Preparation of MnO2-Carbon Materials and Their Applications in Photocatalytic Water Treatment
Abstract
:1. Introduction
2. Preparation Methods of MnO2-Carbon Composites
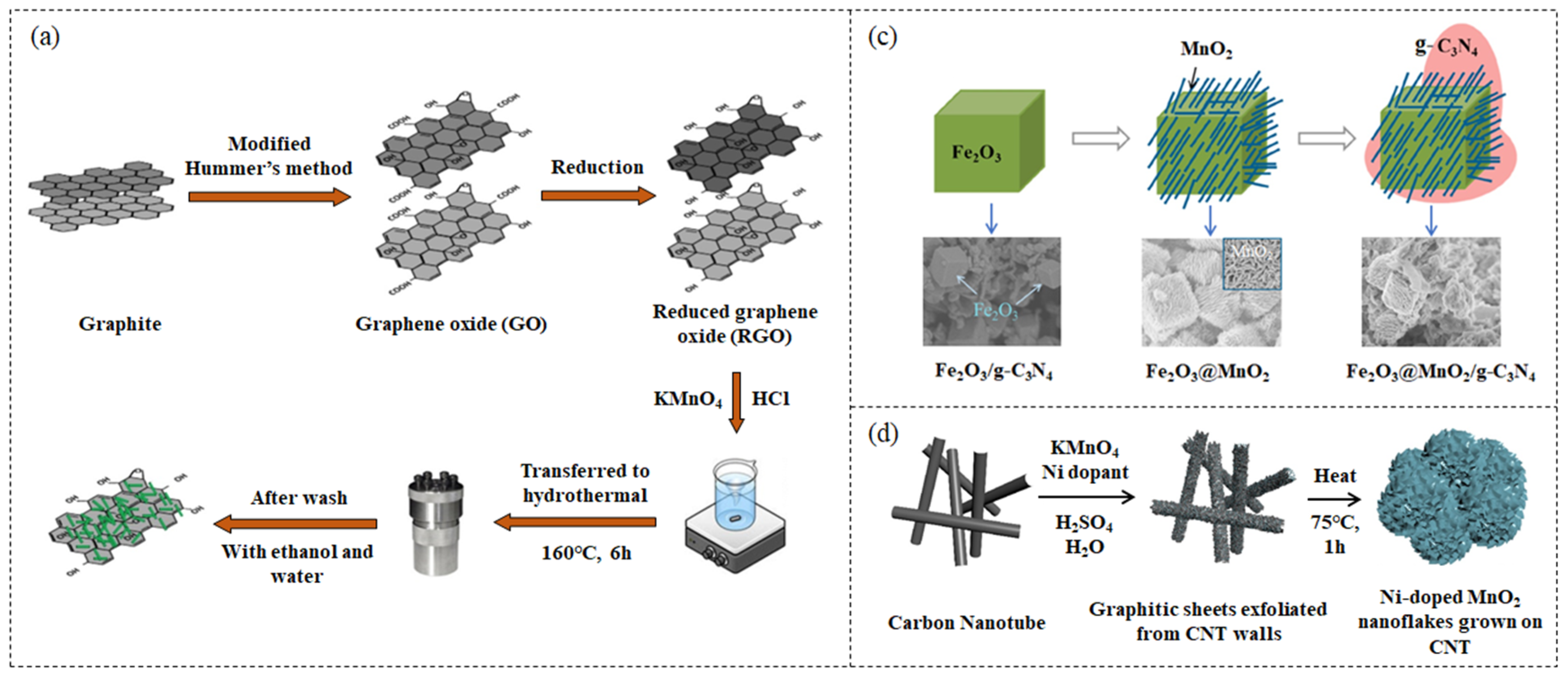
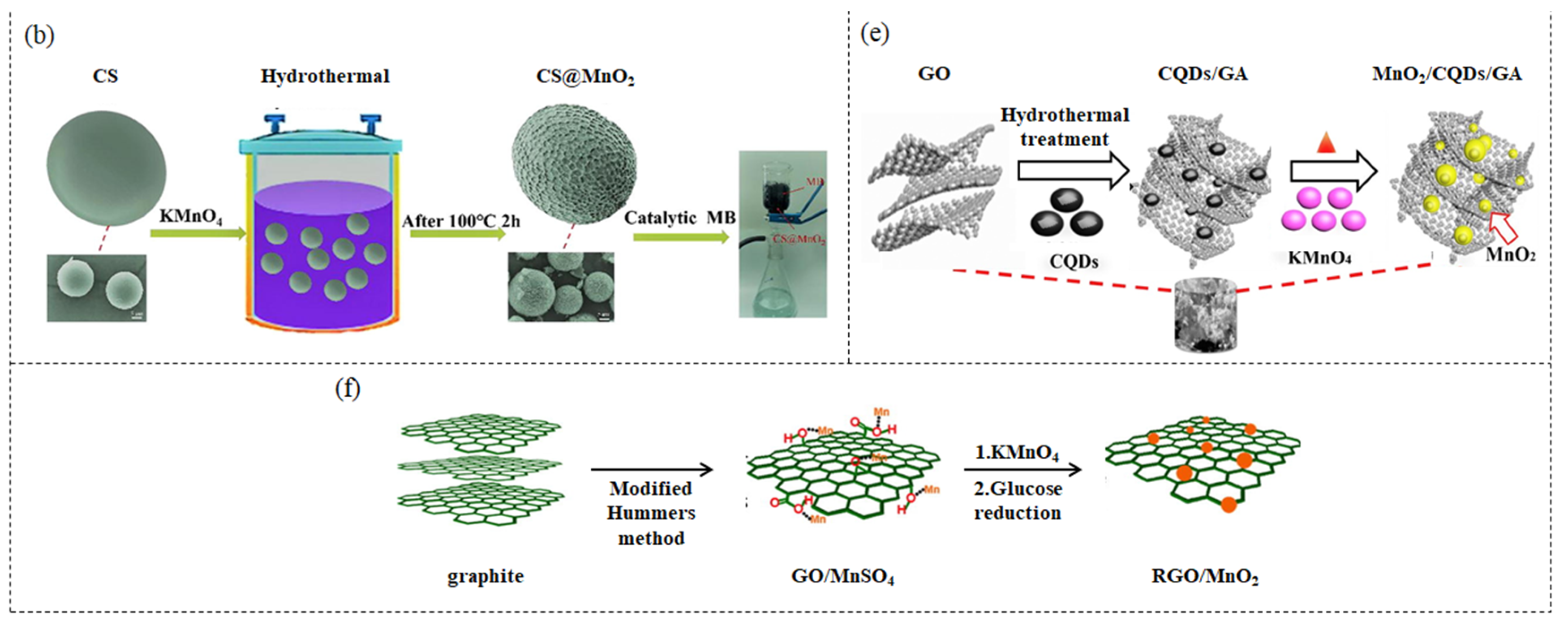
2.1. Hydrothermal Method
2.2. In Situ Redox Deposition
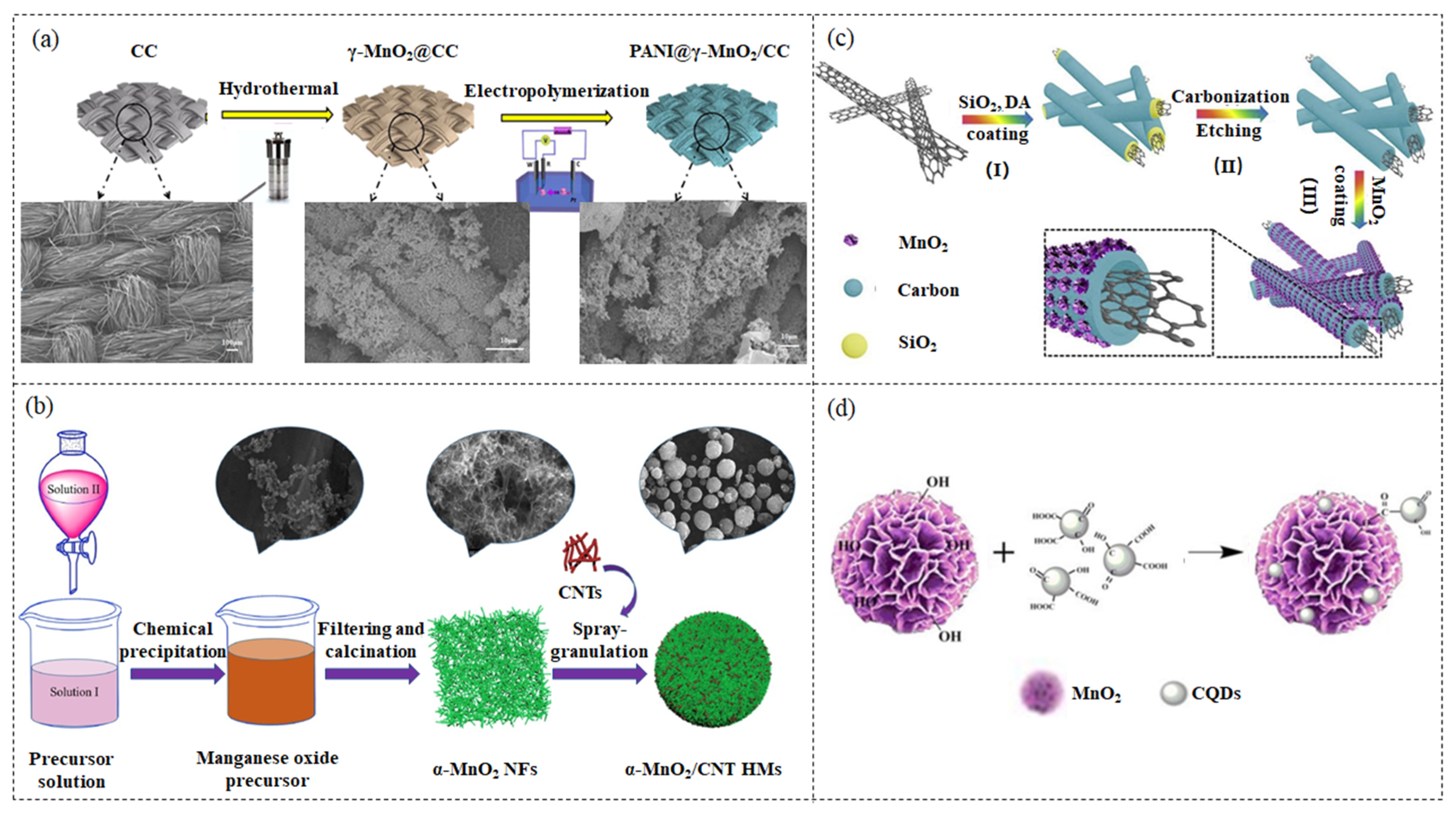
2.3. Electrochemical Deposition
2.4. Co-Precipitating Method
2.5. Template Method
2.6. Ultrasonic-Assisted and Sonochemical Methods
2.7. Other Methods
3. Applications of Photocatalytic Technology in Water Treatment
3.1. Phenolic Wastewater
3.2. Antibiotic Wastewater
3.3. Dye Wastewater
3.4. Heavy Metal Wastewater
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Dong, X.-X.; Lv, Y.-K. Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment. Chemosphere 2020, 242, 125144. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, H.; Han, L.; Mei, J.; Ge, Q.; Long, Z.; Yu, Y. Exploring bacterial communities and biodegradation genes in activated sludge from pesticide wastewater treatment plants via metagenomic analysis. Environ. Pollut. 2018, 243, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wang, J.; Li, J.; Wang, X.; Chen, Z.; Alsaedi, A.; Hayat, T.; Chen, Y.; Wang, X. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: A review. Environ. Sci. Pollut. Res. 2017, 24, 7938–7958. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Wang, J.; Yu, S.; Chen, Z.; Hayat, T.; Wang, X. Magnetic Porous Carbonaceous Material Produced from Tea Waste for Efficient Removal of As(V), Cr(VI), Humic Acid, and Dyes. ACS Sustain. Chem. Eng. 2017, 5, 4371–4380. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef]
- Guo, N.; Zeng, Y.; Li, H.; Xu, X.; Yu, H.; Han, X. Novel mesoporous TiO2@g-C3N4 hollow core@shell heterojunction with enhanced photocatalytic activity for water treatment and H-2 production under simulated sunlight. J. Hazard. Mater. 2018, 353, 80–88. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of Titanium Dioxide (TiO2) Modification on Its Application to Pollution Treatment—A Review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Saha, D.; Hoinkis, T.J.; Van Bramer, S.E. Electrospun, flexible and reusable nanofiber mat of graphitic carbon nitride: Photocatalytic reduction of hexavalent chromium. J. Colloid Interface Sci. 2020, 575, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.-L.; Lin, L.-Y. Applied potential-dependent performance of the nickel cobalt oxysulfide nanotube/nickel molybdenum oxide nanosheet core-shell structure in energy storage and oxygen evolution. J. Mater. Chem. A 2019, 7, 4626–4639. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Chu, Y.-C.; Hsu, C.-S.; Wang, J.; Tung, C.-W.; Chen, H.M. Hetero-Atomic Pairs with a Distal Fe3+-Site Boost Water Oxidation. Angew. Chem.-Int. Ed. 2022, 61, e202211142. [Google Scholar] [CrossRef] [PubMed]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbare, S.; Hsiao, Y.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Photosynthetic Biohybrid Systems. ACS Mater. Lett. 2022, 5, 95–115. [Google Scholar] [CrossRef]
- Das, S.; Sarnanta, A.; Jana, S. Light-Assisted Synthesis of Hierarchical Flower-Like MnO2 Nanocomposites with Solar Light Induced Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2017, 5, 9086–9094. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Wanag, A.; Kusiak-Nejman, E.; Ekiert, E.; Rokicka-Konieczna, P.; Morawski, A.W. Effect of calcination on the photocatalytic activity and stability of TiO2 photocatalysts modified with APTES. J. Environ. Chem. Eng. 2021, 9, 104794. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B-Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Babar, S.; Gayade, N.; Shinde, H.; Mahajan, P.; Lee, K.H.; Mane, N.; Deshmukh, A.; Garadkar, K.; Bhuse, V. Evolution of Waste Iron Rust into Magnetically Separable g-C3N4-Fe2O3 Photocatalyst: An Efficient and Economical Waste Management Approach. ACS Appl. Nano Mater. 2018, 1, 4682–4694. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Li, R.; Chen, P.; Chen, D.; Cai, M.; Liu, H.; Feng, Y.; Lv, W.; Liu, G. Synthesis of a carbon dots modified g-C3N4/SnO2 Z -scheme photocatalyst with superior photocatalytic activity for PPCPs degradation under visible light irradiation. J. Hazard. Mater. 2021, 401, 123257. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Han, T.; Xie, C.M.; Meng, Y.J.; Wei, Y. Synthesized MnO2/Ag/g-C3N4 composite for photoreduction carbon dioxide under visible light. J. Mater. Sci.-Mater. Electron. 2018, 29, 20984–20990. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Sakai, N.; Ebina, Y.; Takada, K.; Sasaki, T. Photocurrent generation from semiconducting manganese oxide nanosheets in response to visible light. J. Phys. Chem. B 2005, 109, 9651–9655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, G.; Zhang, Q. MnO2/CeO2 for catalytic ultrasonic degradation of methyl orange. Ultrason. Sonochem. 2014, 21, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lian, J.; Wu, L.; Duan, Z.; Jiang, J.; Zhao, L. Synthesis of a Thin-Layer MnO2 Nanosheet-Coated Fe3O4 Nanocomposite as a Magnetically Separable Photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Yuan, B.; Fu, M.-L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere 2020, 239, 124745. [Google Scholar] [CrossRef]
- Cao, H.; Suib, S.L. Highly efficient heterogeneous photooxidation of 2-propanol to acetone with amorphous manganese oxide catalysts. J. Am. Chem. Soc. 1994, 116, 5334–5342. [Google Scholar] [CrossRef]
- Hang, Y.; Zhang, C.; Luo, X.; Xie, Y.; Xin, S.; Li, Y.; Zhang, D.; Goodenough, J.B. alpha-MnO2 nanorods supported on porous graphitic carbon nitride as efficient electrocatalysts for lithium-air batteries. J. Power Sources 2018, 392, 15–22. [Google Scholar] [CrossRef]
- Salari, H. Efficient photocatalytic degradation of environmental pollutant with enhanced photocarrier separation in novel Z-scheme a-MnO(2)nanorod/a-MoO3 nanocomposites. J. Photochem. Photobiol. A-Chem. 2020, 401, 112787. [Google Scholar] [CrossRef]
- Singh, M.; Thanh, D.N.; Ulbrich, P.; Strnadova, N.; Stepanek, F. Synthesis, characterization and study of arsenate adsorption from aqueous solution by alpha- and delta-phase manganese dioxide nanoadsorbents. J. Solid State Chem. 2010, 183, 2979–2986. [Google Scholar] [CrossRef]
- Samanta, A.; Pal, S.K.; Jana, S. Exploring flowery MnO2/Ag nanocomposite as an efficient solar-light-driven photocatalyst. New J. Chem. 2022, 46, 4189–4197. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.a.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of alpha-, beta-, gamma- and delta-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B-Environ. 2020, 260, 118150. [Google Scholar] [CrossRef]
- Baral, A.; Satish, L.; Zhang, G.; Ju, S.; Ghosh, M.K. A Review of Recent Progress on Nano MnO2: Synthesis, Surface Modification and Applications. J. Inorg. Organomet. Polym. Mater. 2021, 31, 899–922. [Google Scholar] [CrossRef]
- Chiam, S.-L.; Pung, S.-Y.; Yeoh, F.-Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778. [Google Scholar] [CrossRef] [PubMed]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.M.; Tade, M.O.; Wang, S. Different Crystallographic One-dimensional MnO2 Nanomaterials and Their Superior Performance in Catalytic Phenol Degradation. Environ. Sci. Technol. 2013, 47, 5882–5887. [Google Scholar] [CrossRef]
- Ma, L.; Li, D.; Wang, L.; Ma, X. In situ hydrothermal synthesis of alpha-MnO2 nanowire/activated carbon hollow fibers from cotton stalk composite: Dual-effect cyclic visible light photocatalysis performance. Cellulose 2020, 27, 8937–8948. [Google Scholar] [CrossRef]
- Baral, A.; Das, D.P.; Minakshi, M.; Ghosh, M.K.; Padhi, D.K. Probing Environmental Remediation of RhB Organic Dye Using alpha-MnO2 under Visible- Light Irradiation: Structural, Photocatalytic and Mineralization Studies. Chemistryselect 2016, 1, 4277–4285. [Google Scholar] [CrossRef]
