Photocatalytic Degradation of Ceftriaxone Using TiO2 Coupled with ZnO Micronized by Supercritical Antisolvent Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnO from ZnAc Micronized through Supercritical Antisolvent (SAS) Technique
2.3. Preparation of ZnO/PC50 Composites
- PC50;
- ZnOcomm;
- ZnOSAS;
- ZnOcomm/PC50;
- ZnOSAS/PC50.
2.4. Characterization Methods
2.5. Photocatalytic Activity Tests
3. Results and Discussion
3.1. Chemical–Physical Characterization of Photocatalysts
3.1.1. FESEM Characterization
3.1.2. UV-vis DRS Results
3.1.3. WAXD Results
3.1.4. Specific Surface Area (SSA) Measurement
3.1.5. Raman Spectroscopy Results
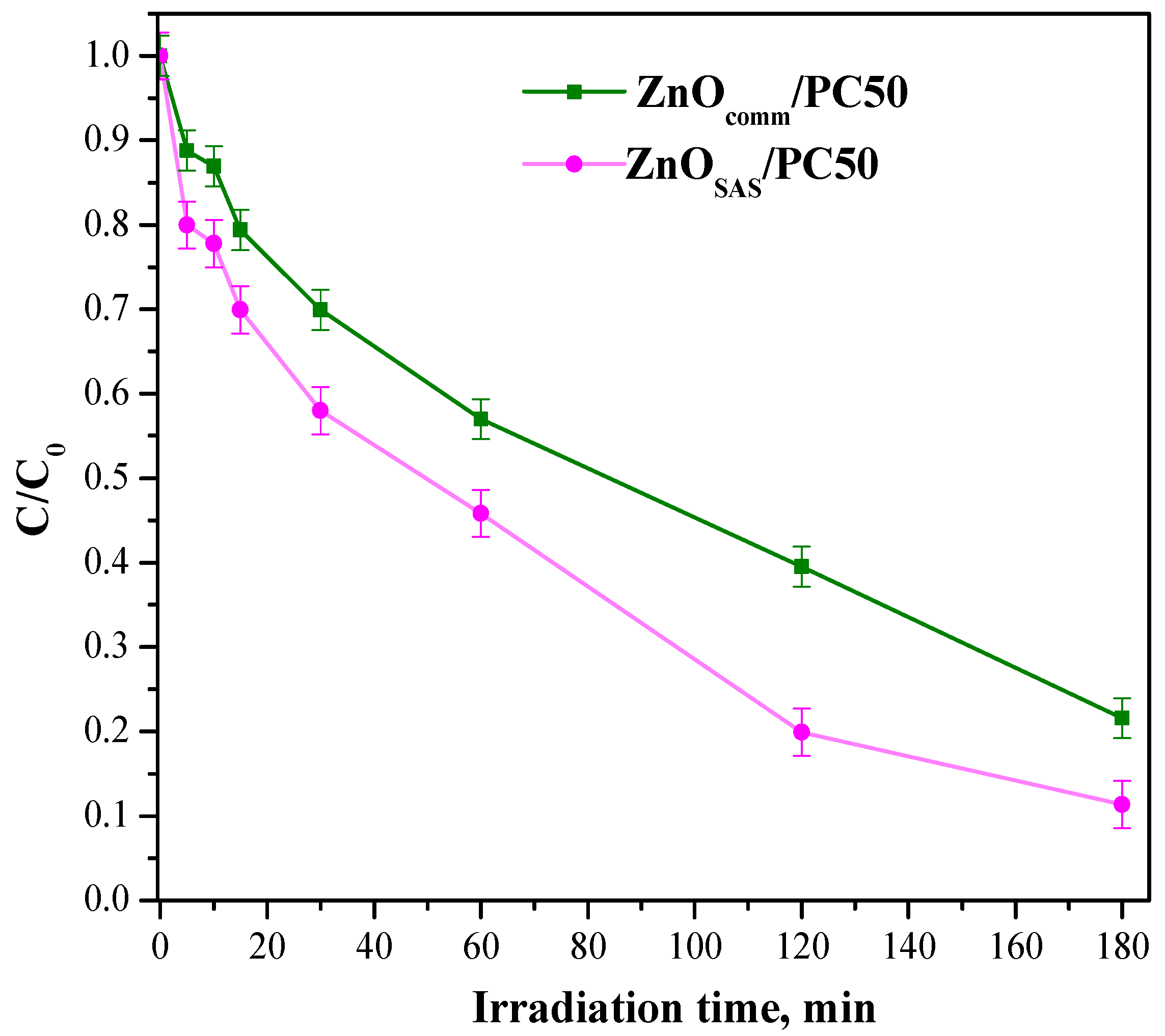
3.2. Photocatalytic Activity Results
3.2.1. Role of Reactive Oxygen Species in Ceftriaxone Photocatalytic Degradation in the Presence of the ZnOSAS/PC50 Composite
3.2.2. Possible Mechanism of the Photocatalytic Activity of the ZnOSAS/PC50 Composite
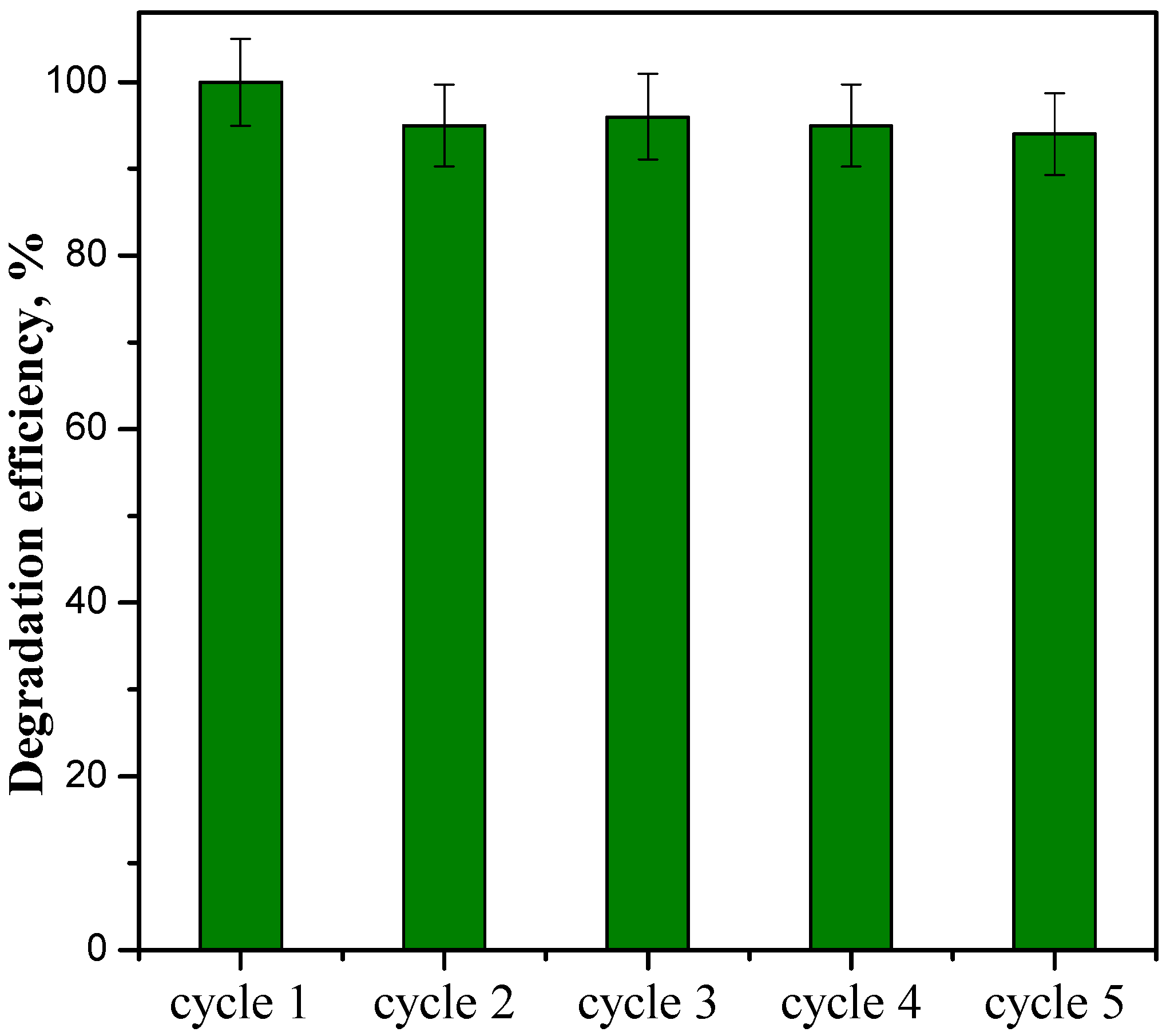
3.2.3. Stability Tests on ZnOSAS/PC50 Composite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vinu, R.; Madras, G. Environmental remediation by photocatalysis. J. Indian Inst. Sci. 2010, 90, 189–230. [Google Scholar]
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, Z.; Qi, S.; Yin, Z.; Deng, S.; Zhang, M.; Sun, Z. The quaternary system of Ag2S/ZnS co-modified ZnO/TiO2 nanotree arrays: Excellent photocatalysis and photoelectrochemistry performance. Appl. Surf. Sci. 2021, 538, 148044. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Mohadesi, M.; Sanavi Fard, M.; Shokri, A. The application of modified nano-TiO2 photocatalyst for wastewater treatment: A review. Int. J. Environ. Anal. Chem. 2022, 1–22. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO based nanomaterials for photocatalytic degradation of aqueous pharmaceutical waste solutions—A contemporary review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Puri, N.; Gupta, A. Water remediation using titanium and zinc oxide nanomaterials through disinfection and photo catalysis process: A review. Environ. Res. 2023, 227, 115786. [Google Scholar] [CrossRef] [PubMed]
- Jafarova, V.N.; Orudzhev, G.S. Structural and electronic properties of ZnO: A first-principles density-functional theory study within LDA(GGA) and LDA(GGA)+U methods. Solid State Commun. 2021, 325, 114166. [Google Scholar] [CrossRef]
- Likozar, B.; Huš, M. Electronic properties of rutile and anatase TiO2 and their effect on CO2 adsorption: A comparison of first principle approaches. Fuel 2022, 328, 125322. [Google Scholar] [CrossRef]
- Pesci, F.M.; Wang, G.; Klug, D.R.; Li, Y.; Cowan, A.J. Efficient suppression of electron-hole recombination in oxygen-deficient hydrogen-treated TiO2 nanowires for photoelectrochemical water splitting. J. Phys. Chem. C 2013, 117, 25837–25844. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Oh, S.G. Controlling the recombination of electron-hole pairs by changing the shape of ZnO nanorods via sol-gel method using water and their enhanced photocatalytic properties. Korean J. Chem. Eng. 2019, 36, 2118–2124. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, D.; Mo, Y.; Song, L.; Brewer, E.; Huang, X.; Xiong, Y. Photocatalytic activity of polymer-modified ZnO under visible light irradiation. J. Hazard. Mater. 2008, 156, 80–85. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Wu, D.; Sun, Y. Visible-light responsive dye-modified TiO2 photocatalyst. J. Solid State Chem. 2008, 181, 593–602. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Hou, B.; Wu, D.; Sun, Y. Synthesis of visible light-activated TiO2 photocatalyst via surface organic modification. J. Solid State Chem. 2007, 180, 1787–1791. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Sannino, D.; Pragliola, S.; Vaiano, V. Enhanced visible-light-driven photodegradation of Acid Orange 7 azo dye in aqueous solution using Fe-N co-doped TiO2. Arab. J. Chem. 2020, 13, 8347–8360. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Pinto da Silva, L.; Zegaoui, O.; Esteves da Silva, J.C.G. Synthesis of Fe- and Co-Doped TiO(2) with Improved Photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef]
- Sharotri, N.; Sud, D. Visible light responsive Mn-S-co-doped TiO2 photocatalyst—Synthesis, characterization and mechanistic aspect of photocatalytic degradation. Sep. Purif. Technol. 2017, 183, 382–391. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Munawar, T.; Mukhtar, F.; Naveed ur Rahman, M.; Riaz, M.; Iqbal, F. Enhancement in the photocatalytic and antimicrobial properties of ZnO nanoparticles by structural variations and energy bandgap tuning through Fe and Co co-doping. Ceram. Int. 2021, 47, 11109–11121. [Google Scholar] [CrossRef]
- Marcì, G.; Augugliaro, V.; López-Muñoz, M.J.; Martín, C.; Palmisano, L.; Rives, V.; Schiavello, M.; Tilley, R.J.D.; Venezia, A.M. Preparation characterization and photocatalytic activity of polycrystalline ZnO/TiO2 systems. 2. Surface, bulk characterization, and 4-nitrophenol photodegradation in liquid-solid regime. J. Phys. Chem. B 2001, 105, 1033–1040. [Google Scholar] [CrossRef]
- Yu, J.; Zhuang, S.; Xu, X.; Zhu, W.; Feng, B.; Hu, J. Photogenerated electron reservoir in hetero-p-n CuO-ZnO nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr(vi). J. Mater. Chem. A 2015, 3, 1199–1207. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alenezi, J.F.; Mohamed, I.M.A.; Yasin, A.S.; Hashem, A.F.M.; Abdal-hay, A. Synthesis of TiO2@ZnO heterojunction for dye photodegradation and wastewater treatment. J. Alloys Compd. 