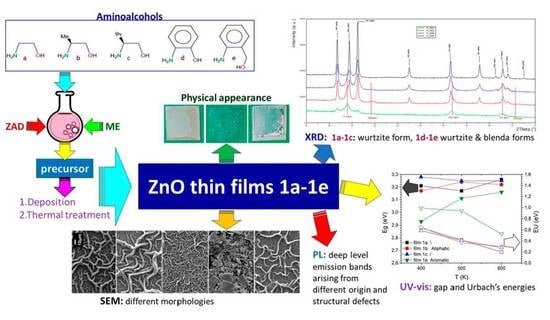
Influence of the Nature of Aminoalcohol on ZnO Films Formed by Sol-Gel Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Characterization Methods
3. Results and Discussion
3.1. Film Characterization
3.1.1. General Appearance and Chemical Analysis
3.1.2. Structural Properties
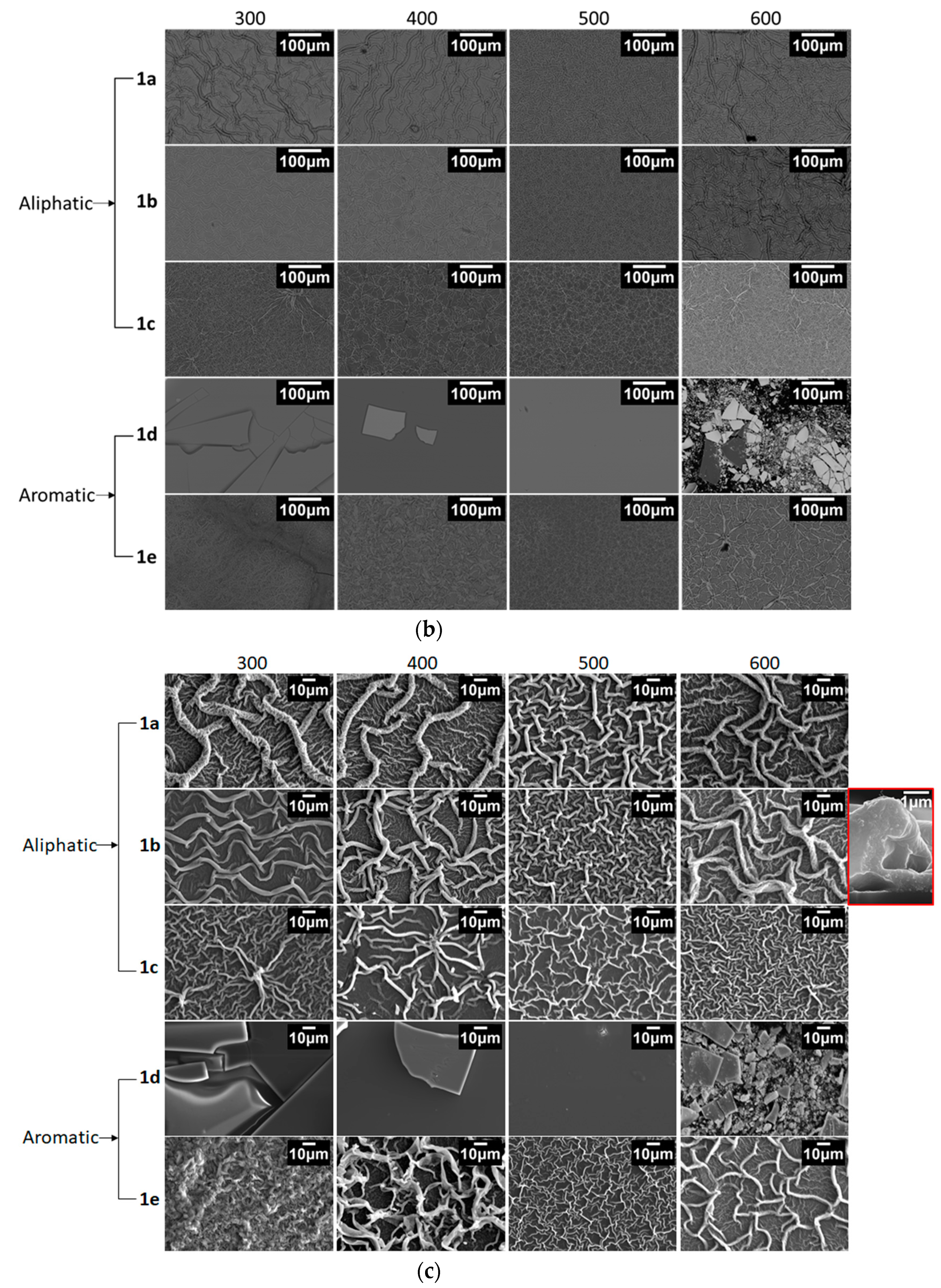
3.1.3. Surface Morphology and Microstructure
3.2. Optical Studies
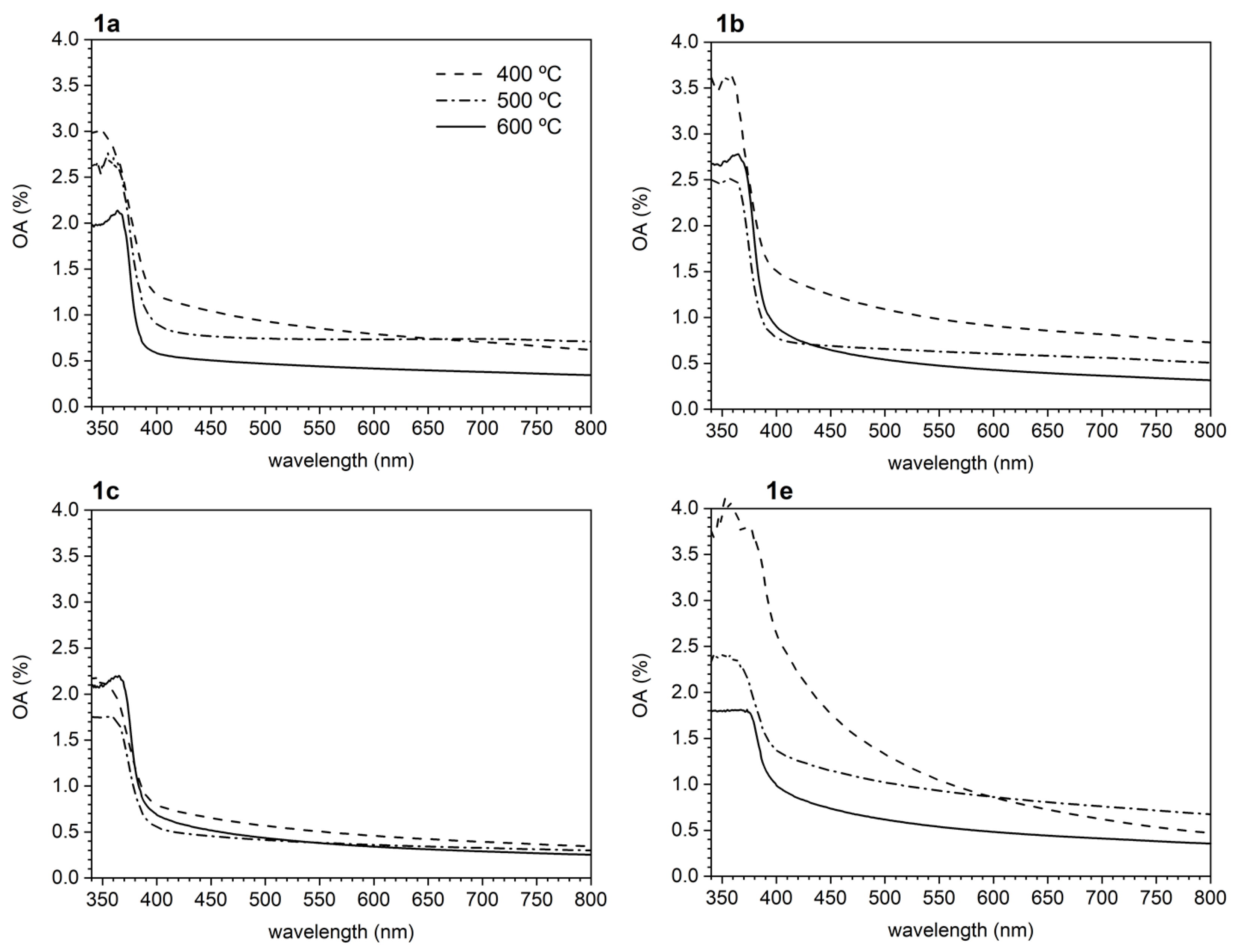
3.2.1. UV-Visible (UV-VIS) Spectroscopy
3.2.2. Photoluminescence (PL)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klingshirn, C.F.; Meyer, B.K.; Waag, A.; Hoffman, A.; Geurts, J. (Eds.) Zinc Oxide: From Fundamental Properties Towards Novel Applications; Springer Series in Materials Sciences n. 120; Springer: Berlin/Heidelberg, Germany, 2010; Chapter 2. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447. [Google Scholar] [CrossRef]
- Fang, X.; Bando, Y.; Gautam, U.K.; Zhai, T.; Zeng, H.; Xu, X.; Liao, M.; Golberg, D. ZnO and ZnS Nanostructures: Ultraviolet-Light Emitters, Lasers, and Sensors. Crit. Rev. Solid State 2009, 34, 190–223. [Google Scholar] [CrossRef]
- Gordon, R.G. Criteria for Choosing Transparent Conductors. MRS Bull. 2000, 25, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Singh, M.; Singh, R.P.P. ZnO Based Diluted Magnetic Semiconductors for Spintronic Device Applications: A review. Int. J. Emerg. Res. Manag. Technol. 2015, 4, 16–20. [Google Scholar]
- Murphy, M.W.; Bovo, L.; Bottaro, G.; Armelao, L.; Sham, T.-K. Electronic and relating behavior of Mn-doped ZnO nanostructures: An x-ray absorption spectroscopy study. AIP Adv. 2021, 11, 065027. [Google Scholar] [CrossRef]
