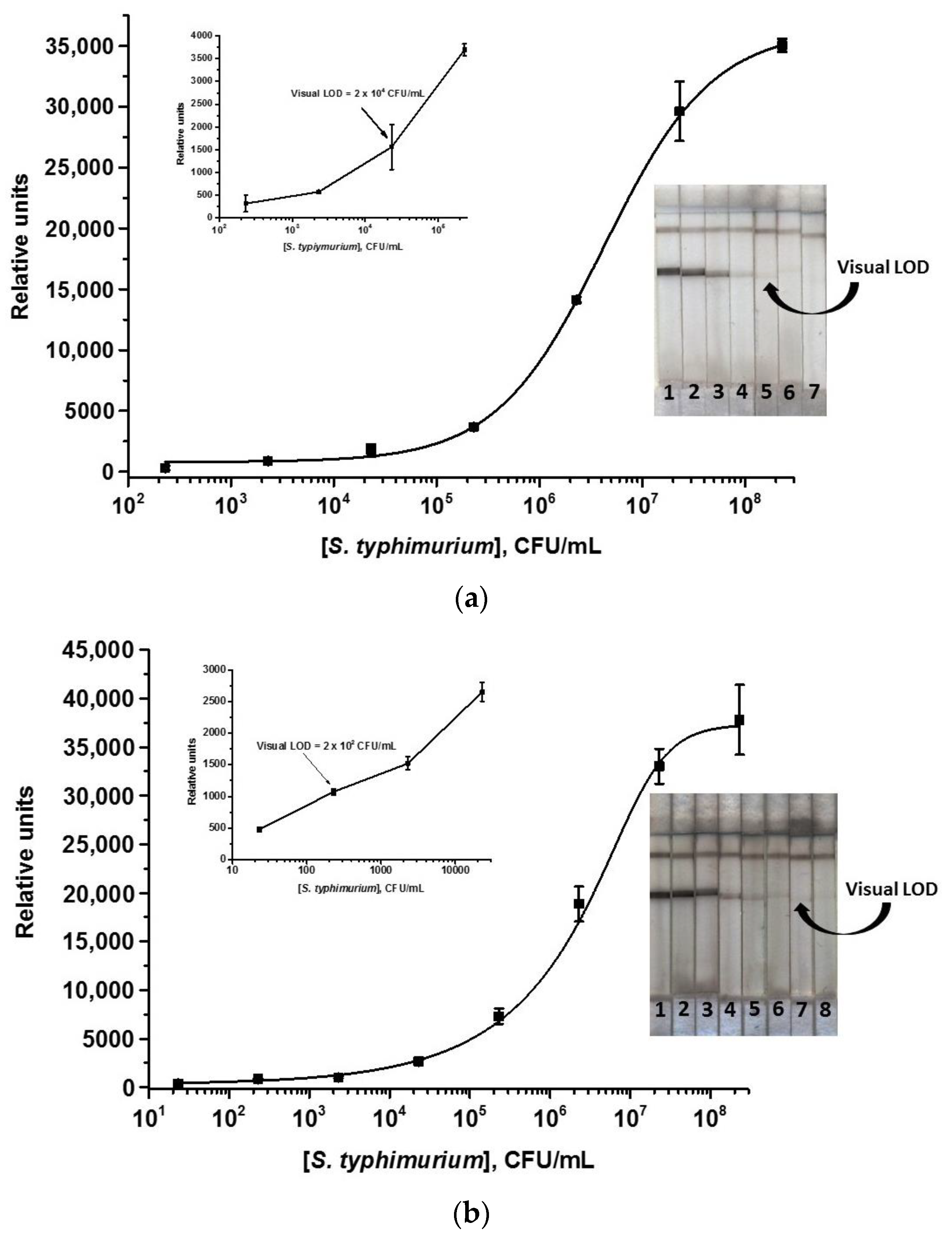
3.3. Common and Enhanced ICAs of S. typhimurium Using Au@Pt Nanozyme
3.4. ICA Specificity
3.5. ICA of S. typhimurium in Cow Milk and Chicken Meat
3.6. Comparison with Other Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Modi, B.; Timilsina, H.; Bhandari, S.; Achhami, A.; Pakka, S.; Shrestha, P.; Kandel, D.; GC, D.B.; Khatri, S.; Chhetri, P.M.; et al. Current trends of food analysis, safety, and packaging. Int. J. Food Sci. 2021, 2021, 9924667. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.D.; Wells, J.E. Reducing foodborne pathogen persistence and transmission in animal production environments: Challenges and opportunities. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Calogero, A.; Montesano, D.; Naviglio, D. Relationships between food and diseases: What to know to ensure food safety. Food Res. Int. 2020, 137, 109414. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.L.; Lynne, A.M. Food animal-associated Salmonella challenges: Pathogenicity and antimicrobial resistance. J. Anim. Sci. 2008, 86, E173–E187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vaddu, S.; Bhumanapalli, S.; Mishra, A.; Applegate, T.; Singh, M.; Thippareddi, H. A systematic review and meta-analysis of the sources of Salmonella in poultry production (pre-harvest) and their relative contributions to the microbial risk of poultry meat. Poult. Sci. 2023, 102, 102566. [Google Scholar] [CrossRef]
- Anderson, C.J.; Kendall, M.M. Salmonella enterica serovar Typhimurium strategies for host adaptation. Front. Microbiol. 2017, 8, 1983. [Google Scholar] [CrossRef]
- Li, Q. Mechanisms for the invasion and dissemination of Salmonella. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 2655801. [Google Scholar] [CrossRef]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.M.C.; Bhowmik, S.; Ali, A. Conventional and advanced detection techniques of foodborne pathogens: A comprehensive review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, L.; Li, Y. Biosensors for rapid detection of Salmonella in food: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 149–197. [Google Scholar] [CrossRef]
- Gao, D.; Yu, J.; Dai, X.; Tian, Y.; Sun, J.; Xu, X.; Cai, X. Development and evaluation of an indirect enzyme-linked immunosorbent assay based on a recombinant SifA protein to detect Salmonella infection in poultry. Poult. Sci. 2023, 102, 102513. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Song, Z.; Zhou, C.; Ma, P.; Li, C.; Lu, Q.; Liao, Z.; Huang, Z.; Tang, Y.; Li, H.; et al. Development of nanobody-horseradish peroxidase-based sandwich ELISA to detect Salmonella enteritidis in milk and in vivo colonization in chicken. J. Nanobiotechnol. 