2021, 886, 161169. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef] [PubMed]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- El Koura, Z.; Cazzanelli, M.; Bazzanella, N.; Patel, N.; Fernandes, R.; Arnaoutakis, G.E.; Gakamsky, A.; Dick, A.; Quaranta, A.; Miotello, A. Synthesis and Characterization of Cu and N Codoped RF-Sputtered TiO2 Films: Photoluminescence Dynamics of Charge Carriers Relevant for Water Splitting. J. Phys. Chem. C 2016, 120, 12042–12050. [Google Scholar] [CrossRef]
- Shifu, C.; Lei, C.; Shen, G.; Gengyu, C. The preparation of coupled SnO2/TiO2 photocatalyst by ball milling. Mater. Chem. Phys. 2006, 98, 116–120. [Google Scholar] [CrossRef]
- Chen, C.; Mei, W.; Wang, C.; Yang, Z.; Chen, X.; Chen, X.; Liu, T. Synthesis of a flower-like SnO/ZnO nanostructure with high catalytic activity and stability under natural sunlight. J. Alloys Compd. 2020, 826, 154122. [Google Scholar] [CrossRef]
- Sakthivel, S.; Geissen, S.U.; Bahnemann, D.W.; Murugesan, V.; Vogelpohl, A. Enhancement of photocatalytic activity by semiconductor heterojunctions: α-Fe2O3, WO3 and CdS deposited on ZnO. J. Photochem. Photobiol. A 2002, 148, 283–293. [Google Scholar] [CrossRef]
- Sapkota, B.B.; Mishra, S.R. A simple ball milling method for the preparation of p-CuO/n-ZnO nanocomposite photocatalysts with high photocatalytic activity. J. Nanosci. Nanotechnol. 2013, 13, 6588–6596. [Google Scholar] [CrossRef]
- Mottola, S.; Mancuso, A.; Sacco, O.; Vaiano, V.; De Marco, I. Photocatalytic Systems Based on ZnO Produced by Supercritical Antisolvent for Ceftriaxone Degradation. Catalysts 2023, 13, 1173. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Mottola, S.; Pragliola, S.; Moretta, A.; Vaiano, V.; De Marco, I. Synthesis of Fe-doped ZnO by supercritical antisolvent precipitation for the degradation of azo dyes under visible light. Inorg. Chim. Acta 2023, 549, 121407. [Google Scholar] [CrossRef]