- Srujana, S.; Bhagat, D. Chemical-based synthesis of ZnO nanoparticles and their applications in agriculture. Nanotechnol. Environ. Eng. 2022, 7, 269–275. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basner, P.; Muherjee, J. ZnO based nanomaterials for photo-catalytic degradation of aqueous pharmaceutical waste solutions—A contemporary review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Medina Cruz, D.; Mostafavi1, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: A review. J. Phys. Mater. 2020, 3, 034005. [Google Scholar] [CrossRef]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO nanostructured materials for emerging solar cell applications. RSC Adv. 2020, 42838–42859. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Mele, P. (Ed.) ZnO Thin Films: Properties, Performance and Applications; Mat. Sci. Tech. Ser.; Nova Science Publishers: Hauppauge, NY, USA, 2019; ISBN 978-1-53616-086-4. [Google Scholar]
- Vyas, S. A Short Review on Properties and Applications of Zinc Oxide Based Thin Films and Devices. Johns. Matthey Technol. Rev. 2020, 64, 202–218. [Google Scholar] [CrossRef]
- Muslih, E.Y.; Munir, B. Fabrication of ZnO thin film Through Chemical Preparation. In Emerging Solar Energy Materials; Ameen, S., Akhtar, M.S., Shin, H.-S., Eds.; IntechOpen: London, UK, 2017; Chapter 3. [Google Scholar] [CrossRef] [Green Version]
- Amin, N.; Husna, J.; Alam, M.M. ZnO Thin Films: The Most Potential Semiconductor Material as Buffer Layers in Thin Film Solar Cells. In Two-Dimensional Nanostructures for Energy-Related Applications, 1st ed.; Cheong, K.Y., Ed.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 8. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Al-Dossary, O.; Umar, A. ZnO nanostructured thin films: Depositions, properties and applications—A review. Mater. Express 2015, 5, 3–23. [Google Scholar] [CrossRef]
- Gupta, V.; Sreenivas, K. Pulsed Laser Deposition of Zinc Oxide (ZnO). In Zinc Oxide Bulk, Thin Films and Nanostructures; Jagadish, C., Pearton, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 2; pp. 85–174. [Google Scholar] [CrossRef]
- Ahmadpour, G.; Nilforoushan, M.R.; Boroujeny, B.S.; Tayebi, M.; Jesmani, S.M. Effect of substrate surface treatment on the hydrothermal synthesis of zinc oxide nanostructures. Ceram. Int. 2022, 48, 2323–2329. [Google Scholar] [CrossRef]