2022, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.G.; Falkenhorst, G.; Ceper, T.H.; Dalby, T.; Ethelberg, S.; Mølbak, K.; Krogfelt, K.A. Detecting non-typhoid Salmonella in humans by ELISAs: A literature review. J. Med. Microbiol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Song, S.; Tang, L.; Kuang, H.; Xu, C. A highly sensitive ELISA and immunochromatographic strip for the detection of Salmonella typhimurium in milk samples. Sensors 2015, 15, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.; Yassine, H.M.; Kourentzi, K.; Tang, P.; Litvinov, D.; Willson, R.C.; Abu-Raddad, L.J.; Nasrallah, G.K. A review of rapid food safety testing: Using lateral flow assay platform to detect foodborne pathogens. Crit. Rev. Food Sci. Nutr. 2023, 23, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wu, A.; Xu, L.; Xu, C.; Liu, L.; Kuang, H.; Xu, X. Recent progress on lateral flow immunoassays in foodborne pathogen detection. Food Biosci. 2023, 52, 102475. [Google Scholar] [CrossRef]
- Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Immunochromatographic technique for express determination of ampicillin in milk and dairy products. Appl. Biochem. Microbiol. 2011, 47, 627–634. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Sensitive lateral flow immunoassay of an antibiotic neomycin in foodstuffs. J. Food Sci. Technol. 2021, 58, 292–301. [Google Scholar] [CrossRef]
- Çam, D.; Öktem, H.A. Development of rapid dipstick assay for food pathogens, Salmonella, by optimized parameters. J. Food Sci. Technol. 2019, 56, 140–148. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Yang, H.; Liang, S.; Pan, J.; Zou, Y.; Li, S.; Li, Q.; Zhao, S. Versatile Au@Ru nanocomposites for the rapid detection of Salmonella typhimurium and photothermal sterilization. J. Colloid Interface Sci. 2022, 621, 489–498. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, M.; Liu, C.; Tian, Y.; Fang, S.; Yang, H.; Li, B.; Liu, Q. Colloidal gold immunochromatographic test strips for broad-spectrum detection of Salmonella. Food Control 2021, 126, 108052. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Yin, X.; Deng, Z.; Liu, X.; Yang, D.; Zhao, L.; Sun, J.; Wang, J.; Zhang, D. Joint-detection of Salmonella typhimurium and Escherichia coli O157:H7 by an immersible amplification dip-stick immunoassay. Biosens. Bioelectron. 2023, 224, 115075. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Ding, S.; Zhang, N.; Zhou, Y.; Cheng, N.; Wang, M.; Xu, M.; Feng, Z.; Niu, X.; Cheng, Y.; et al. Single-atom nanozymes linked immunosorbent assay for sensitive detection of Aβ 1–40: A biomarker of Alzheimer’s disease. Research 2020, 2020, 4724505. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Leem, H.; Kim, M. Development of a liposome-based immunochromatographic strip assay for the detection of Salmonella. Anal. Bioanal. Chem. 2011, 401, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Leem, H.; Lee, J.-S.; Kim, M. Immunochromatographic strip assay for the rapid and sensitive detection of Salmonella typhimurium in artificially contaminated tomato samples. Can. J. Microbiol. 2014, 60, 399–406. [Google Scholar] [CrossRef]