- Franco, P.; Navarra, W.; Sacco, O.; De Marco, I.; Mancuso, A.; Vaiano, V.; Venditto, V. Photocatalytic degradation of atrazine under visible light using Gd-doped ZnO prepared by supercritical antisolvent precipitation route. Catal. Today 2022, 397–399, 240–248. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Sannino, D.; Vaiano, V. Photocatalytic Degradation of Eriochrome Black-T Azo Dye Using Eu-Doped ZnO Prepared by Supercritical Antisolvent Precipitation Route: A Preliminary Investigation. Top. Catal. 2020, 63, 1193–1205. [Google Scholar] [CrossRef]
- Navarra, W.; Ritacco, I.; Sacco, O.; Caporaso, L.; Farnesi Camellone, M.; Venditto, V.; Vaiano, V. Density Functional Theory Study and Photocatalytic Activity of ZnO/N-Doped TiO2Heterojunctions. J. Phys. Chem. C 2022, 126, 7000–7011. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Shoneye, A.; Tang, J. Highly dispersed FeOOH to enhance photocatalytic activity of TiO2 for complete mineralisation of herbicides. Appl. Surf. Sci. 2020, 511, 145479. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A highly efficient TiO2@ZnO n-p-n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Radingoana, P.M.; Guillemet-Fritsch, S.; Olubambi, P.A.; Chevallier, G.; Estournès, C. Influence of processing parameters on the densification and the microstructure of pure zinc oxide ceramics prepared by spark plasma sintering. Ceram. Int. 2019, 45, 10035–10043. [Google Scholar] [CrossRef]
- Arora, A.K.; Devi, S.; Jaswal, V.S.; Singh, J.; Kinger, M.; Gupta, V.D. Synthesis and characterization of ZnO nanoparticles. Orient. J. Chem. 2014, 30, 1671–1679. [Google Scholar] [CrossRef]