- Mishra, B.P.; Acharya, L.; Subudhi, S.; Parida, K. Oxygen vacancy rich a-MnO2 @B/O-g-C3N4 photocatalyst: A thriving 1D-2D surface interaction effective towards photocatalytic O(2) and H-2 evolution through Z-scheme charge dynamics. Int. J. Hydrog. Energy 2022, 47, 32107–32120. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, S.; Vijayarengan, P.; Kalirajan, K.M.; Santhakumar, T.; Sekar, S.; Sadhasivam, S. Upcycling of Wastewater via Effective Photocatalytic Hydrogen Production Using MnO2 Nanoparticles-Decorated Activated Carbon Nanoflakes. Nanomaterials 2020, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, J.; Guo, Y.; Qu, Z.; Liao, Y.; Xie, J.; Shangguan, W.; Yan, N. Design of 3D MnO2/Carbon sphere composite for the catalytic oxidation and adsorption of elemental mercury. J. Hazard. Mater. 2018, 342, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, T.; Kumar, A.; Bahuguna, A.; Krishnan, V. Reduced graphene oxide supported MnO2 nanorods as recyclable and efficient adsorptive photocatalysts for pollutants removal. Vacuum 2019, 160, 333–346. [Google Scholar] [CrossRef]
- Ong, W.-J.; Putri, L.K.; Mohamed, A.R. Rational Design of Carbon-Based 2D Nanostructures for Enhanced Photocatalytic CO(2)Reduction: A Dimensionality Perspective. Chem.-A Eur. J. 2020, 26, 9710–9748. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.-J.; Liao, F.; Ke, Q.-F.; Guo, Y.-J.; Guo, Y.-P. Synergetic effect of titanium dioxide ultralong nanofibers and activated carbon fibers on adsorption and photodegradation of toluene. Chem. Eng. J. 2017, 328, 962–976. [Google Scholar] [CrossRef]
- Teng, F.; Zhang, G.; Wang, Y.; Gao, C.; Chen, L.; Zhang, P.; Zhang, Z.; Xie, E. The role of carbon in the photocatalytic reaction of carbon/TiO2 photocatalysts. Appl. Surf. Sci. 2014, 320, 703–709. [Google Scholar] [CrossRef]
- Cui, G.-W.; Wang, W.-L.; Ma, M.-Y.; Zhang, M.; Xia, X.-Y.; Han, F.-Y.; Shi, X.-F.; Zhao, Y.-Q.; Dong, Y.-B.; Tang, B. Rational design of carbon and TiO2 assembly materials: Covered or strewn, which is better for photocatalysis? Chem. Commun. 2013, 49, 6415–6417. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Chandrasekaran, S.; Salla, S.; Elakkiya, V.; Senthil, R.A.; Nithyadharseni, P.; Maiyalagan, T.; Micheal, K.; Ayeshamariam, A.; Arasu, M.V.; et al. Recent developments of metal oxide based heterostructures for photocatalytic applications towards environmental remediation. J. Solid State Chem. 2018, 267, 35–52. [Google Scholar] [CrossRef]
- Park, S.K.; Suh, D.H.; Park, H.S. Electrochemical assembly of reduced graphene oxide/manganese dioxide nanocomposites into hierarchical sea urchin-like structures for supercapacitive electrodes. J. Alloys Compd. 2016, 668, 146–151. [Google Scholar] [CrossRef]
- Guan, S.; Li, W.; Ma, J.; Lei, Y.; Zhu, Y.; Huang, Q.; Dou, X. A review of the preparation and applications of MnO2 composites in formaldehyde oxidation. J. Ind. Eng. Chem. 2018, 66, 126–140. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Zeng, G.; Zhou, C.; Xue, W.; Gong, X.; Yan, X.; Chen, S.; Wang, W.; Cheng, M. Megamerger in photocatalytic field: 2D g-C3N4 nanosheets serve as support of 0D nanomaterials for improving photocatalytic performance. Appl. Catal. B-Environ. 2019, 240, 153–173. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A-Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Fernando, K.A.S.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.-P. Carbon Quantum Dots and Applications in Photocatalytic Energy Conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef]
- Hung, M.-C.; Yuan, S.-Y.; Hung, C.-C.; Cheng, C.-L.; Ho, H.-C.; Ko, T.-H. Effectiveness of ZnO/carbon-based material as a catalyst for photodegradation of acrolein. Carbon 2014, 66, 93–104. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Tang, K.; Hong, T.Z.X.; You, L.; Zhou, K. Carbon-metal compound composite electrodes for capacitive deionization: Synthesis, development and applications. J. Mater. Chem. A 2019, 7, 26693–26743. [Google Scholar] [CrossRef]
- Zhu, S.; Huo, W.; Liu, X.; Zhang, Y. Birnessite based nanostructures for supercapacitors: Challenges, strategies and prospects. Nanoscale Adv. 2020, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Wang, G.; Cheng, B.; Zhou, M. Effects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders. Appl. Catal. B-Environ. 2007, 69, 171–180. [Google Scholar] [CrossRef]
- Pang, H.; Wu, Y.; Wang, X.; Hu, B.; Wang, X. Recent Advances in Composites of Graphene and Layered Double Hydroxides for Water Remediation: A Review. Chem.-Asian J. 2019, 14, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wu, H. Graphene-based nanocomposites: Preparation, functionalization, and energy and environmental applications. Energy Environ. Sci. 2013, 6, 3483–3507. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. CdS/Graphene Nanocomposite Photocatalysts. Adv. Energy Mater. 2015, 5, 1500010. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Y.-L.; Li, C.-E. The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite. Sci. Total Environ. 2019, 651, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Lian, J.; Zhu, X.; Yan, P.; Lei, Y.; Yuan, S.; et al. Construction of MnO2/Monolayer g-C3N4 with Mn vacancies for Z-scheme overall water splitting. Appl. Catal. B-Environ. 2019, 241, 452–460. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Li, Y.; Liu, G.; Jin, R.; Wang, Q.; Xu, H.; Gao, S. 2D/1D protonated g-C3N4/alpha-MnO2 Z-scheme heterojunction with enhanced visible-light photocatalytic efficiency. Ceram. Int. 2020, 46, 25905–25914. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, S.; Li, L.; Qian, K.; Sun, J.; Guo, W.; Cai, X.; Yu, K. Vertically aligned MnO2 nanostructures on carbon fibers with tunable electromagnetic wave absorption performance. Appl. Surf. Sci. 2022, 589, 152858. [Google Scholar] [CrossRef]
- Lv, H.; Gao, X.; Xu, Q.; Liu, H.; Wang, Y.-G.; Xia, Y. Carbon Quantum Dot-Induced MnO2 Nanowire Formation and Construction of a Binder-Free Flexible Membrane with Excellent Superhydrophilicity and Enhanced Supercapacitor Performance. ACS Appl. Mater. Interfaces 2017, 9, 40394–40403. [Google Scholar] [CrossRef]
- Wu, M.; Kwok, Y.H.; Zhang, Y.; Szeto, W.; Huang, H.; Leung, D.Y.C. Synergetic effect of vacuum ultraviolet photolysis and ozone catalytic oxidation for toluene degradation over MnO2-rGO composite catalyst. Chem. Eng. Sci. 2021, 231, 116288c. [Google Scholar] [CrossRef]
- Hao, L.; Li, S.-S.; Wang, J.; Tan, Y.; Bai, L.; Liu, A. MnO2/multi-walled carbon nanotubes based nanocomposite with enhanced electrocatalytic activity for sensitive amperometric glucose biosensing. J. Electroanal. Chem. 2020, 878, 114602. [Google Scholar] [CrossRef]
- Yu, L.; Mo, Z.; Zhu, X.; Deng, J.; Xu, F.; Song, Y.; She, Y.; Li, H.; Xu, H. Construction of 2D/2D Z-scheme MnO2-x/g-C3N4 photocatalyst for efficient nitrogen fixation to ammonia. Green Energy Environ. 2021, 6, 538–545. [Google Scholar] [CrossRef]
- Shi, J.; Wang, S.; Wang, Q.; Chen, X.; Du, X.; Wang, M.; Zhao, Y.; Dong, C.; Ruan, L.; Zeng, W. A new flexible zinc-ion capacitor based on delta-MnO2@Carbon cloth battery-type cathode and MXene@Cotton cloth capacitor-type anode. J. Power Sources 2020, 446, 227345. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, S.; Cui, W.; Lv, J.; Geng, Y.; Li, H.; Deng, J. Interconnected network of ultrafine MnO2 nanowires on carbon cloth with weed-like morphology for high-performance supercapacitor electrodes. Electrochim. Acta 2018, 268, 340–346. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Zhang, L.; Guo, L.; Dong, X. Photothermal conversion of graphene/layered manganese oxide 2D/2D composites for room-temperature catalytic purification of gaseous formaldehyde. J. Taiwan Inst. Chem. Eng. 2020, 107, 119–128. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Liang, X.; Zhang, D.; Miao, M. Flexible supercapacitors based on carbon nanotube-MnO2 nanocomposite film electrode. Chem. Eng. J. 2019, 371, 145–153. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Tashi, L.; Khajuria, H.; Sheikh, H.N. Hydrothermal synthesis of manganese oxide and nitrogen doped graphene (NG-MnO2) nanohybrid for visible light degradation of methyl orange dye. Mol. Phys. 2019, 117, 2477–2486. [Google Scholar] [CrossRef]