- Bu, T.; Huang, Q.; Yan, L.; Huang, L.; Zhang, M.; Yang, Q.; Yang, B.; Wang, J.; Zhang, D. Ultra technically-simple and sensitive detection for Salmonella enteritidis by immunochromatographic assay based on gold growth. Food Control 2018, 84, 536–543. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, J.; He, Y.; Wang, Y.; Bai, M.; Zhang, C.; Luo, L.; Wang, J. Ultrasensitive label-free immunochromatographic strip sensor for Salmonella determination based on salt-induced aggregated gold nanoparticles. Food Chem. 2021, 343, 128518. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Y.; Gao, Y.; Zhu, Z. Rational design strategies for nanozyme. ACS Nano 2023, 17, 13062–13080. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Q.; Gu, J. Recent advances in colorimetric sensors based on nanozymes with peroxidase-like activity. Analyst 2023, 148, 487–506. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Safenkova, I.V.; Slutskaya, E.S.; Panferov, V.G.; Zherdev, A.V.; Dzantiev, B.B. Complex analysis of concentrated antibody-gold nanoparticle conjugates’ mixtures using asymmetric flow field-flow fractionation. J. Chromatogr. A 2016, 1477, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Vostrikova, N.L.; Chernukha, I.M.; Dzantiev, B.B.; Zherdev, A.V. Lateral flow immunoassay for sensitive detection of undeclared chicken meat in meat products. Food Chem. 2021, 344, 128598. [Google Scholar] [CrossRef] [PubMed]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values–Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef]
- Oliveira, B.B.; Ferreira, D.; Fernandes, A.R.; Baptista, P.V. Engineering gold nanoparticles for molecular diagnostics and biosensing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1836. [Google Scholar] [CrossRef] [PubMed]
- Sotnikov, D.V.; Byzova, N.A.; Zherdev, A.V.; Dzantiev, B.B. Retention of activity by antibodies immobilized on gold nanoparticles of different sizes: Fluorometric method of determination and comparative evaluation. Nanomaterials 2021, 11, 3117. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Panferov, V.G.; Solopova, O.N.; Zherdev, A.V.; Sveshnikov, P.G.; Dzantiev, B.B. Application of Au@Pt nanozyme as enhancing label for the sensitive lateral flow immunoassay of okadaic acid. Biosensors 2022, 12, 1137. [Google Scholar] [CrossRef]
- Siva, S.; Bodkhe, G.A.; Cong, C.; Kim, S.H.; Kim, M. Heterostructured Au@Pt/Nb2C-MXene nanocomposite as a colorimetric sensor with peroxidase-like activity for reactive oxygen species of H2O2. J. Ind. Eng. Chem. 2023, 124, 523–531. [Google Scholar] [CrossRef]