- Vlazan, P.; Ursu, D.H.; Irina-Moisescu, C.; Miron, I.; Sfirloaga, P.; Rusu, E. Structural and electrical properties of TiO2/ZnO core–shell nanoparticles synthesized by hydrothermal method. Mater. Charact. 2015, 101, 153–158. [Google Scholar] [CrossRef]
- Sacco, O.; Mancuso, A.; Venditto, V.; Pragliola, S.; Vaiano, V. Behavior of N-Doped TiO2 and N-Doped ZnO in Photocatalytic Azo Dye Degradation under UV and Visible Light Irradiation: A Preliminary Investigation. Catalysts 2022, 12, 1208. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, B.P.; Dhar, S.; Gondorf, A.; Spasova, M. Effect of surface groups on the luminescence property of ZnO nanoparticles synthesized by sol-gel route. Surf. Sci. 2012, 606, L13–L17. [Google Scholar] [CrossRef]
- Šćepanović, M.; Grujić-Brojčin, M.; Vojisavljević, K.; Bernikc, S.; Srećković, T. Raman study of structural disorder in ZnO nanopowders. J. Raman Spectrosc. 2010, 41, 914–921. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Z.; Zhang, M. Investigation of structural transformations in nanophase titanium dioxide by Raman spectroscopy. Appl. Phys. A Mater. Sci. Process. 1998, 66, 621–627. [Google Scholar] [CrossRef]
- Gajovi, A.; Stubičar, M.; Ivanda, M.; Furi, K. Raman spectroscopy of ball-milled TiO2. J. Mol. Struct. 2001, 563–564, 315–320. [Google Scholar] [CrossRef]
- Bazzanella, N.; Bajpai, O.P.; Fendrich, M.; Guella, G.; Miotello, A.; Orlandi, M. Ciprofloxacin degradation with a defective TiO2-x nanomaterial under sunlight. MRS Commun. 2023, 13, 1252–1259. [Google Scholar] [CrossRef]
- Shokri, M.; Isapour, G.; Shamsvand, S.; Kavousi, B. Photocatalytic degradation of ceftriaxone in aqueous solutions by immobilized TiO2 and ZnO nanoparticles: Investigating operational parameters. J. Mater. Environ. Sci. 2016, 7, 2843–2851. [Google Scholar]