- Liu, W.-X.; Zhu, X.-L.; Li, S.-Q.; Gu, Q.-Q.; Meng, Z.-D. Near-Infrared-Driven Selective Photocatalytic Removal of Ammonia Based on Valence Band Recognition of an alpha-MnO2/N-Doped Graphene Hybrid Catalyst. ACS Omega 2018, 3, 5537–5546. [Google Scholar] [CrossRef]
- Dong, J.; Lu, G.; Wu, F.; Xu, C.; Kang, X.; Cheng, Z. Facile synthesis of a nitrogen-doped graphene flower-like MnO2 nanocomposite and its application in supercapacitors. Appl. Surf. Sci. 2018, 427, 986–993. [Google Scholar] [CrossRef]
- Poochai, C.; Sriprachuabwong, C.; Sodtipinta, J.; Lohitkarn, J.; Pasakon, P.; Primpray, V.; Maeboonruan, N.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. Alpha-MnO2 nanofibers/nitrogen and sulfur-co-doped reduced graphene oxide for 4.5 V quasi-solid state supercapacitors using ionic liquid-based polymer electrolyte. J. Colloid Interface Sci. 2021, 583, 734–745. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Hu, J.; Wei, L.; Liu, J.; Zheng, M. Facile synthesis of MnO2 grown on nitrogen-doped carbon nanotubes for asymmetric supercapacitors with enhanced electrochemical performance. J. Power Sources 2018, 393, 135–144. [Google Scholar] [CrossRef]
- Dewangan, L.; Korram, J.; Karbhal, I.; Nagwanshi, R.; Satnami, M.L. N-Doped Carbon Quantum Dot-MnO2 Nanowire FRET Pairs: Detection of Cholesterol, Glutathione, Acetylcholinesterase, and Chlorpyrifos. ACS Appl. Nano Mater. 2021, 4, 13612–13624. [Google Scholar] [CrossRef]
- Bano, D.; Chandra, S.; Yadav, P.K.; Singh, V.K.; Hasan, S.H. Off-on detection of glutathione based on the nitrogen, sulfur codoped carbon quantum dots@MnO2 nano-composite in human lung cancer cells and blood serum. J. Photochem. Photobiol. A-Chem. 2020, 398, 112558. [Google Scholar] [CrossRef]
- Li, J.; Luo, S.; Liu, G.; Wan, J.; Lu, J.; Li, B.; Han, X.; Hu, C. A high-performance asymmetric supercapacitor achieved by surface-regulated MnO2 and organic-framework-derived N-doped carbon cloth. Mater. Today Chem. 2021, 22, 100620. [Google Scholar] [CrossRef]
- Zhong, R.; Xu, M.; Fu, N.; Liu, R.; Zhou, A.A.; Wang, X.; Yang, Z. A flexible high-performance symmetric quasi-solid supercapacitor based on Ni-doped MnO2 nano-array @ carbon cloth. Electrochim. Acta 2020, 348, 136209. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Wang, Z.; Bai, W.; Cao, Y.; Wei, Y. Constructing hierarchical structure based on LDH anchored boron-doped g-C3N4 assembled with MnO2 nanosheets towards reducing toxicants generation and fire hazard of epoxy resin. Compos. Part B-Eng. 2022, 229, 109453. [Google Scholar] [CrossRef]
- Shan, Q.Y.; Guo, X.L.; Dong, F.; Zhang, Y.X. Single atom (K/Na) doped graphitic carbon Nitride@ MnO2 as an efficient electrode Material for supercapacitor. Mater. Lett. 2017, 202, 103–106. [Google Scholar] [CrossRef]
- Wan, H.; Ge, H.; Zhang, L.; Duan, T. CS@MnO2 core-shell nanospheres with enhanced visible light photocatalytic degradation. Mater. Lett. 2019, 237, 290–293. [Google Scholar] [CrossRef]
- Wang, N.; Wu, L.; Li, J.; Mo, J.; Peng, Q.; Li, X. Construction of hierarchical Fe2O3@MnO2 core/shell nanocube supported C3N4 for dual Z-scheme photocatalytic water splitting. Sol. Energy Mater. Sol. Cells 2020, 215, 110624. [Google Scholar] [CrossRef]
- Asif, M.; Rashad, M.; Ali, Z.; Qiu, H.; Li, W.; Pan, L.; Hou, Y. Ni-doped MnO2/CNT nanoarchitectures as a cathode material for ultra-long life magnesium/lithium hybrid ion batteries. Mater. Today Energy 2018, 10, 108–117. [Google Scholar] [CrossRef]
- Qu, J.; Shi, L.; He, C.; Gao, F.; Li, B.; Zhou, Q.; Hu, H.; Shao, G.; Wang, X.; Qiu, J. Highly efficient synthesis of graphene/MnO2 hybrids and their application for ultrafast oxidative decomposition of methylene blue. Carbon 2014, 66, 485–492. [Google Scholar] [CrossRef]
- Lv, H.; Yuan, Y.; Xu, Q.; Liu, H.; Wang, Y.-G.; Xia, Y. Carbon quantum dots anchoring MnO2/graphene aerogel exhibits excellent performance as electrode materials for supercapacitor. J. Power Sources 2018, 398, 167–174. [Google Scholar] [CrossRef]
- Tuan Sang, T.; Tripathi, K.M.; Kim, B.N.; You, I.-K.; Park, B.J.; Han, Y.H.; Kim, T. Three-dimensionally assembled Graphene/alpha-MnO2 nanowire hybrid hydrogels for high performance supercapacitors. Mater. Res. Bull. 2017, 96, 395–404. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, Q.; Zhang, J.; Yang, H.; Wang, B.; Yang, D.; Hu, J.; Liu, Z. Functionalization of Biomass Carbonaceous Aerogels: Selective Preparation of MnO2@CA Composites for Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9689–9697. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Shi, Y.; Zhang, Q.; Xu, X. Green synthesis of ultrafine Methyl-cellulose-derived porous carbon/MnO2 nanowires for asymmetric supercapacitors and flexible pattern stamping. Appl. Surf. Sci. 2018, 462, 923–931. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, C.; Liu, D.; Li, Y.; Zhou, H.; Kuang, Y. Three-Dimensional Pompon-like MnO2/Graphene Hydrogel Composite for Supercapacitor. Electrochim. Acta 2016, 210, 804–811. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Payami, E.; Piriniya, A.; Hadi, R. Green synthesis of ferrocenyl-modified MnO2/carbon-based nanocomposite as an outstanding supercapacitor electrode material. Appl. Organomet. Chem. 2022, 36, e6620. [Google Scholar] [CrossRef]
- Niu, Z.; Yue, T.; Hu, W.; Sun, W.; Hu, Y.; Xu, Z. Covalent bonding of MnO2 onto graphene aerogel forwards: Efficiently catalytic degradation of organic wastewater. Appl. Surf. Sci. 2019, 496, 143585. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, J.; Duan, X.; Tan, X.; Liu, S.; Wang, S. Self-assembly of 3D MnO2/N-doped graphene hybrid aerogel for catalytic degradation of water pollutants: Structure-dependent activity. Chem. Eng. J. 2019, 369, 1049–1058. [Google Scholar] [CrossRef]
- Wang, Z.; Han, Y.; Fan, W.; Wang, Y.; Huang, L. Shell-core MnO2/Carbon@Carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline. Sep. Purif. Technol. 2022, 278, 119558. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, Y.; Wu, F.; Fang, L.; Luo, H.; Zhang, Y.; Zhou, M. MnO2 nanorods/MXene/CC composite electrode for flexible supercapacitors with enhanced electrochemical performance. J. Alloys Compd. 2019, 802, 259–268. [Google Scholar] [CrossRef]
- Ghosh, K.; Yue, C.Y.; Sk, M.M.; Jena, R.K.; Bi, S. Development of a 3D graphene aerogel and 3D porous graphene/MnO2@polyaniline hybrid film for all-solid-state flexible asymmetric supercapacitors. Sustain. Energy Fuels 2018, 2, 280–293. [Google Scholar] [CrossRef]
- Iqbal, J.; Ansari, M.O.; Numan, A.; Wageh, S.; Al-Ghamdi, A.; Alam, M.G.; Kumar, P.; Jafer, R.; Bashir, S.; Rajpar, A.H. Hydrothermally Assisted Synthesis of Porous Polyaniline@Carbon Nanotubes-Manganese Dioxide Ternary Composite for Potential Application in Supercapattery. Polymers 2020, 12, 2918. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, S.; Cui, W.; Yu, D.; Deng, J. Ultrafine MnO2 nanowires grown on RGO-coated carbon cloth as a binder-free and flexible supercapacitor electrode with high performance. RSC Adv. 2018, 8, 38631–38640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhao, Y.; Liu, Z.; Yang, L.; Zhang, J.; Wang, M.; Che, R. Flexible Graphene-Wrapped Carbon Nanotube/Graphene@MnO2 3D Multilevel Porous Film for High-Performance Lithium-Ion Batteries. Small 2018, 14, e1801007. [Google Scholar] [CrossRef]
- Tong, L.; Qiu, F.; Zeng, T.; Long, J.; Yang, J.; Wang, R.; Zhang, J.; Wang, C.; Sun, T.; Yang, Y. Recent progress in the preparation and application of quantum dots/graphene composite materials. RSC Adv. 2017, 7, 47999–48018. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Liu, J.; Qiao, J.; Huang, H.; Zhou, X.-D. Interweaving between MnO2 nanowires/nanorods and carbon nanotubes as robust multifunctional electrode for both liquid and flexible electrochemical energy devices. J. Power Sources 2020, 455, 227992. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, L.; Li, Z.; Qu, Y.; Wu, W.; Jing, L. Porous two-dimension MnO2-C3N4/titanium phosphate nanocomposites as efficient photocatalsyts for CO oxidation and mechanisms. Appl. Catal. B-Environ. 2021, 282, 119563. [Google Scholar] [CrossRef]