- Krajian, H.; Abdallah, B.; Kakhia, M.; AlKafri, N. Hydrothermal growth method for the deposition of ZnO films: Structural, chemical and optical studies. Microelectron. Reliab. 2021, 125, 114352. [Google Scholar] [CrossRef]
- Echendu, O.K.; Werta, S.Z.; Dejene, F.B.; Cracium, V. Electrochemical deposition and characterization of ZnOS thin films for photovoltaic and photocatalysis applications. J. Alloys Compd. 2018, 769, 201–209. [Google Scholar] [CrossRef]
- Biswas, I.; Majumder, M.; Roy (Kundu), P.; Mukherjee, D.; Chakraborty, A.K. Nanostructured ZnO thin film with improved optical and electrochemical properties prepared by hydrothermal electrochemical deposition technique. Micro Nano Lett. 2016, 11, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Alehdaghi, H.; Kazemi, M.; Zirak, M. Facile preparation of ZnO nanostructured thin films via oblique angle ultrasonic mist vapor deposition (OA-UMVD): A systematic investigation. Appl. Phys. A 2020, 126, 103. [Google Scholar] [CrossRef]
- Ayouchi, R.; Leinen, D.; Martın, F.; Gabas, M.; Dalchiele, E.; Ramos-Barrado, J.R. Preparation and characterization of transparent ZnO thin films obtained by spray pyrolysis. Thin Solid Films 2003, 426, 68–77. [Google Scholar] [CrossRef]
- Hosseinnejad, M.T.; Shirazi, M.; Ghoranneviss, M.; Hantehzadeh, M.R. Preparation of Nanostructured ZnO Thin Films Using Magnetron Sputtering for the Gas Sensors Applications. J. Inorg. Organomet. Polym. 2016, 26, 405–412. [Google Scholar] [CrossRef]
- Rini, A.S.; Nabilla, A.; Rati, Y. Microwave-assisted biosynthesis and characterization of ZnO film for photocatalytic application in methylene blue degradation. Commun. Sci. Technol. 2021, 6, 69–73. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mat. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Valverde Aguilar, G.; Fonseca, M.R.J.; Mantilla Ramírez, Á.; Juárez Gracia, A.G. Photoluminescence Studies on ZnO Thin Films Obtained by Sol-Gel Method. In Recent Applications in Sol-Gel Synthesis; Chandra, U., Ed.; IntechOpen: London, UK, 2017; Chapter 9. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Fang, L.; Ruan, H.B.; Wu, F.; Huang, Q.L.; Xu, C.L.; Kong, C.Y. Effect of zinc acetate concentration on the structural and optical properties of ZnO thin films deposited by sol-gel method. Int. J. Phys. Sci. 2021, 7, 2971–2979. [Google Scholar] [CrossRef]
- Khan, Z.R.; Khan, M.S.; Zulfequar, M.; Khan, M.S. Optical and Structural Properties of ZnO Thin Films Fabricated by Sol-Gel Method. Mater. Sci. Appl. 2011, 2, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Amari, R.; Mahroug, A.; Boukhari, A.; Deghfel, B.; Selmi, N. Structural, Optical and Luminescence Properties of ZnO Thin Films Prepared by Sol-Gel Spin-Coating Method: Effect of Precursor Concentration. Chin. Phys. Lett. 2018, 35, 016801. [Google Scholar] [CrossRef]
- Gómez-Núñez, A.; López, C.; Alonso-Gil, S.; Roura, P.; Vilà, A. Study of a sol-gel precursor and its evolution towards ZnO. Mater. Chem. Phys. 2015, 162, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Núñez, A.; López, C.; Alonso-Gil, S.; Roura, P.; Vilà, A. Role of ethanolamine on the stability of a sol-gel ZnO ink. J. Phys. Chem. C 2017, 121, 23839–23846. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Núñez, A.; Alonso-Gil, S.; López, C.; Roura, P.; Vilà, A. From ethanolamine precursor towards ZnO: How N is released from the experimental and theoretical points of view. Nanomaterials 2019, 9, 1415. [Google Scholar] [CrossRef] [Green Version]
- Vajargah, P.H.; Abdizadeh, H.; Ebrahimifard, R.; Golobostanfard, M.R. Sol-gel derived ZnO thin films: Effect of amino-additives. Appl. Surf. Sci. 2013, 285P, 732. [Google Scholar] [CrossRef]
- Chu, M.C.; You, H.C.; Meena, J.; Shieh, S.H.; Shao, C.Y.; Chang, F.C.; Ko, F.H. Facile electroless deposition of zinc oxide ultrathin film for zinc acetate solution-processed transistors. Int. J. Electrochem. Sc. 2012, 7, 5977–5989. [Google Scholar]