- Byzova, N.A.; Zherdev, A.V.; Sveshnikov, P.G.; Sadykhov, E.G.; Dzantiev, B.B. Development of an immunochromatographic test system for the detection of Helicobacter pylori antigens. Appl. Biochem. Microbiol. 2015, 51, 608–617. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Du, D.; Smith, J.; Timchalk, C.; Liu, D.; Lin, Y. Direct analysis of trichloropyridinol in human saliva using an Au nanoparticles-based immunochromatographic test strip for biomonitoring of exposure to chlorpyrifos. Talanta 2013, 114, 261–267. [Google Scholar] [CrossRef]
- Peng, T.; Wang, J.; Zhao, S.; Zeng, Y.; Zheng, P.; Liang, D.; Mari, G.M.; Jiang, H. Highly luminescent green-emitting Au nanocluster-based multiplex lateral flow immunoassay for ultrasensitive detection of clenbuterol and ractopamine. Anal. Chim. Acta 2018, 1040, 143–149. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Rodpai, E.; Kanarat, S. Evaluation of an immunochromatographic assay for rapid detection of Salmonella enterica serovars typhimurium and enteritidis. J. Vet. Diagn. Investig. 2011, 23, 797–801. [Google Scholar] [CrossRef]
- Preechakasedkit, P.; Pinwattana, K.; Dungchai, W.; Siangproh, W.; Chaicumpa, W.; Tongtawe, P.; Chailapakul, O. Development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosens. Bioelectron. 2012, 31, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, L.Y.; Nor, D.Z.; Rahmah, N.; Khairunisak, A.R. Development and evaluation of colloidal gold lateral flow immunoassays for detection of Escherichia Coli O157 and Salmonella Typhi. J. Phys. Conf. Ser. 2018, 1082, 012049. [Google Scholar] [CrossRef]
- Mahari, S.; Prakashan, D.; Gandhi, S. Immunochromatographic assay for the point-of-care diagnosis of food borne Salmonella strains using smartphone application. Colloids Surf. B Biointerfaces 2023, 226, 113319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, F.; Wang, G.; Liao, T.; Hao, Y.; Zhang, H. Rapid and sensitive pathogen detection platform based on a lanthanide-labeled immunochromatographic strip test combined with immunomagnetic separation. Sens. Actuators B Chem. 2021, 329, 129273. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Bu, T.; Deng, Z.; Zhao, L.; Tian, Y.; Jia, C.; Li, Y.; Wang, R.; Wang, J.; et al. Nanosheet antibody mimics based label-free and dual-readout lateral flow immunoassay for Salmonella enteritidis rapid detection. Biosens. Bioelectron. 2023, 229, 115239. [Google Scholar] [CrossRef]
- Bu, T.; Wang, J.; Huang, L.; Dou, L.; Zhao, B.; Li, T.; Zhang, D. New functional tracer—Two-dimensional nanosheet-based immunochromatographic assay for Salmonella enteritidis detection. J. Agric. Food Chem. 2019, 67, 6642–6649. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Zhao, L.-J.; Wang, Y.; Wang, K.; Li, H.-W.; Li, X.; Zi, M.; Zeng, J.-B. Colorimetric and photothermal dual-mode lateral flow immunoassay based on Au-Fe3O4 multifunctional nanoparticles for detection of Salmonella typhimurium. Microchim. Acta 2023, 190, 57. [Google Scholar] [CrossRef]