- Chao, G.; Zhang, L.; Yuan, S.; Xue, T.; Yang, F.; Huang, Y.; Fan, W.; Liu, T. Ultrathin MnO2 Sheet Arrays Grown on Hollow Carbon Fibers as Effective Polysulfide-Blocking Interlayers for High-Performance Li-S Batteries. ACS Appl. Energy Mater. 2020, 3, 12703–12708. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K. Preferential killing of bacterial cells by hybrid carbon nanotube-MnO2 nanocomposite synthesized by novel microwave assisted processing. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 81, 469–477. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Zhan, H. Ultrathin manganese dioxide nanosheets grown on partially unzipped nitrogen-doped carbon nanotubes for high-performance asymmetric supercapacitors. J. Alloys Compd. 2017, 702, 236–243. [Google Scholar] [CrossRef]
- Sridhar, V.; Lee, I.; Jung, K.H.; Park, H. Metal Organic Framework Derived MnO2-Carbon Nanotubes for Efficient Oxygen Reduction Reaction and Arsenic Removal from Contaminated Water. Nanomaterials 2020, 10, 1895. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, G.; Duan, X. Self-Assembled Three-Dimensional Graphene Macrostructures: Synthesis and Applications in Supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef]
- Khamsanga, S.; Nguyen, M.T.; Yonezawa, T.; Thamyongkit, P.; Pornprasertsuk, R.; Pattananuwat, P.; Tuantranont, A.; Siwamogsatham, S.; Kheawhom, S. MnO(2)Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2020, 21, 4689. [Google Scholar] [CrossRef]
- Guo, J.; Chen, T.; Zhou, X.; Zheng, T.; Xia, W.; Zhong, C.; Liu, Y. Preparation and Pb (II) adsorption in aqueous of 2D/2D g-C3N4/MnO2 composite. Appl. Organomet. Chem. 2019, 33, e5119. [Google Scholar] [CrossRef]
- Xu, J.; Li, D.; Chen, Y.; Tan, L.; Kou, B.; Wan, F.; Jiang, W.; Li, F. Constructing Sheet-On-Sheet Structured Graphitic Carbon Nitride/Reduced Graphene Oxide/Layered MnO2 Ternary Nanocomposite with Outstanding Catalytic Properties on Thermal Decomposition of Ammonium Perchlorate. Nanomaterials 2017, 7, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.; Zhang, W.; Han, Z.; Wang, F.; Geng, D.; Li, X.; Li, Y.; Zhang, X. Preparation of PAN-based carbon fiber@MnO2 composite as an anode material for structural lithium-ion batteries. J. Mater. Sci. 2019, 54, 11972–11982. [Google Scholar] [CrossRef]
- Ji, T.; Zhang, S.; He, Y.; Zhang, X.; Zhang, X.; Li, W. Enhanced thermoelectric property of cement-based materials with the synthesized MnO2/carbon fiber composite. J. Build. Eng. 2021, 43, 103190. [Google Scholar] [CrossRef]
- Corpuz, R.D.; De Juan, L.M.Z.; Praserthdam, S.; Pomprasertsuk, R.; Yonezawa, T.; Mai Thanh, N.; Kheawhom, S. Annealing induced a well-ordered single crystal delta-MnO2 and its electrochemical performance in zinc-ion battery. Sci. Rep. 2019, 9, 15107. [Google Scholar] [CrossRef] [Green Version]
- Ou, X.; Li, Q.; Xu, D.; Guo, J.; Yan, F. In Situ Growth of MnO2 Nanosheets on N-Doped Carbon Nanotubes Derived from Polypyrrole Tubes for Supercapacitors. Chem. -Asian J. 2018, 13, 545–551. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; Sun, L.; Wu, H.; Wang, J.; Zhao, Y.; Yan, L.; Luo, Y.; Jiang, K.; Li, Q.; et al. MnO2 nanoparticles anchored on carbon nanotubes with hybrid supercapacitor-battery behavior for ultrafast lithium storage. Carbon 2018, 139, 145–155. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Ding, Y.; Luo, C.; Du, X.; Lin, J. MnO2 spontaneously coated on carbon nanotubes for enhanced water oxidation. Chem. Commun. 2014, 50, 11938–11941. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Song, C.; Qiu, H.; Xue, H. Study on preparation and performance of flexible all-solid-state supercapacitor based on nitrogen-doped RGO/CNT/MnO2 composite fibers. J. Alloys Compd. 2021, 859, 157816. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Li, L.; Li, W.; Zou, C.; Jin, H.; Wang, S.; Chou, S.-L. Facile Synthesis of Birnessite delta-MnO2 and Carbon Nanotube Composites as Effective Catalysts for Li-CO2 Batteries. ACS Appl. Mater. Interfaces 2021, 13, 16585–16593. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, Y.; Su, K.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; et al. The enhanced photocatalytic properties of MnO2/g-C3N4 heterostructure for rapid sterilization under visible light. J. Hazard. Mater. 2019, 377, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Singu, B.S.; Goda, E.S.; Yoon, K.R. Carbon Nanotube-Manganese oxide nanorods hybrid composites for high-performance supercapacitor materials. J. Ind. Eng. Chem. 2021, 97, 239–249. [Google Scholar] [CrossRef]
- Wang, M.; Shen, M.; Zhang, L.; Tian, J.; Jin, X.; Zhou, Y.; Shi, J. 2D-2D MnO2/g-C3N4 heterojunction photocatalyst: In-situ synthesis and enhanced CO2 reduction activity. Carbon 2017, 120, 23–31. [Google Scholar] [CrossRef]
- Peng, S.; Yang, X.; Strong, J.; Sarkar, B.; Jiang, Q.; Peng, F.; Liu, D.; Wang, H. MnO2-decorated N-doped carbon nanotube with boosted activity for low-temperature oxidation of formaldehyde. J. Hazard. Mater. 2020, 396, 122750. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, B.; Xing, B. Wrinkles and Folds of Activated Graphene Nanosheets as Fast and Efficient Adsorptive Sites for Hydrophobic Organic Contaminants. Environ. Sci. Technol. 2016, 50, 3798–3808. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, B. Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem. Eng. J. 2015, 281, 379–388. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the Aquatic Environment: Adsorption, Dispersion, Toxicity and Transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Zhang, H.; Zhong, G.-J.; Yao, G.; Lai, B. Cellulose/carbon Composites and their Applications in Water Treatment—A Review. Chem. Eng. J. 2021, 405, 126980. [Google Scholar] [CrossRef]
- Liang, J.; Xu, Y.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Li, F.; Guo, T.; Chen, Y. Infrared-Triggered Actuators from Graphene-Based Nanocomposites. J. Phys. Chem. C 2009, 113, 9921–9927. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, Ultra-Flyweight, Synergistically Assembled Carbon Aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ge, X.; Ye, X.; Wang, G.; Zhang, H.; Zhou, H.; Zhang, Y.; Zhao, H. 3D graphene/delta-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 2016, 4, 1970–1979. [Google Scholar] [CrossRef]
- Zhou, J.; Pei, Z.; Li, N.; Han, S.; Li, Y.; Chen, Q.; Sui, Z. Synthesis of 3D graphene/MnO2 nanocomposites with hierarchically porous structure for water purification. J. Porous Mater. 2022, 29, 983–990. [Google Scholar] [CrossRef]
- Lai, F.; Huang, Y.; Zuo, L.; Gu, H.; Miao, Y.-E.; Liu, T. Electrospun nanofiber-supported carbon aerogel as a versatile platform toward asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 15861–15869. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Wang, R.-H.; Ong, K.; Ong, J.H.L.; Lee, R.-H. Cellulose/carbon nanotube/MnO2 composite electrodes with high mass loadings for symmetric supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, K.; Tang, Y.; Li, F.; Meng, Q. Forest-like carbon foam templated rGO/CNTs/MnO2 electrode for high-performance supercapacitor. Electrochim. Acta 2021, 375, 137960. [Google Scholar] [CrossRef]
- He, S.; Xiao, K.; Chen, X.-Z.; Li, T.; Ouyang, T.; Wang, Z.; Guo, M.-L.; Liu, Z.-Q. Enhanced photoelectrocatalytic activity of direct Z-scheme porous amorphous carbon nitride/manganese dioxide nanorod arrays. J. Colloid Interface Sci. 2019, 557, 644–654. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Shifa, T.A.; Wang, D.; Wu, X.; Cui, Y. Hierarchical MnO2/activated carbon cloth electrode prepared by synchronized electrochemical activation and oxidation for flexible asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 1047–1055. [Google Scholar] [CrossRef]
- Kataoka, F.; Ishida, T.; Nagita, K.; Kumbhar, V.; Yamabuki, K.; Nakayama, M. Cobalt-Doped Layered MnO2 Thin Film Electrochemically Grown on Nitrogen-Doped Carbon Cloth for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4720–4726. [Google Scholar] [CrossRef]