- Speaks, D.T. Effect of concentration, aging, and annealing on sol gel ZnO and Al-doped ZnO thin films. Int. J. Mech. Mater. Eng. 2020, 15, 2. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci.-Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Gómez-Núñez, A.; Roura, P.; López, C.; Vilà, A. Comparison of the thermal decomposition processes of several aminoalcohol-based ZnO inks with one containing ethanolamine. Appl. Surf. Sci. 2016, 381, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Núñez, A.; Alonso-Gil, S.; López, C.; Vilà, A. Electronic and dynamic DFT studies on the substituent effects of aminoalcohol stabilizers in sol-gel ZnO precursor. Phys. Status Solidi A 2016, 213, 2329–2335. [Google Scholar] [CrossRef]
- Sypniewska, M.; Szczesny, R.; Popielarski, P.; Strzalkowski, K.; Derkowska-Zielinska, B. Structural, morphological and photoluminescent properties of annealed ZnO thin layers obtained by the rapid sol-gel spin-coating method. Opto-Electron. Rev. 2020, 28, 182–190. [Google Scholar] [CrossRef]
- Nowak, E.; Chlopocka, E.; Szybowicz, M.; Stachowiak, A.; Koczorowski, W.; Piechowiak, D.; Miklaszewski, A. The influence of aminoalcohols on ZnO films’ structure. Gels 2022, 8, 512. [Google Scholar] [CrossRef]
- Szczesny, R.; Scigala, A.; Derkowska-Zielinska, B.; Skowronski, L.; Cassagne, C.; Boudebs, G.; Viter, R.; Szlyk, E. Synthesis, optical, and morphological studies of ZnO powders and thin films fabricated by wet chemical methods. Materials 2020, 13, 2559. [Google Scholar] [CrossRef]
- Minakshi, M.; Appadoo, D.; Martin, D.E. The anodic behavior of planar and porous zinc electrodes in alkaline electrolyte. Electrochem. Solid-State Lett. 2010, 13, A77–A80. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Coelho, A.A.; Evans, J.; Evans, I.; Kern, A.; Parsons, S. The TOPAS symbolic computation system. Powder Diffr. 2011, 26, S22–S25. [Google Scholar] [CrossRef]