- Duan, M.-L.; Huang, Y.-M.; Wu, S.-S.; Li, G.-Q.; Wang, S.-Y.; Chen, M.-H.; Wang, C.; Liu, D.-F.; Liu, C.-W.; Lai, W.-H. Rapid and sensitive detection of Salmonella enteritidis by a pre-concentrated immunochromatographic assay in a large-volume sample system. RSC Adv. 2017, 7, 55141–55147. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Y.; He, D.; Xu, E.; Chai, Q.; Jin, Z.; Wu, Z.; Cui, B. Improving the sensitivity of lateral flow immunoassay for Salmonella typhimurium detection via flow-rate regulation. Food Chem. 2022, 397, 133756. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, C.; Zheng, S.; Jiang, B.; Li, J.; Pang, Y.; Wang, C.; Hao, R.; Xiao, R. Ultrasensitive multichannel immunochromatographic assay for rapid detection of foodborne bacteria based on two-dimensional film-like SERS labels. J. Hazard. Mater. 2022, 437, 129347. [Google Scholar] [CrossRef] [PubMed]
| Added S. typhimurium, CFU/g | Revealed S. typhimurium, CFU/g | Recovery ± SD 1 (%) |
|---|---|---|
| ICA data | ||
| Lactose-free milk with a fat content of 1.5% | ||
| 6 × 106 | 4.5 × 106 ± 0.4 × 106 | 74.8 ± 7.2 |
| 2 × 107 | 1.7 × 107 ± 0.06 × 107 | 82.5 ± 2.8 |
| Milk with a fat content of 3.2% | ||
| 6 × 106 | 5.1 × 106 ± 0.2 × 106 | 85.0 ± 2.9 |
| 2 × 107 | 1.6 × 107 ± 0.1 × 107 | 78.8 ± 5.6 |
| Baked milk with a fat content of 4% | ||
| 6 × 106 | 5.0 × 106 ± 0.3 × 106 | 82.8 ± 5.6 |
| 2 × 107 | 1.7 × 107 ± 0.02 × 107 | 86.0 ± 0.9 |
| Chicken meat | ||
| 2.8 × 103 | 2.6 × 103 ± 0.07 × 103 | 94.5 ± 2.4 |
| 0.8 × 106 | 0.74 × 106 ± 0.002 × 106 | 92.2 ± 0.2 |
| ELISA data | ||
| Lactose-free milk with a fat content of 1.5% | ||
| 6 × 106 | 5.5 × 105 ± 0.4 × 105 | 91.7 ± 7.7 |
| 2 × 107 | 1.6 × 106 ± 0.09 × 106 | 80.0 ± 1.5 |
| Milk with a fat content of 3.2% | ||
| 6 × 106 | 5.2 × 106 ± 0.25 × 106 | 86.7 ± 4.2 |
| 2 × 107 | 1.55 × 107 ± 0.14 × 107 | 77.5 ± 7.0 |
| Baked milk with a fat content of 4% | ||
| 6 × 106 | 5.4 × 106 ± 0.3 × 106 | 90.0 ± 5.0 |
| 2 × 107 | 1.65 × 107 ± 0.06 × 107 | 82.5 ± 3.0 |
| Chicken meat | ||
| 0.8 × 106 | 0.65 × 106 ± 0.04 × 106 | 81.3 ± 5.0 |
| No | Detected Antigen | Label | LOD | Assay Duration, min | Matrix | Reference |
|---|---|---|---|---|---|---|
| Non-instrumental analysis | ||||||
| 1 | S. enteritidis, S. typhimurium | AuNPs | 104, 106 CFU/mL | 5–15 | Cell culture | [43] |
| 2 | S. typhimurium | Liposomes with encapsulated fluorescent dye | 102 CFU/mL | 10 | Buffer | [24] |
| 3 | S. typhi | AuNPs | 1.14 × 105 CFU/mL | 15 | Human serum | [44] |
| 4 | S. typhimurium | Liposomes with encapsulated fluorescent dye | 1.2 cells/g | 10–15 | Tomatoes | [25] |
| 5 | S. enteritidis | AuNPs with signal amplification based on gold growth | 104 CFU/mL | 360 | Milk | [26] |
| 6 | S. typhi | AuNPs | Water: 3 × 108 CFU/mL Milk: 3 × 107 CFU/mL | 10–15 | Water and milk | [45] |