- Ko, W.-Y.; Liu, Y.-C.; Lai, J.-Y.; Chung, C.-C.; Lin, K.-J. Vertically Standing MnO2 Nanowalls Grown on AgCNT-Modified Carbon Fibers for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 669. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, Q.; Qian, Y.; He, J.; Dong, X. Alkali cation incorporated MnO2 cathode and carbon cloth anode for flexible aqueous supercapacitor with high wide-voltage and power density. Electrochim. Acta 2020, 342, 136046. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wei, T.-Y.; Chien, H.-C.; Lu, S.-Y. Manganese Oxide/Carbon Aerogel Composite: An Outstanding Supercapacitor Electrode Material. Adv. Energy Mater. 2011, 1, 901–907. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding Three-Dimensional Graphene/MnO2 Composite Networks As Ultra light and Flexible Supercapacitor Electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Chen, P.; Bao, Y.; Jiang, X.; Chen, Y. Electrochemical performance of polyaniline-coated gamma-MnO2 on carbon cloth as flexible electrode for supercapacitor. Electrochim. Acta 2022, 413, 140146. [Google Scholar] [CrossRef]
- Li, G.-R.; Feng, Z.-P.; Ou, Y.-N.; Wu, D.; Fu, R.; Tong, Y.-X. Mesoporous MnO2/Carbon Aerogel Composites as Promising Electrode Materials for High-Performance Supercapacitors. Langmuir 2010, 26, 2209–2213. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, X.; Han, Q.; Du, Y.; Huang, J.; Liu, Y.; Yang, J. alpha-MnO2 nanofibers/carbon nanotubes hierarchically assembled microspheres: Approaching practical applications of high-performance aqueous Zn-ion batteries. J. Power Sources 2019, 443, 227244. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Lu, Y.; Wang, W.; Peng, T.; Zhang, Y.; Guo, Y.; Wang, Y.; Huo, K.; Kim, J.-K.; et al. Cable-like double-carbon layers for fast ion and electron transport: An example of CNT@NCT@MnO2 3D nanostructure for high-performance supercapacitors. Carbon 2019, 143, 335–342. [Google Scholar] [CrossRef]
- Xu, J.; Hou, K.; Ju, Z.; Ma, C.; Wang, W.; Wang, C.; Cao, J.; Chen, Z. Synthesis and Electrochemical Properties of Carbon Dots/Manganese Dioxide (CQDs/MnO2) Nanoflowers for Supercapacitor Applications. J. Electrochem. Soc. 2017, 164, A430–A437. [Google Scholar] [CrossRef]
- Saharan, P.; Sharma, A.K.; Kumar, V.; Kaushal, I. Multifunctional CNT supported metal doped MnO2 composite for adsorptive removal of anionic dye and thiourea sensing. Mater. Chem. Phys. 2019, 221, 239–249. [Google Scholar] [CrossRef]
- Wadi, V.S.; Ibrahim, Y.; Arangadi, A.F.; Kilybay, A.; Mavukkandy, M.O.; Alhseinat, E.; Hasan, S.W. Three-dimensional graphene/MWCNT-MnO2 nanocomposites for high-performance capacitive deionization (CDI) application. J. Electroanal. Chem. 2022, 914, 116318. [Google Scholar] [CrossRef]
- Hong, S.; Huang, X.; Liu, H.; Gao, Z. In Situ Chemical Synthesis of MnO2/HMCNT Nanocomposite with a Uniquely Developed Three-Dimensional Open Porous Architecture for Supercapacitors. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1587–1596. [Google Scholar] [CrossRef]
- Zeng, X.; Shan, C.; Sun, M.; Ding, D.; Rong, S. Graphene enhanced α-MnO2 for photothermal catalytic decomposition of carcinogen formaldehyde. Chin. Chem. Lett. 2022, 33, 4771–4775. [Google Scholar]
- Kumar, V.; Saharan, P.; Sharma, A.K.; Umar, A.; Kaushal, I.; Mittal, A.; Al-Hadeethi, Y.; Rashad, B. Silver doped manganese oxide-carbon nanotube nanocomposite for enhanced dye-sequestration: Isotherm studies and RSM modelling approach. Ceram. Int. 2020, 46, 10309–10319. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g-C3N4/MnO2 Nanocomposite as a Direct Z-Scheme Photocatalyst for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2018, 6, 965–973. [Google Scholar] [CrossRef]
- Wu, F.; Gao, X.; Xu, X.; Jiang, Y.; Gao, X.; Yin, R.; Shi, W.; Liu, W.; Lu, G.; Cao, X. MnO2 Nanosheet-Assembled Hollow Polyhedron Grown on Carbon Cloth for Flexible Aqueous Zinc-Ion Batteries. Chemsuschem 2020, 13, 1537–1545. [Google Scholar] [CrossRef]
- Lee, K.G.; Jeong, J.-M.; Lee, S.J.; Yeom, B.; Lee, M.-K.; Choi, B.G. Sonochemical-assisted synthesis of 3D graphene/nanoparticle foams and their application in supercapacitor. Ultrason. Sonochem. 2015, 22, 422–428. [Google Scholar] [CrossRef]
- Le, Q.J.; Huang, M.; Wang, T.; Liu, X.Y.; Sun, L.; Guo, X.L.; Jiang, D.B.; Wang, J.; Dong, F.; Zhang, Y.X. Biotemplate derived three dimensional nitrogen doped graphene@MnO2 as bifunctional material for supercapacitor and oxygen reduction reaction catalyst. J. Colloid Interface Sci. 2019, 544, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.Y.; Guan, B.; Zhu, S.J.; Zhang, H.J.; Zhang, Y.X. Facile synthesis of carbon-doped graphitic C3N4@MnO2 with enhanced electrochemical performance. RSC Adv. 2016, 6, 83209–83216. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xia, Y.; Wan, X.; Yang, S.; Cai, Z.; Ye, Y.; Li, G. Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 109, 110615. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.J.; Moholkar, V.S. Ultrasound-assisted facile one-pot synthesis of ternary MWCNT/MnO2/rGO nanocomposite for high performance supercapacitors with commercial-level mass loadings. Ultrason. Sonochem. 2022, 82, 105896. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Bhattacharya, S.K. Sonochemically synthesized hydroxy-functionalized graphene-MnO2 nanocomposite for supercapacitor applications. J. Appl. Electrochem. 2017, 47, 789–801. [Google Scholar] [CrossRef]
- Naderi, H.R.; Norouzi, P.; Ganjali, M.R. Electrochemical study of a novel high performance supercapacitor based on MnO2/nitrogen-doped graphene nanocomposite. Appl. Surf. Sci. 2016, 366, 552–560. [Google Scholar] [CrossRef]
- Majumdar, D.; Bhattacharya, S.K. Synthesis, Characterization and Electrochemical Study of Hydroxy-Functionalized Graphene/MnO2 Nanocomposite. In Proceedings of the International Conference on Materials Research and Applications (ICMRA), Hyderabad, India, 11–13 March 2016; pp. 3872–3877. [Google Scholar]
- Wang, M.; Yan, Q.; Xue, F.; Zhang, J.; Wang, J. Design and synthesis of carbon nanotubes/carbon fiber/reduced graphene oxide/MnO2 flexible electrode material for supercapacitors. J. Phys. Chem. Solids 2018, 119, 29–35. [Google Scholar] [CrossRef]
- Yashas, S.R.; Shivaraju, H.P.; Pema, G.; Swamy, N.K.; Namratha, K.; Gurupadayya, B.; Madhusudan, P. Sonochemical synthesis of graphitic carbon nitride-manganese oxide interfaces for enhanced photocatalytic degradation of tetracycline hydrochloride. Environ. Sci. Pollut. Res. 2021, 28, 4778–4789. [Google Scholar] [CrossRef]
- Chai, C.; Yang, X.; Yang, X.; Dong, C.; Bian, W.; Choi, M.M.F. An ultrasensitive MnO2-S,O-doped g-C3N4 nanoprobe for "turn-on" detection of glutathione and cell imaging. J. Mater. Sci. 2022, 57, 7909–7922. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, L.; Gao, R.; Dai, W.-L. Construction of Highly Efficient 3D/2D MnO2/g-C3N4 Nanocomposite in the Epoxidation of Styrene with TBHP. ACS Sustain. Chem. Eng. 2019, 7, 17008–17019. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, Y.; Deng, F.; Wang, M.; Chen, D. Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants. Sep. Purif. Technol. 2020, 246, 116890. [Google Scholar] [CrossRef]
- Anbumannan, V.; Dinesh, M.; Kumar, R.T.R.; Suresh, K. Hierarchical alpha-MnO2 wrapped MWCNTs sensor for low level detection of p-nitrophenol in water. Ceram. Int. 2019, 45, 23097–23103. [Google Scholar] [CrossRef]
- Jia, H.; Cai, Y.; Zheng, X.; Lin, J.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Mesostructured Carbon Nanotube-on-MnO2 Nanosheet Composite for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 38963–38969. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Othman, F.E.C.; Yusof, N.; Matsuura, T.; Lau, W.J.; Jaafar, J.; Ismail, A.F.; Salleh, W.N.W.; Aziz, F. Preparation of nanocomposite activated carbon nanofiber/manganese oxide and its adsorptive performance toward leads (II) from aqueous solution. J. Water Process Eng. 2020, 37, 101430. [Google Scholar] [CrossRef]
- Wei, B.; Wang, L.; Wang, Y.; Yuan, Y.; Miao, Q.; Yang, Z.; Fei, W. In situ growth of manganese oxide on 3D graphene by a reverse microemulsion method for supercapacitors. J. Power Sources 2016, 307, 129–137. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, P.; Xu, S.; Yan, X.; Xue, Q. Free-Standing Three-Dimensional Graphene/Manganese Oxide Hybrids As Binder-Free Electrode Materials for Energy Storage Applications. ACS Appl. Mater. Interfaces 2014, 6, 11665–11674. [Google Scholar] [CrossRef]