- Balzar, D.; Audebrand, N.; Daymond, M.R.; Fitch, A.; Hewat, A.; Langford, J.I.; Le Bail, A.; Louer, D.; Masson, P.; McCowan, C.N.; et al. Size–strain line-broadening analysis of the ceria round-robin sample. J. Appl. Crystallogr. 2004, 37, 911–924. [Google Scholar] [CrossRef]
- Whitfield, P.S. Spherical harmonics preferential orientation corrections and structure solution from powder diffraction data—A possible avenue of last resort. J. Appl. Crystallogr. 2009, 42, 134–136. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A.A.; Cline, J.P. Fundamental Parameters Line Profile Fitting in Laboratory Diffractometers. J. Res. Natl. Inst. Stand. Technol. 2007, 109, 1. [Google Scholar] [CrossRef]
- Tokumoto, M.S.; Briois, V.; Santilli, C.V.; Pulcinelli, S.H. Preparation of ZnO Nanoparticles: Structural Study of the Molecular Precursor. J. Sol-Gel. Sci. Technol. 2003, 26, 547–551. [Google Scholar] [CrossRef]
- Kalsi, P.S. Spectroscopy of Organic Compounds, 6th ed.; New Age International Publishers Ltd.: Delhi, India, 2004; Chapter 3; pp. 65–184. [Google Scholar]
- Baizar, D.; Ledbetter, H. Accurate Modeling of Size and Strain Broadening in the Rietveld Refinement: The “Double-Voigt” Approach. Adv. X-ray Anal. 1994, 38, 397–404. [Google Scholar] [CrossRef]
- Amakali, T.; Daniel, L.S.; Uahengo, L.S.; Dzade, N.Y.; de Leeuw, N.H. Structural and Optical Properties of ZnO Thin Films Prepared by Molecular Precursor and Sol–Gel Methods. Crystals 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Nesheva, D.; Dzhurkov, V.; Stambolova, I.; Blaskov, V.; Bineva, I.; Calderon Moreno, I.; Preda, J.S.; Gartner, M.; Hristova-Vasileva, T.; Shipochka, M. Surface modification and chemical sensitivity of sol gel deposited nanocrystalline ZnO films. Mater. Chem. Phys. 2018, 209, 165–171. [Google Scholar] [CrossRef]