- Sun, X.; Pan, Y.; Song, Y.; Liu, W.; Nghiem, L.D.; Wang, Q.; Cai, Z. Ceftriaxone sodium degradation by carbon quantum dots (CQDs)-decorated C-doped α-Bi2O3 nanorods. Environ. Sci. Ecotechnol. 2023, 13, 100219. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Synthesis of Ag3PO4/G-C3N4 composite with enhanced photocatalytic performance for the photodegradation of diclofenac under visible light irradiation. Catalysts 2018, 8, 45. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Fabrication of novel visible-light-driven AgI/g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Colloid Interface Sci. 2017, 496, 167–176. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, X.; Zhou, W.; Wu, J.; Liu, Q. First-principle study on anatase TiO2 codoped with nitrogen and ytterbium. J. Semicond. 2010, 31, 032001. [Google Scholar] [CrossRef]
- Sacco, O.; Murcia, J.J.; Lara, A.E.; Hernández-Laverde, M.; Rojas, H.; Navío, J.A.; Hidalgo, M.C.; Vaiano, V. Pt–TiO2–Nb2O5 heterojunction as effective photocatalyst for the degradation of diclofenac and ketoprofen. Mat. Sci. Semicon. Proc. 2020, 107, 104839. [Google Scholar] [CrossRef]
- Martha, S.; Reddy, K.H.; Parida, K.M. Fabrication of In2O3 modified zno for enhancing stability, optical behaviour, electronic properties and photocatalytic activity for hydrogen production under visible light. J. Mater. Chem. A 2014, 2, 3621–3631. [Google Scholar] [CrossRef]
- Singha, B.; Ray, K. Density functional theory insights on photocatalytic ability of CuO/TiO2 and CuO/ZnO. Mater. Today Proc. 2023, 72, 451–458. [Google Scholar] [CrossRef]