- Pang, H.; Abdalla, A.M.; Sahu, R.P.; Duan, Y.; Puri, I.K. Low-temperature synthesis of manganese oxide-carbon nanotube-enhanced microwave-absorbing nanocomposites. J. Mater. Sci. 2018, 53, 16288–16302. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xiao, Y.; Zhang, L.; Guo, L.; Zhang, L.; Dong, X. Free-standing composite films of multiple 2D nanosheets: Synergetic photothermocatalysis/photocatalysis for efficient removal of formaldehyde under ambient condition. Chem. Eng. J. 2020, 394, 125014. [Google Scholar] [CrossRef]
- Hasija, V.; Nguyen, V.-H.; Kumar, A.; Raizada, P.; Krishnan, V.; Khan, A.A.P.; Singh, P.; Lichtfouse, E.; Wang, C.; Huong, P.T. Advanced activation of persulfate by polymeric g-C3N4 based photocatalysts for environmental remediation: A review. J. Hazard. Mater. 2021, 413, 125324. [Google Scholar] [CrossRef]
- Sonu; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar] [CrossRef]
- Zuo, W.; Zhang, L.; Zhang, Z.; Tang, S.; Sun, Y.; Huang, H.; Yu, Y. Degradation of organic pollutants by intimately coupling photocatalytic materials with microbes: A review. Crit. Rev. Biotechnol. 2021, 41, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pastora, J.; Dominguez, S.; Bringas, E.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Review and perspectives on the use of magnetic nanophotocatalysts (MNPCs) in water treatment. Chem. Eng. J. 2017, 310, 407–427. [Google Scholar] [CrossRef]
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water-A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Tho Chau Minh Vinh, D.; Duy Quoc, N.; Kien Trung, N.; Phuoc Huu, L. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef] [Green Version]
- Jalloul, G.; Keniar, I.; Tehrani, A.; Boyadjian, C. Antibiotics Contaminated Irrigation Water: An Overview on Its Impact on Edible Crops and Visible Light Active Titania as Potential Photocatalysts for Irrigation Water Treatment. Front. Environ. Sci. 2021, 9, 767963. [Google Scholar] [CrossRef]
- Pandiyan, R.; Dharmaraj, S.; Ayyaru, S.; Sugumaran, A.; Somasundaram, J.; Kazi, A.S.; Samiappan, S.C.; Ashokkumar, V.; Ngamcharussrivichai, C. Ameliorative photocatalytic dye degradation of hydrothermally synthesized bimetallic Ag-Sn hybrid nanocomposite treated upon domestic wastewater under visible light irradiation. J. Hazard. Mater. 2022, 421, 126734. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Yin, J.; Zhao, J.; Li, X.; Wang, S.; Bai, Z.; Jiao, T. Facile preparation and highly efficient photodegradation performances of self-assembled Artemia eggshell-ZnO nanocomposites for wastewater treatment. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 610, 125752. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Sudhaik, A.; Sharma, K.; Kumar, A.; Singh, P.; Jonnalagadda, S.B.; Thakur, V.K. Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today 2019, 15, 494–524. [Google Scholar] [CrossRef]
- Yang, F.; Du, M.; Yin, K.; Qiu, Z.; Zhao, J.; Liu, C.; Zhang, G.; Gao, Y.; Pang, H. Applications of Metal-Organic Frameworks in Water Treatment: A Review. Small 2022, 18, 2105715. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Aathira, M.S.; Pal, U.; Jain, S.L. Photochemical Oxidative Coupling of 2-Naphthols using a Hybrid Reduced Graphene Oxide/Manganese Dioxide Nanocomposite under Visible-Light Irradiation. Chemcatchem 2018, 10, 1844–1852. [Google Scholar] [CrossRef]
- Teh, C.M.; Mohamed, A.R. Roles of titanium dioxide and ion-doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: A review. J. Alloys Compd. 2011, 509, 1648–1660. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Biglari, H.; Afsharnia, M.; Alipour, V.; Khosravi, R.; Sharafi, K.; Mahvi, A.H. A review and investigation of the effect of nanophotocatalytic ozonation process for phenolic compound removal from real effluent of pulp and paper industry. Environ. Sci. Pollut. Res. 2017, 24, 4105–4116. [Google Scholar] [CrossRef]
- Said, K.A.M.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Ramos-Ramirez, E.; Tzompantzi-Morales, F.; Gutierrez-Ortega, N.; Mojica-Calvillo, H.G.; Castillo-Rodriguez, J. Photocatalytic Degradation of 2,4,6-Trichlorophenol by MgO-MgFe2O4 Derived from Layered Double Hydroxide Structures. Catalysts 2019, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Shobha, P.; Paul Winston, A.J.P.; Sunil, S.; David, T.M.; Margaret, S.M.; Muthupandi, S.; Sagayaraj, P. Facile Synthesis of rGO/Mn3O4 Composite for Efficient Photodegradation of Phenol under Visible Light. J. Nanomater. 2021, 2021, 5576048. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H. Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Process Eng. 2022, 47, 102671. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Sun, C. Occurrence, Ecological and Human Health Risks, and Seasonal Variations of Phenolic Compounds in Surface Water and Sediment of a Potential Polluted River Basin in China. Int. J. Environ. Res. Public Health 2017, 14, 1140. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. Recent developments in photocatalysis of industrial effluents: A review and example of phenolic compounds degradation. Chemosphere 2022, 296, 133688. [Google Scholar] [CrossRef]
- Malakootian, M.; Heidari, M.R. Removal of phenol from steel wastewater by combined electrocoagulation with photo-Fenton. Water Sci. Technol. 2018, 78, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, G.; Chen, X.; Huang, J.; Li, M.; Yin, J.; Liang, Y.; Yao, Y.; Li, Y. A review on graphitic carbon nitride (g-C3N4) based hybrid membranes for water and wastewater treatment. Sci. Total Environ. 2021, 792, 148462. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, C.; Genc, A. Review of Treatment Technologies for the Removal of Phenol from Wastewaters. J. Water Chem. Technol. 2021, 43, 145–154. [Google Scholar] [CrossRef]
- Mehta, A.; Mishra, A.; Basu, S. Fluorescent carbon dot decorated MnO2 nanorods for complete photomineralization of phenol from water. Environ. Sci.-Water Res. Technol. 2018, 4, 2012–2020. [Google Scholar] [CrossRef]
- Salam, M.A.; Mohamed, R.M.; Obaid, A.Y. Enhancement of Titanium Dioxide-Manganese Oxide Nanoparticles Photocatalytic Activity by Doping with Multi-walled Carbon Nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 765–779. [Google Scholar] [CrossRef]
- Xavier, S.S.J.; Siva, G.; Ranjani, M.; Rani, S.D.; Priyanga, N.; Srinivasan, R.; Pannipara, M.; Al-Sehemi, A.G.; Kumar, G.G. Turn-on fluorescence sensing of hydrazine using MnO2 nanotube-decorated g-C3N4 nanosheets. New J. Chem. 2019, 43, 13196–13204. [Google Scholar] [CrossRef]
- Pan, X.; Kong, F.; Xing, M. Spatial separation of photo-generated carriers in g-C3N4/MnO2/Pt with enhanced H-2 evolution and organic pollutant control. Res. Chem. Intermed. 2022, 48, 2837–2855. [Google Scholar] [CrossRef]
- Yasmeen, H.; Zada, A.; Liu, S. Dye loaded MnO2 and chlorine intercalated g-C3N4 coupling impart enhanced visible light photoactivities for pollutants degradation. J. Photochem. Photobiol. A-Chem. 2019, 380, 111867. [Google Scholar] [CrossRef]
- Pradhan, M.R.; Rath, D.; Sethi, R.; Nanda, B.B.; Nanda, B. alpha-MnO2 modified exfoliated porous g-C3N4 nanosheet (2D) for enhanced photocatalytic oxidation efficiency of aromatic alcohols. Inorg. Chem. Commun. 2021, 130, 108717. [Google Scholar] [CrossRef]
- Dong, J.; Xie, H.; Feng, R.; Lai, X.; Duan, H.; Xu, L.; Xia, X. Transport and fate of antibiotics in a typical aqua-agricultural catchment explained by rainfall events: Implications for catchment management. J. Environ. Manag. 2021, 293, 112953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chen, L.; Yang, L.; Sun, L.; Li, S.; Li, M.; Feng, Q. Effects of land use and rainfall on sequestration of veterinary antibiotics in soils at the hillslope scale. Environ. Pollut. 2020, 260, 114112. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Ahmadijokani, F.; Molavi, H.; Tajahmadi, S.; Rezakazemi, M.; Amini, M.; Kamkar, M.; Rojas, O.J.; Arjmand, M. Coordination chemistry of metal-organic frameworks: Detection, adsorption, and photodegradation of tetracycline antibiotics and beyond. Coord. Chem. Rev. 2022, 464, 214562. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. A review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef]