- Bish, D.L.; Howard, S.A. Quantitativ Analysis using the Rietveld method. J. Appl. Crystallogr. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Muñoz-Aguirre, N.; Martínez-Pérez, L.; Muñoz-Aguirre, S.; Flores-Herrera, L.A.; Vergara Hernández, E.; Zelaya-Angel, O. Luminescent properties of (004) highly oriented cubic zinc blende ZnO thin films. Materials 2019, 12, 3314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dijken, A.; Meulenkamp, E.A.; Vanmaekkelberh, D.; Meijerink, A. The luminescence of nanocrystalline ZnO particles: The mechanism of the ultraviolet and visible emission. J. Lumin. 2000, 87–89, 454–456. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ghobadi, N. Band gap determination using absorption spectrum fitting procedure. Int. Nano Lett. 2013, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Panigrahy, B.; Bahadur, D. P-type phosphorus doped ZnO nanostructures: An electrical, optical and magnetic properties study. RSC Adv. 2012, 2, 6222–6227. [Google Scholar] [CrossRef]
- Caglar, M.; Ilican, S.; Caglar, Y. Influence of dopant concentration on the optical properties of ZnO:In films by sol-gel method. Thin Solid Films 2009, 517, 5023–5028. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today-Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Chen, X.; Xie, Q.; Li, J. Significantly improved photoluminescence properties of ZnO thin films by lithium doping. Ceram. Int. 2020, 46, 2309–2316. [Google Scholar] [CrossRef]
- Quy, C.T.; Thai, N.X.; Hoa, N.D.; Le, D.T.T.; Hung, C.M.; Duy, N.V.; Hieu, N.V. C2H5OH and NO2 sensing properties of ZnO nanostructures: Correlation between crystal size, defect level and sensing performance. RSC Adv. 2018, 8, 5629. [Google Scholar] [CrossRef] [Green Version]
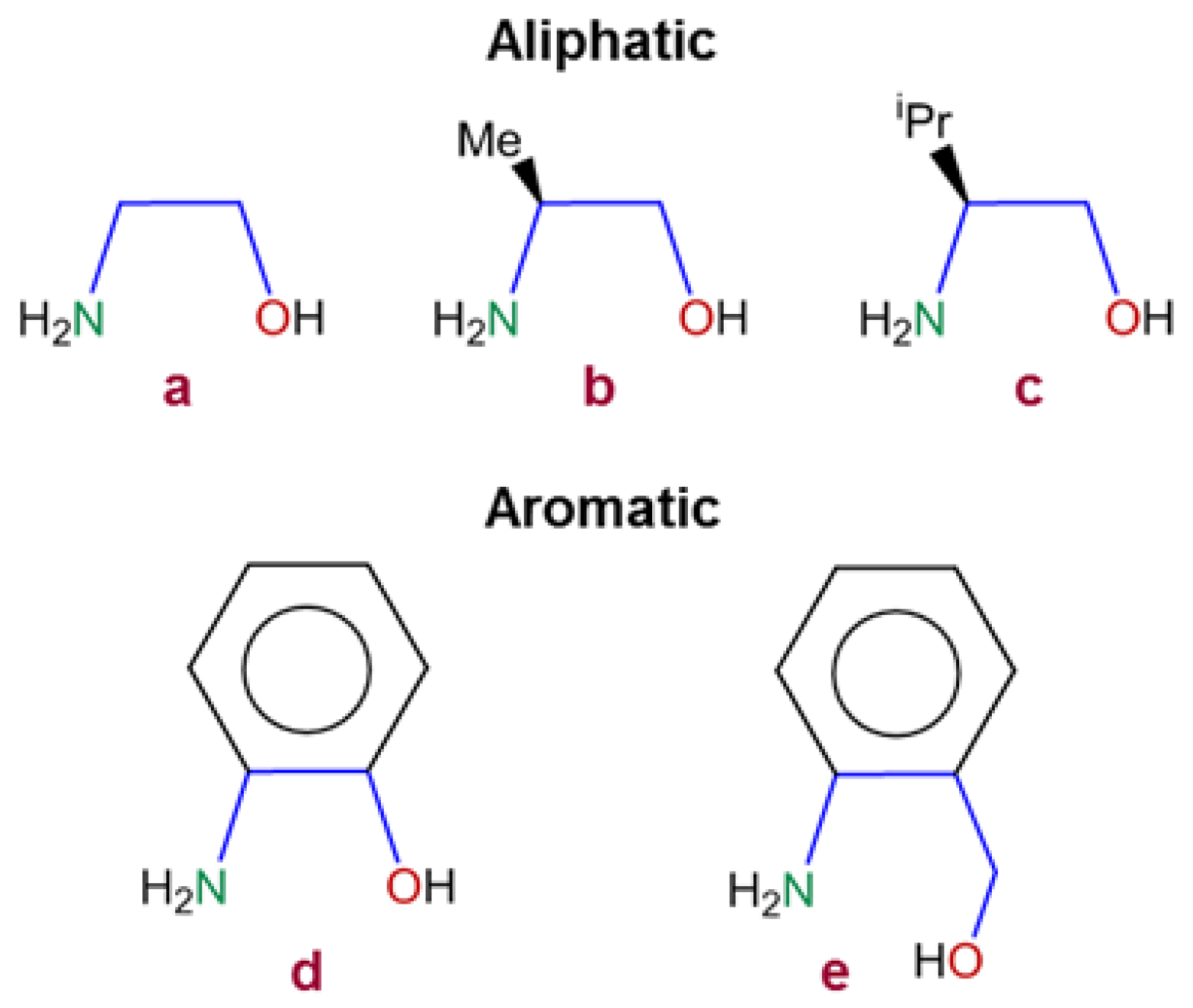







| Aminoalcohol | Film | Elemental Analyses (%) | Temperature (°C) | |||
|---|---|---|---|---|---|---|
| 300 | 400 | 500 | 600 | |||
| Aliphatic | 1a | N | 1.23 | 1.34 | 0.06 | 0.01 |
| C | 6.90 | 6.89 | 2.94 | 0.24 | ||
| H | 0.91 | 0.53 | 0.53 | 0.00 | ||