| 7 | S. enteritidis | MoS2 or graphene | 103 CFU/mL | 10 | Drinking water and watermelon juice | [49] |
| 8 | S. enteritidis, S. infantis | AuNPs | 106 CFU/mL | 4–5 | Buffer | [19] |
| 9 | S. typhimurium | AuNPs | 4 × 105 CFU/mL | 5–15 | Chicken meat | [21] |
| 10 | S. enteritidis, S. typhimurium | Salt-induced aggregated AuNPs | 103 CFU/mL | 14 | Cabbage and drinking water | [27] |
| 11 | S. gallinarum, S. pullorum, S. enteritidis | AuNPs | Feces: 103 CFU/mL; Meat: 102 CFU/mL; Milk: 104 CFU/mL | 10 | Fecal, meat, and milk | [46] |
| 12 | S. typhimurium | Au@Pt nanozyme with peroxidase-mimic activity | 2 × 102 CFU/mL | 15 | Milk of different fat content and chicken meat | This study |
| Analysis required additional equipment including that for magnetic separation | ||||||
| 13 | S. enteritidis | Magnetic nanoparticles | 1.95 × 105 CFU/mL | 30 | Milk | [51] |
| 14 | S. typhimurium | Tris (dibenzoylmethane)mono(1,10-phenanthroline)europium(III) in polystyrene nanoparticles | 103 CFU/mL | 90 | Milk and human serum | [47] |
| 15 | S. typhimurium | Multifunctional Au shell-coated graphene oxide nanosheets as a label for SERS signal enhancement | 20 cells/mL | 20 | Milk, vegetable juice, and fruit juice | [53] |
| 16 | S. typhimurium | Gold-ruthenium nanocomposites | 9.8 × 104 CFU/mL | 10 | Drinking water, orange juice, and milk | [20] |
| 17 | S. typhimurium | Platinum-coated gold nanorods, magnetic Fe3O4 nanoparticles | 50 CFU/mL (color mode), 75 CFU/mL (magnetic mode) | 15 | Milk | [52] |
| 18 | S. typhimurium | CoFe2O4 having catalase-like activity and magnetic properties | 102 CFU/mL | 15 | Milk and beef | [28] |
| 19 | S. enteritidis | Glucan-functionalized two-dimensional transition metal dichalcogenides tungsten disulfide as SERS-probe | 103 CFU/mL | 10 | Milk and drinking water | [48] |
| 20 | S. typhimurium | Au-Fe3O4 multifunctional nanoparticles | 5 × 105 CFU/mL (visual); 5 × 104 CFU/mL (photothermal) | 15 | Milk | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrickson, O.D.; Byzova, N.A.; Safenkova, I.V.; Panferov, V.G.; Dzantiev, B.B.; Zherdev, A.V. Sensitive Immunochromatographic Determination of Salmonella typhimurium in Food Products Using Au@Pt Nanozyme. Nanomaterials 2023, 13, 3074. https://doi.org/10.3390/nano13233074
Hendrickson OD, Byzova NA, Safenkova IV, Panferov VG, Dzantiev BB, Zherdev AV. Sensitive Immunochromatographic Determination of Salmonella typhimurium in Food Products Using Au@Pt Nanozyme. Nanomaterials. 2023; 13(23):3074. https://doi.org/10.3390/nano13233074
Chicago/Turabian StyleHendrickson, Olga D., Nadezhda A. Byzova, Irina V. Safenkova, Vasily G. Panferov, Boris B. Dzantiev, and Anatoly V. Zherdev. 2023. "Sensitive Immunochromatographic Determination of Salmonella typhimurium in Food Products Using Au@Pt Nanozyme" Nanomaterials 13, no. 23: 3074. https://doi.org/10.3390/nano13233074