- Haffad, S.; Korir Kiprono, K. Interfacial structure and electronic properties of TiO2/ZnO/TiO2 for photocatalytic and photovoltaic applications: A theoretical study. Surf. Sci. 2019, 686, 10–16. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wei, X.; Liu, G.; Li, J. ZnO nanoparticles implanted in TiO2 macrochannels as an effective direct Z-scheme heterojunction photocatalyst for degradation of RhB. Appl. Surf. Sci. 2018, 456, 666–675. [Google Scholar] [CrossRef]

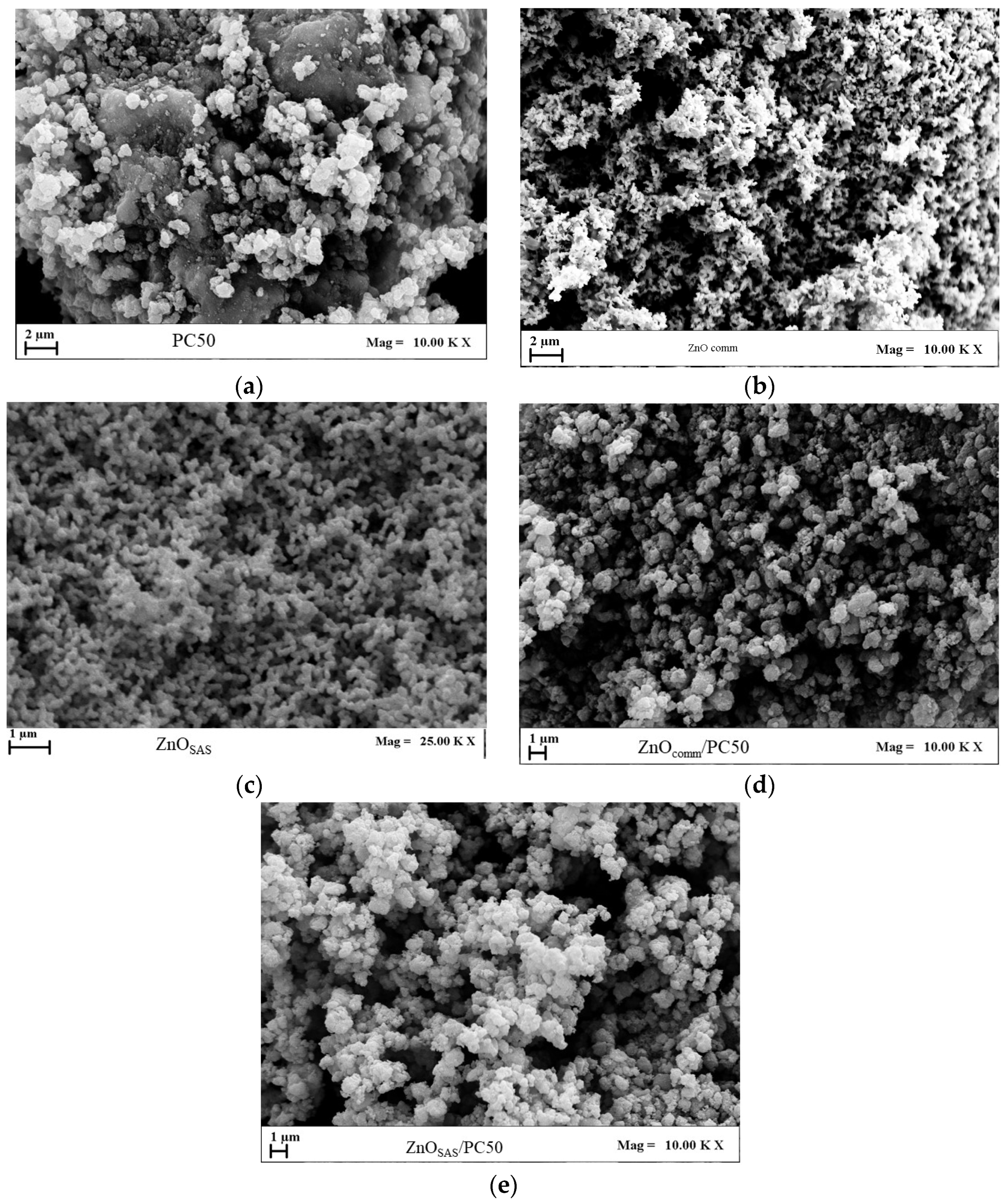
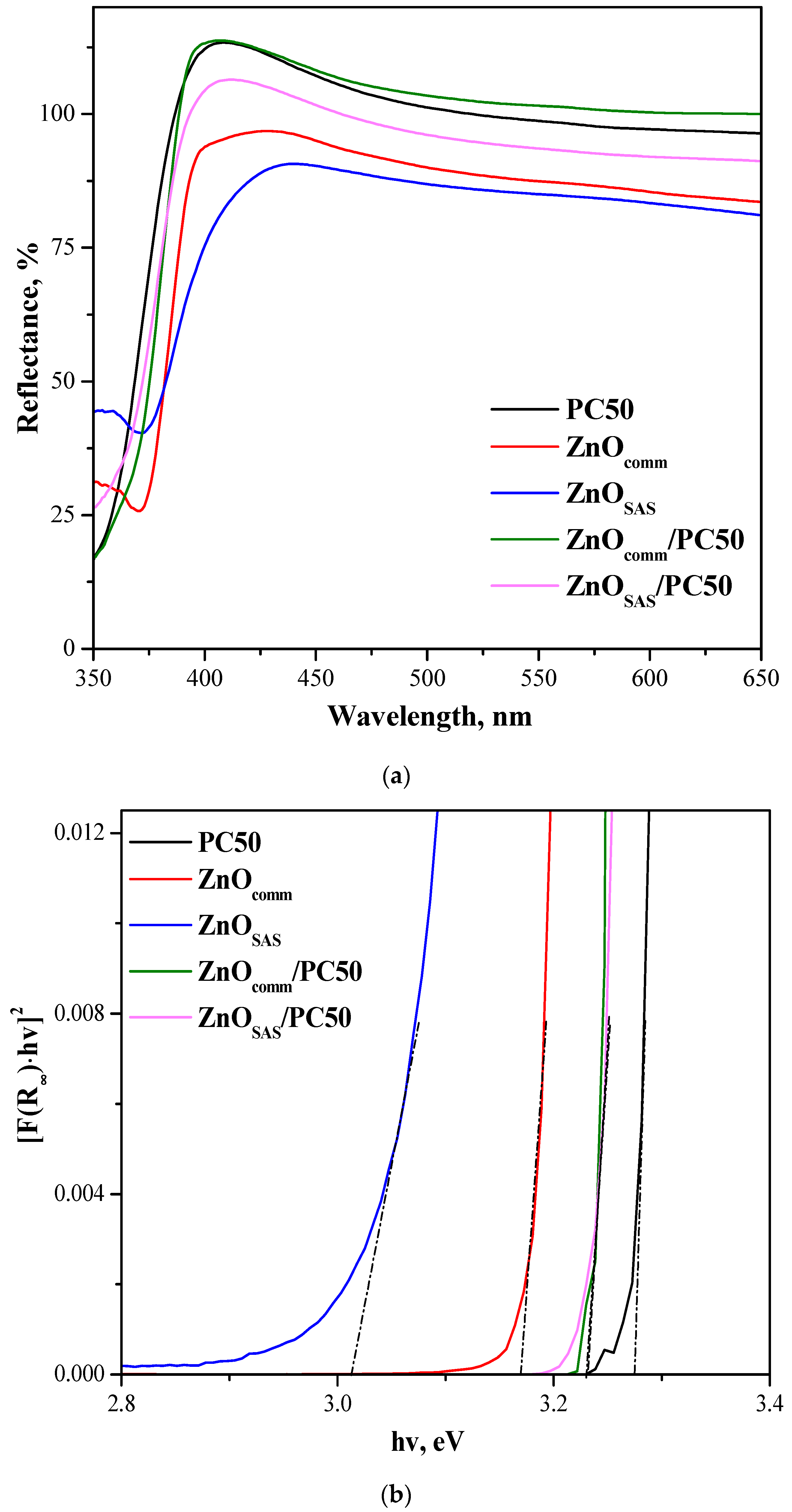
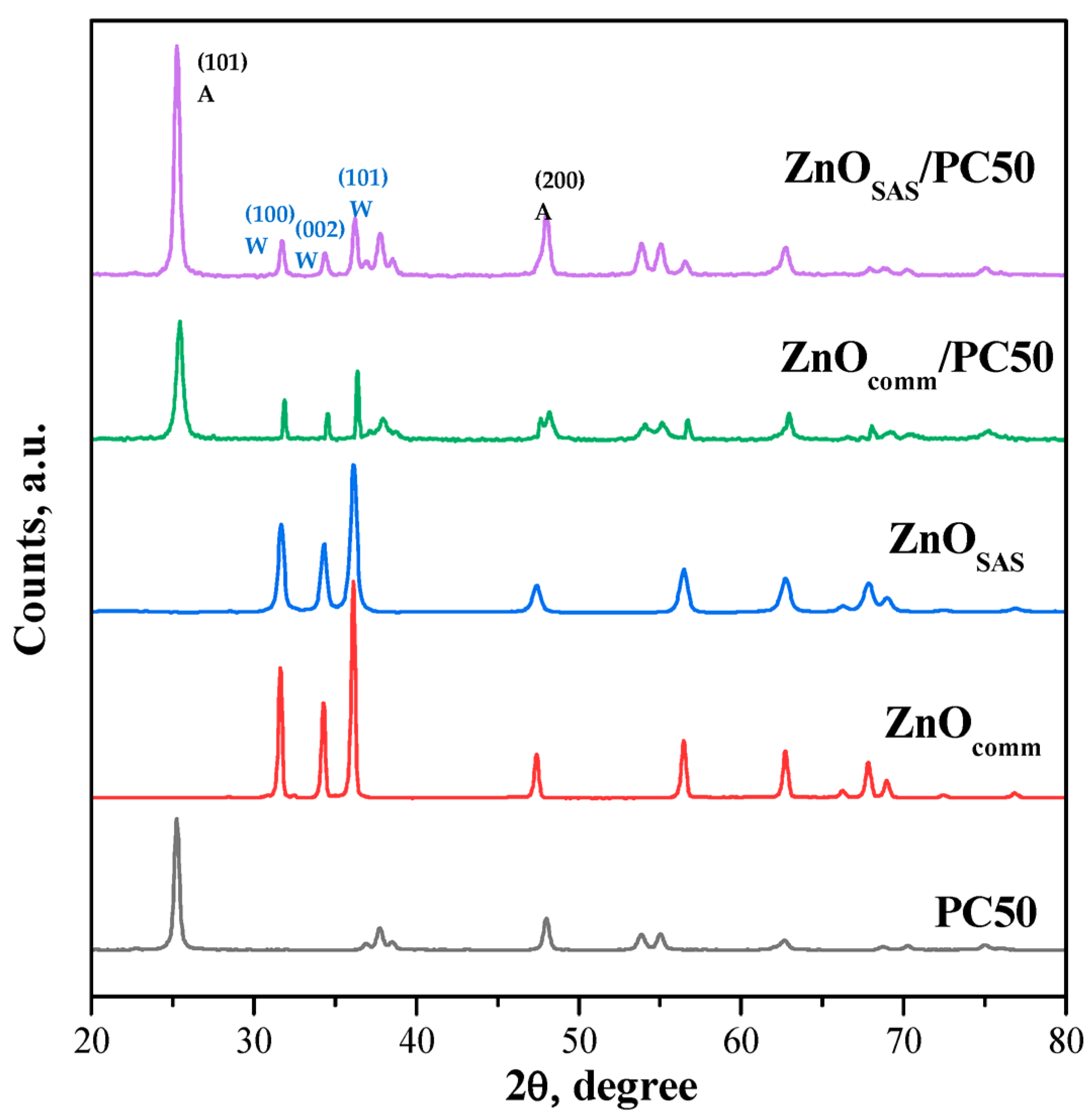
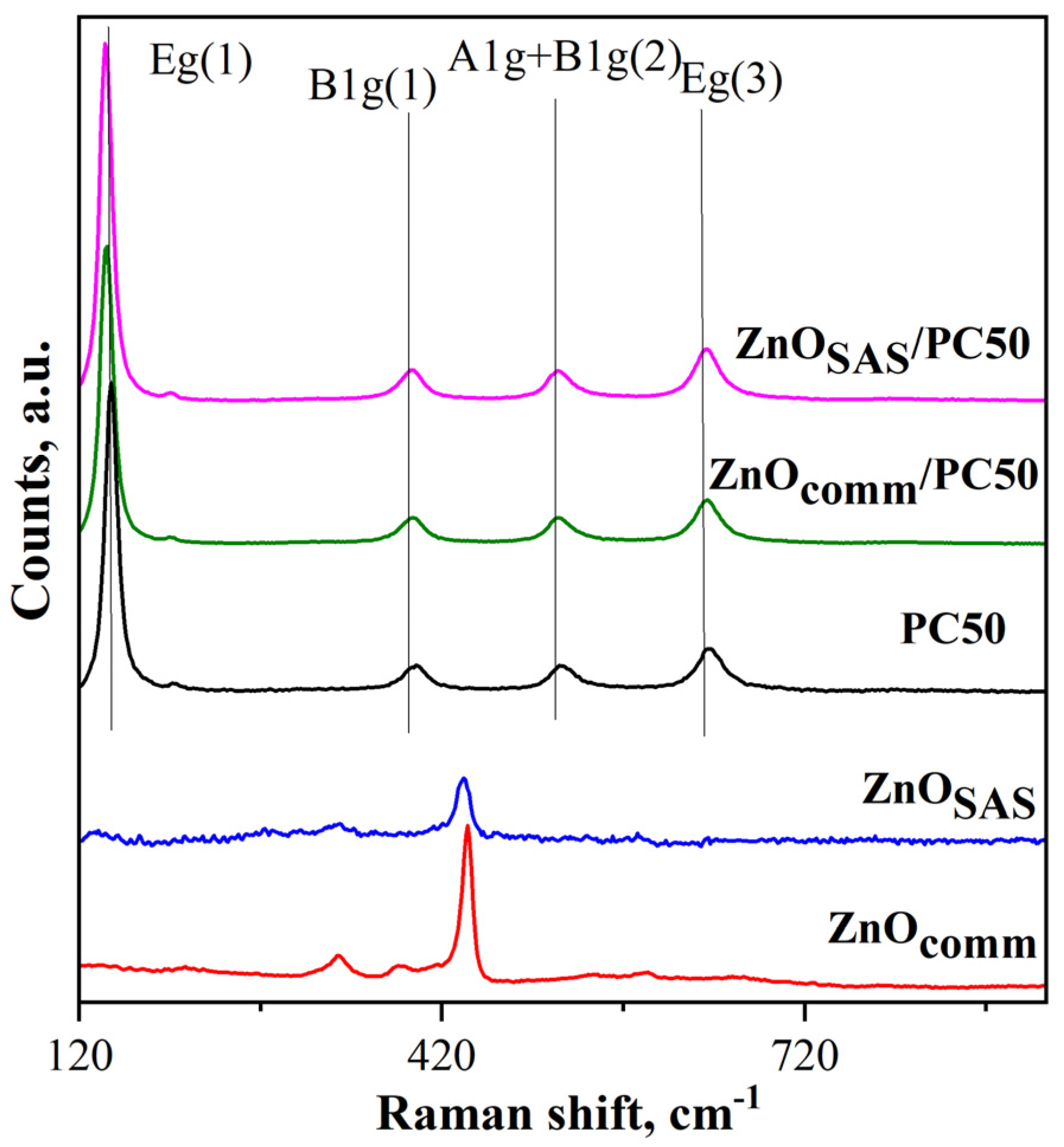



| Sample | Morphology | md ± sd [μm] | D [nm] Anatase Wurtzite | SSA [m2g−1] | Eg [eV] |
|---|---|---|---|---|---|
| PC50 | C | - | 26 | 44 | 3.27 |
| ZnOcomm | C | - | 24 | 6 | 3.17 |
| ZnOSAS | NP | 0.08 ± 0.007 | 22 | 20 | 3.03 |
| ZnOcomm/PC50 | cMP | - | 25 25 | 40 | 3.22 |
| ZnOSAS/PC50 | cMP | - | 27 23 | 39 | 3.22 |
| Sample | kUV [min−1] * UV Light Tests | kVis [min−1] ** Visible-Light Tests |
|---|---|---|
| PC50 | 1.18 | - |
| ZnOcomm | 0.40 | - |
| ZnOSAS | 0.32 | - |
| ZnOcomm/PC50 | 1.43 | 0.0085 |
| ZnOSAS/PC50 | 2.00 | 0.0131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, A.; Mottola, S.; Sacco, O.; Vaiano, V.; De Marco, I. Photocatalytic Degradation of Ceftriaxone Using TiO2 Coupled with ZnO Micronized by Supercritical Antisolvent Route. Nanomaterials 2023, 13, 3130. https://doi.org/10.3390/nano13243130
Mancuso A, Mottola S, Sacco O, Vaiano V, De Marco I. Photocatalytic Degradation of Ceftriaxone Using TiO2 Coupled with ZnO Micronized by Supercritical Antisolvent Route. Nanomaterials. 2023; 13(24):3130. https://doi.org/10.3390/nano13243130
Chicago/Turabian StyleMancuso, Antonietta, Stefania Mottola, Olga Sacco, Vincenzo Vaiano, and Iolanda De Marco. 2023. "Photocatalytic Degradation of Ceftriaxone Using TiO2 Coupled with ZnO Micronized by Supercritical Antisolvent Route" Nanomaterials 13, no. 24: 3130. https://doi.org/10.3390/nano13243130