- Ma, W.; Xu, X.; An, B.; Zhou, K.; Mi, K.; Huo, M.; Liu, H.; Wang, H.; Liu, Z.; Cheng, G.; et al. Single and ternary competitive adsorption-desorption and degradation of amphenicol antibiotics in three agricultural soils. J. Environ. Manag. 2021, 297, 113366. [Google Scholar] [CrossRef]
- Wahab, M.; Zahoor, M.; Salman, S.M.; Kamran, A.W.; Naz, S.; Burlakovs, J.; Kallistova, A.; Pimenov, N.; Zekker, I. Adsorption-Membrane Hybrid Approach for the Removal of Azithromycin from Water: An Attempt to Minimize Drug Resistance Problem. Water 2021, 13, 1969. [Google Scholar] [CrossRef]
- Bai, X.; Chen, W.; Wang, B.; Sun, T.; Wu, B.; Wang, Y. Photocatalytic Degradation of Some Typical Antibiotics: Recent Advances and Future Outlooks. Int. J. Mol. Sci. 2022, 23, 8130. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zeng, L.; Zhu, M. Recent progress on the removal of antibiotic pollutants using photocatalytic oxidation process. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1401–1448. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Hu, Y.H. Strategies of tuning catalysts for efficient photodegradation of antibiotics in water environments: A review. J. Mater. Chem. A 2021, 9, 2592–2611. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Pal, D.; Mishra, J.; Thakur, C. Noble metal-free doped graphitic carbon nitride (g-C3N4) for efficient photodegradation of antibiotics: Progress, limitations, and future directions. Environ. Sci. Pollut. Res. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bisaria, K.; Sinha, S.; Singh, R.; Iqbal, H.M.N. Recent advances in structural modifications of photo-catalysts for organic pollutants degradation-A comprehensive review. Chemosphere 2021, 284, 131263. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Li, Y.; Wang, X.; Long, J.; Liang, B. Carbon nanosheet/MnO2/BiOCl ternary composite for degradation of organic pollutants. J. Alloys Compd. 2022, 891, 162090. [Google Scholar] [CrossRef]
- Chen, R.R. Preparation and Degradation of g-C3N4 Based Photocatalysts; Anhui Jianzhu University: Hefei, China, 2021. [Google Scholar]
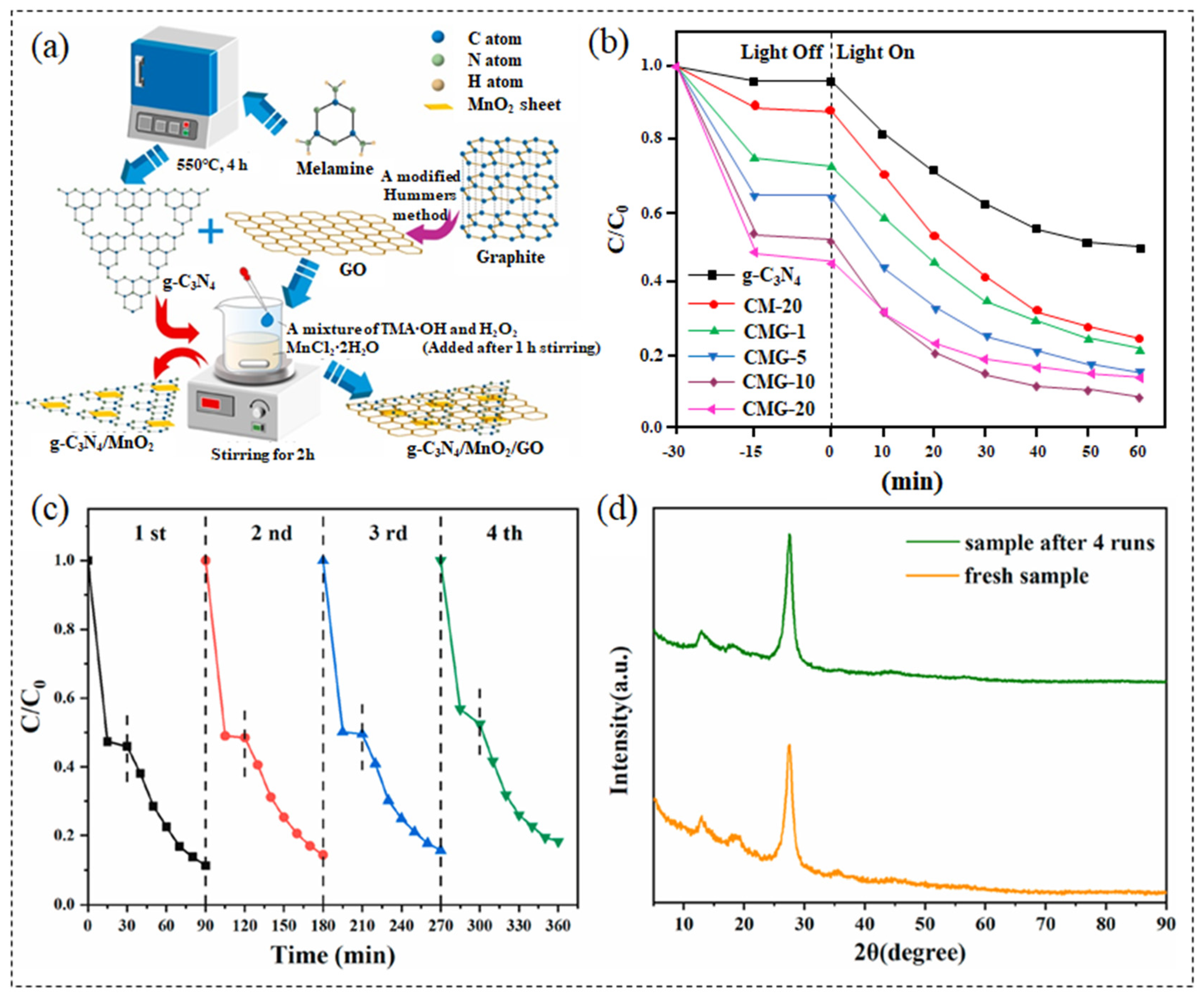
- Du, C.; Zhang, Z.; Tan, S.; Yu, G.; Chen, H.; Zhou, L.; Yu, L.; Su, Y.; Zhang, Y.; Deng, F.; et al. Construction of Z-scheme g-CN4/MnO2/GO ternary photocatalyst with enhanced photodegradation ability of tetracycline hydrochloride under visible light radiation. Environ. Res. 2021, 200, 111427. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Chen, Q.; Fan, W.; Gong, Z. Pumice-loaded rGO@MnO2 nanomesh photocatalyst with visible light response for rapid degradation of ciprofloxacin. Sep. Purif. Technol. 2022, 297, 121502. [Google Scholar] [CrossRef]
- Kaur, P.K.; Badru, R.; Singh, P.P.; Kaushal, S. Photodegradation of organic pollutants using heterojunctions: A review. J. Environ. Chem. Eng. 2020, 8, 103666. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Jabeen, S.; Khan, M.S.; Khattak, R.; Zekker, I.; Burlakovs, J.; Rubin, S.S.d.; Ghangrekar, M.M.; Kallistova, A.; Pimenov, N.; Zahoor, M.; et al. Palladium-Supported Zirconia-Based Catalytic Degradation of Rhodamine-B Dye from Wastewater. Water 2021, 13, 1522. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Rahman, N.U.; Ullah, I.; Alam, S.; Khan, M.S.; Shah, L.A.; Zekker, I.; Burlakovs, J.; Kallistova, A.; Pimenov, N.; Vincevica-Gaile, Z.; et al. Activated Ailanthus altissima Sawdust as Adsorbent for Removal of Acid Yellow 29 from Wastewater: Kinetics Approach. Water 2021, 13, 2136. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Saroyan, H.; Kyzas, G.Z.; Deliyanni, E.A. Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide. Processes 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Warsi, M.F.; Bilal, M.; Zulfiqar, S.; Khalid, M.U.; Agboola, P.O.; Shakir, I. Enhanced visible light driven Photocatalytic activity of MnO2 nanomaterials and their hybrid structure with carbon nanotubes. Mater. Res. Express 2020, 7, 105015. [Google Scholar] [CrossRef]
- Warsi, M.F.; Bashir, B.; Zulfiqar, S.; Aadil, M.; Khalid, M.U.; Agboola, P.O.; Shakir, I.; Yousuf, M.A.; Shahid, M. Mn1-xCuxO2/ reduced graphene oxide nanocomposites: Synthesis, characterization, and evaluation of visible light mediated catalytic studies. Ceram. Int. 2021, 47, 5044–5053. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Manzoor, O.; Mohsin, M.; Chaudhry, S.A. Nigella sativa seed based nanocomposite-MnO2/BC: An antibacterial material for photocatalytic degradation, and adsorptive removal of Methylene blue from water. Environ. Res. 2019, 171, 328–340. [Google Scholar] [CrossRef]
- Zhang, L.; Jamal, R.; Zhao, Q.; Wang, M.; Abdiryim, T. Preparation of PEDOT/GO, PEDOT/MnO2, and PEDOT/GO/MnO2 nanocomposites and their application in catalytic degradation of methylene blue. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Siddeswara, D.M.K.; Venkatesh, T.; Mahesh, K.R.V.; Mylarappa, M.; Anantharaju, K.S.; Kumara, K.N.S.; Raghavendra, N.; Shivakumar, M.S. One Step Synthesis of Ternary Composite of GNS/CNT/MnO2 for the Applications of Electrochemical and Photocatalytic Studies. In Proceedings of the International Conference on Nanotechnology (ICNano), Karnataka, India, 21–23 April 2017; pp. 11799–11805. [Google Scholar]
- Singh, A.K.; Gautam, R.K.; Agrahari, S.; Prajapati, J.; Tiwari, I. Graphene oxide supported Fe3O4-MnO2 nanocomposites for adsorption and photocatalytic degradation of dyestuff: Ultrasound effect, surfactants role and real sample analysis. Int. J. Environ. Anal. Chem. 2022, 1–27. [Google Scholar] [CrossRef]
- Chen, R.-R.; Ren, Q.-F.; Liu, Y.-X.; Ding, Y.; Zhu, H.-T.; Xiong, C.-Y.; Jin, Z.; Oh, W.-C. Synthesis of g-C3N4/diatomite/MnO2 composites and their enhanced photo-catalytic activity driven by visible light. J. Korean Ceram. Soc. 2021, 58, 548–558. [Google Scholar] [CrossRef]
- Ahmad, J.; Wahid, M.; Majid, K. In situconstruction of hybrid MnO2@GO heterostructures for enhanced visible light photocatalytic, anti-inflammatory and anti-oxidant activity. New J. Chem. 2020, 44, 11092–11104. [Google Scholar] [CrossRef]
- Vikal, M.; Shah, S.; Singh, N.; Singh, P.; Gupta, M.; Singh, M.J.; Kumar, A.; Kumar, Y. Efficient MnO2 decorated graphitic carbon nitride-based nanocomposite for application in water purification. Mater. Today Proc. 2022, 67, 777–783. [Google Scholar] [CrossRef]
- Gayathri, M.; Shanthi, M.; Satheeshkumar, E.; Jayaprakash, N.; Sundaravadivel, E. Preparation and characterization of boron doped CN's/MnO2 and its photocatalytic application of dye degradation. In Proceedings of the 2nd International Conference on Recent Advances in Materials and Manufacturing (ICRAMM), Tamil Nadu, India, 20–21 November 2021; pp. 1506–1512. [Google Scholar]
- Ma, M.; Yang, Y.; Chen, Y.; Jiang, J.; Ma, Y.; Wang, Z.; Huang, W.; Wang, S.; Liu, M.; Ma, D.; et al. Fabrication of hollow flower-like magnetic Fe3O4/C/MnO2/C3N4 composite with enhanced photocatalytic activity. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Numan, A.; Ponomarev, N.; Iqbal, J.; Khalid, M. Enhanced photocatalytic performance of PANI-rGO-MnO2 ternary composite for degradation of organic contaminants under visible light. J. Environ. Chem. Eng. 2021, 9, 106006. [Google Scholar] [CrossRef]
- Panimalar, S.; Uthrakumar, R.; Selvi, E.T.; Gomathy, P.; Inmozhi, C.; Kaviyarasu, K.; Kennedy, J. Studies of MnO2/g-C3N4 hetrostructure efficient of visible light photocatalyst for pollutants degradation by sol-gel technique. Surf. Interfaces 2020, 20, 100512. [Google Scholar] [CrossRef]
- Tahir, M.B.; Kiran, H.; Iqbal, T. The detoxification of heavy metals from aqueous environment using nano-photocatalysis approach: A review. Environ. Sci. Pollut. Res. 2019, 26, 10515–10528. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Fadlalla, M.I.; Kumar, P.S.; Selvam, V.; Babu, S.G. Emerging energy and environmental application of graphene and their composites: A review. J. Mater. Sci. 2020, 55, 7156–7183. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Guo, Y.; Gao, H.; Li, H.; Yan, S. Introduction of alpha-MnO2 nanosheets to NH2 graphene to remove Cr6+ from aqueous solutions. RSC Adv. 2015, 5, 44096–44106. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, V.; Kim, K.-H.; Kwon, E.E.; Younis, S.A. Metal-organic frameworks for photocatalytic detoxification of chromium and uranium in water. Coord. Chem. Rev. 2021, 447, 214148. [Google Scholar] [CrossRef]
- Jafarzadeh, M. Recent Progress in the Development of MOF-Based Photocatalysts for the Photoreduction of Cr-(VI). ACS Appl. Mater. Interfaces 2022, 14, 24993–25024. [Google Scholar] [CrossRef]
- Padhi, D.K.; Baral, A.; Parida, K.; Singh, S.K.; Ghosh, M.K. Visible Light Active Single-Crystal Nanorod/Needle-like alpha-MnO2@RGO Nanocomposites for Efficient Photoreduction of Cr(VI). J. Phys. Chem. C 2017, 121, 6039–6049. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, L.; Xu, L.; Xie, Z.J.; Liu, Y.H. Preparation of MnO2@g-C3N4 and Its Photoreduction Performance for Uranium (Ⅵ). Hydrometall. China 2021, 40, 148–154. [Google Scholar] [CrossRef]
| MnO2 | Carbon Material | Synthesis Method | Composite Product | Morphology | Ref. |
|---|---|---|---|---|---|
| ultrafine MnO2 nanowires | CC | hydrothermal | MnO2@CC | Weedy 1D ultrafine MnO2 nanowire interconnection network covered on the surface of CC. | [74] |
| MnO2 | g-C3N4 | In situ redox deposition | MnO2/g-C3N4 | flower-like MnO2 nanosheets deposited on g-C3N4, resulting in surface roughness. | [125] |
| MnO2 | 3D Graphene Networks | Electrochemical deposition | 3D Graphene/MnO2 | MnO2 nanoporous structures were uniformly coated on a 3D graphene network skeleton. | [146] |
| α-MnO2 | HMCNTs | Co-precipitating | MnO2/HMCNTs | MnO2 was deposited on the surface of CNTs and provided active sites. | [154] |
| MnO2 | g-C3N4 | Sonochemical | g-C3N4/MnO2 | Different sizes of materials were obtained by ultrasound with different amplitudes. | [169] |
| MnO2 Polyhedron Precursors | Bulk-g-C3N4 nanosheets | Calcination | 3D/2D MnO2/g-C3N4 Nanocomposite | MnO2 was wrapped by the g-C3N4 layers. | [171] |
| MnO2 Nanorods | Mn-modified alkalinized g-C3N4 | Impregnation | Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction | In the process of Mn modifying alkalinized g-C3N4, slender rod-shaped MnO2 was formed. | [172] |
| layered MnOX | GO | hydrothermal | GO/MnOX composites | nanosheets | [75] |
| α-MnO2 nanorods | MWCNTs | direct pyrolysis | MWCNTs/MnO2 nanocomposite | MnO2 nanorods are uniformly attached to the surface of MWCNTs. | [173] |
| Photocatalyst | Target Pollutant | Light Source | Photocatalyst Amount | Initial Concentration | Activity | Ref. |
|---|---|---|---|---|---|---|
| Titanium dioxide-manganese oxide/multi-walled CNT (TiO2-MnO2/ MWCNT) | phenol | UV light 150 W fluorescent lamp | 90 mg | 300 mL 100 mg/L | 40 min 100% | [208] |
| CQDs decorated MnO2 nanorods (MnO2@CQDs) | phenol | visible light | / | 100 mg/L | 50 min 90% | [207] |
| MnO2/g-C3N4 (MG3) | phenol | visible light | 50 mg | 100 mL 5 mg/L | 100 min 98% | [209] |
| 2D g-C3N4/MnO2 heterojunctions (2D g-C3N4/MnO2) | phenol | visible light 300 W Xenon lamp | 50 mg | 50 mL 50 mg/L | 180 min 73.6% | [157] |
| 2D/1D protonated g-C3N4/α-MnO2 (CNM) | phenol | visible light 300 W Xe arc lamp | 40 mg | 80 mL 10 mg/L | 120 min 93.8% | [67] |
| g-C3N4/MnO2/Pt | Phenol; Bisphenol A | Solar source 300 W Xenon lamp | 50 mg 20 mg PMS | 100 mL 20 mg/L | 30 min 20%→57%; 13%→97% | [210] |
| Dye-loaded MnO2 and chlorine-intercalated g-C3N4 (MO/CN-Cl) | Phenol; 2,4-dichlorophenol | visible light 150 W Xe lamp | 200 mg | 50 mL 20 mg/L | 1 h 47%; 1 h 60% | [211] |
| Graphene oxide/MnO2 nanocomposite (rGO/MnO2) | 2-naphthols | visible light 20 W LED | 100 mg | 144 mg | 12 h 97.2% | [193] |
| 3 wt% MnO2 modified exfoliated porous g-C3N4 nanosheet (GM3) | aromatic alcohols | visible light 150 W xenon lamp | / | 20 mL 100 mg/L | 80 min 78% | [212] |
| Photocatalyst | Target Pollutant | Light Source | Photocatalyst Amount | Initial Concentration | Activity | Ref. |
|---|---|---|---|---|---|---|
| Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction (MnO2/CNK-OH-Mn15%) | tetracycline | visible light 300 W Xe lamp | 50 mg | 100 mL 10 mg/L | 120 min 96.7% | [172] |
| Carbon nanosheet/MnO2/BiOCl (Cs/Mn/Bi-1/1) | tetracycline hydrochloride | UV light 300 W mercury lamp | 20 mg | 100 mL 20 mg/L | 30 min 80% | [225] |
| g-C3N4/diatomite/MnO2 | tetracycline hydrochloride | visible light | 30 mg | 100 mL 50 mg/L | 60 min 87% | [226] |
| g-C3N4/MnO2/GO (CMG-10) | tetracycline hydrochloride | visible light 300 W xenon lamp | 50 mg | 100 mL 10 mg/L | 60 min 91.4% | [227] |
| g-C3N4-MnO2 (CMn2) | tetracycline hydrochloride | visible light LED | 30 mg | 75 mL 20 mg/L | 135 min 92.47% | [169] |
| Pumice-loaded rGO@MnO2 PS@rGO@MnO2 | ciprofloxacin | sunlight 300 W xenon lamp | 300 mg | 30 mL 5 mg/L | 6 h 80% | [228] |
| g-C3N4/MnO2/Pt | sulfadiazine | Solar source 300 W Xenon lamp | 50 mg 20 mg PMS | 100 mL 20 mg/L | 30 min 11%→68% | [210] |
| Photocatalyst | Target Pollutant | Light Source | Photocatalyst Amount | Initial Concentration | Activity | Ref. |
|---|---|---|---|---|---|---|
| MnO2/CNT | MB | visible light solar radiation | 20 mg | 50 mL 20 mg/L | 75 min 70% | [239] |
| Cu-doped MnO2/r-GO | MB | visible light 200 W tungsten bulb | 20 mg | 50 mL 5 mg/L | 90 min 86.69% | [240] |
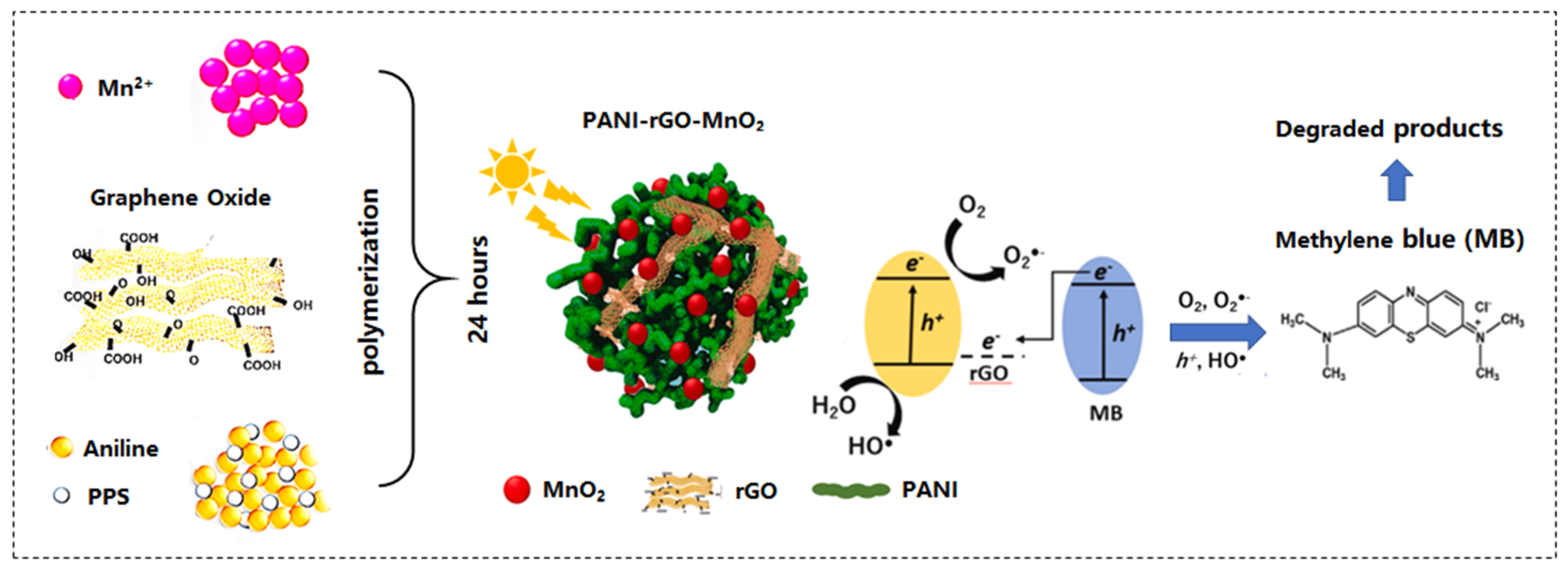
| PANI-rGO-MnO2 | MB | visible light 150 W halogen bulb with Halogen cold light source | 10 mg | 5 mg/L | 120 min 91% | [250] |
| MnO2/BC | MB | 27 °C sunlight 45 °C | 10 mg | 10 mL 10 mg/L | 120 min 85% 97% | [241] |
| α-MnO2 nanowire/activated carbon hollow fibers (MnO2@ACHF) | MB | visible light | 20 mg | 33 mg/L | 240 min 99.8% | [38] |
| poly(3, 4-ethylenedioxythiophene)/GO/MnO2 (PEDOT/GO/MnO2) | MB | UV light sunlight | 20 mg | 50 mL | 7 h 97.1% 7 h 98.9% | [242] |
| graphene nano sheets/CNT/MnO2 (GNS/CNT/MnO2) | MB MG | visible light 400 W metal Philips lamp | 60 mg | 250 mL 60 mg/L | 60 min 71% 60 min 89% | [243] |
| GO@Fe3O4-MnO2 | MG tartrazine | sunlight | 10 mg | 50 mL 10 mg/L | 70 min 99.9% 80 min 98% | [244] |
| Carbon nanosheet/MnO2/BiOCl (Cs/Mn/Bi-1/1) | RhB MB | UV light 300 W mercury lamp | 10 mg | 100 mL 10 mg/L | 25 min 97% 40 min 98% | [225] |
| g-C3N4/diatomite/MnO2 | RhB | visible light | 30 mg | 100 mL 10 mg/L | 50 min 94% | [245] |
| 2D/1D protonated g-C3N4/α-MnO2 (CNM) | RhB | visible light 300 W Xe arc lamp | 40 mg | 80 mL 10 mg/L | 60 min 98.8% | [67] |
| 2D g-C3N4/MnO2 | RhB | visible light 300 W Xenon lamp | 50 mg | 50 mL 10 mg/L | 60 min 91.3% | [157] |
| MnO2@GO (MG 0.4) | RhB | visible light 500 W xenon–mercury lamp | 40 mg | 50 mL 20 mg/L | 65 min 93.86% | [246] |
| g-C3N4/MnO2 (GCN/MnO2) | RhB | sunlight | 4 mg | 20 mL 9.6 mg/L | 90 min 100% | [247] |
| Boron-doped carbon nitrides/MnO2 (BCN/MnO2) | RhB | visible light | 25 mg | 50 mL 10 mg/L | 180 min 61.1% | [248] |
| g-C3N4/MnO2/Pt | RhB MO | Solar source 300 W Xenon lamp | 50 mg 20 mg PMS | 100 mL 20 mg/L | 30 min 99% 30 min 97% | [210] |
| nitrogen-doped grapheme/MnO2 NG-MnO2 | MO | visible light | 5 mg | 5 mL 20 mg/L | 70 min 95% | [77] |
| MnO2/g-C3N4 (MG3) | MO | visible light | 50 mg | 100 mL 5 mg/L | 100 min 92% | [251] |
| Fe3O4/C/MnO2/C3N4 | MO | 400 W metal halide lamp | 20 mg | 20 mL 10 mg/L | 140 min 94.11% | [249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.; Chen, Q.; Zhao, J.; Liu, Y. Preparation of MnO2-Carbon Materials and Their Applications in Photocatalytic Water Treatment. Nanomaterials 2023, 13, 541. https://doi.org/10.3390/nano13030541
Fan K, Chen Q, Zhao J, Liu Y. Preparation of MnO2-Carbon Materials and Their Applications in Photocatalytic Water Treatment. Nanomaterials. 2023; 13(3):541. https://doi.org/10.3390/nano13030541
Chicago/Turabian StyleFan, Kun, Qing Chen, Jian Zhao, and Yue Liu. 2023. "Preparation of MnO2-Carbon Materials and Their Applications in Photocatalytic Water Treatment" Nanomaterials 13, no. 3: 541. https://doi.org/10.3390/nano13030541