| 1b | N | 1.32 | 1.01 | 0.04 | 0.01 | |
| C | 11.96 | 5.83 | 0.80 | 0.27 | ||
| H | 1.28 | 0.72 | 0.13 | 0.00 | ||
| 1c | N | 0.68 | 0.58 | 0.09 | 0.01 | |
| C | 12.28 | 5.80 | 3.64 | 0.41 | ||
| H | 1.34 | 0.00 | 0.73 | 0.00 | ||
| Aromatic | 1d | N | 5.18 | 4.49 | 3.03 | 0.01 |
| C | 42.23 | 33.27 | 20.70 | 0.71 | ||
| H | 3.21 | 2.35 | 1.88 | 0.00 | ||
| 1e | N | 2.24 | 4.43 | 0.57 | 0.43 | |
| C | 32.16 | 29.32 | 7.14 | 3.49 | ||
| H | 3.54 | 2.03 | 0.86 | 0.00 | ||
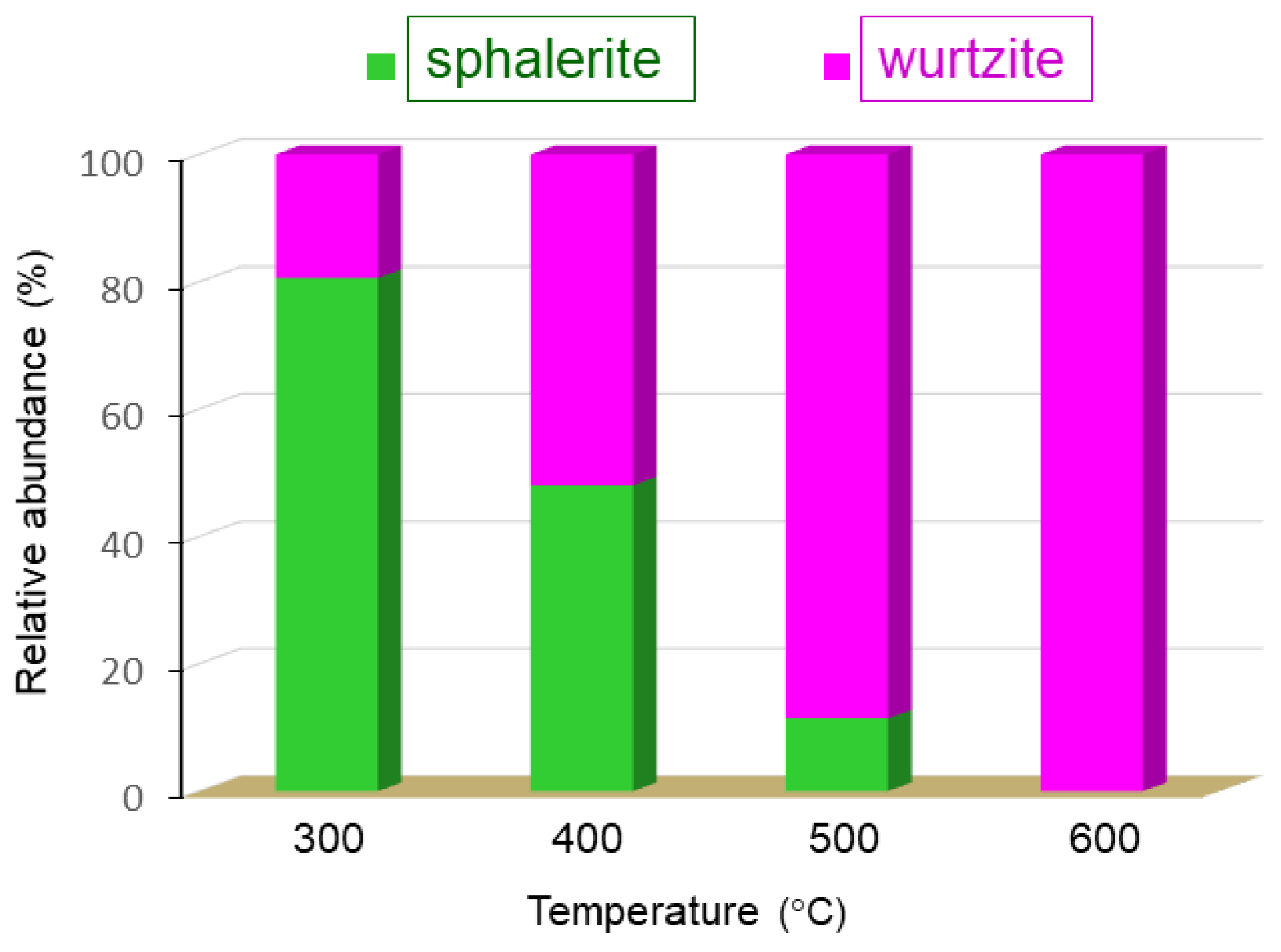
| Aminoalcohol | T | a | c | c/a | Phase, % | D | ε × 10−3 | |
|---|---|---|---|---|---|---|---|---|
| Aliphatic | 1a | 400 | 3.2398 | 5.2410 | 1.618 | wurtzite, 100 | 12.6 | 2.8 |
| 500 | 3.2485 | 5.2096 | 1.604 | 14.4 | 0.3 | |||
| 600 | 3.2494 | 5.2079 | 1.603 | 34.4 | 0.6 | |||
| 1b | 400 | 3.2453 | 5.2319 | 1.612 | wurtzite, 100 | 12.7 | 2.2 | |
| 500 | 3.2487 | 5.2088 | 1.603 | 16.7 | 0.3 | |||
| 600 | 3.2495 | 5.2065 | 1.602 | 37.6 | 0.6 | |||
| 1c | 400 | 3.2418 | 5.2423 | 1.617 | wurtzite, 100 | 12.8 | 2.7 | |
| 500 | 3.2467 | 5.2125 | 1.605 | 10.3 | 0.4 | |||
| 600 | 3.2490 | 5.2075 | 1.603 | 32.0 | 0.6 | |||
| Aromatic | 1d | 400 | 3.2526 4.5656 | 5.2105 - | 1.602 - | wurtzite, 77.5 sphalerite, 22.5 | 26.1 10.6 | 0.1 - |
| 500 | 3.2493 | 5.2083 | 1.603 | wurtzite, 100.0 | 10.5 | 0.0 | ||
| 600 | 3.2490 | 5.2066 | 1.603 | wurtzite, 100.0 | 45.8 | 0.3 | ||
| 1e | 400 | 3.2494 4.5687 | 5.2103 - | 1.603 - | wurtzite, 52.2 sphalerite, 47.8 | 25.3 8.3 | 0.3 - | |
| 500 | 3.2488 4.5632 | 5.2137 - | 1.605 - | wurtzite, 88.6 sphalerite, 11.4 | 12.1 4.7 | 0.0 0.0 | ||
| 600 | 3.2488 | 5.2061 | 1.602 | wurtzite, 100.0 | 30.4 | 0.5 | ||
| Aminoalcohol | Film | Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|
| 400 | 500 | 600 | |||||
| Eg | EU | Eg | EU | Eg | EU | ||
| Aliphatic | 1a | 3.21 | 0.64 | 3.17 | 0.42 | 3.26 | 0.22 |
| 1b | 3.17 | 0.65 | 3.24 | 0.37 | 3.22 | 0.30 | |
| 1c | 3.28 | 0.59 | 3.25 | 0.41 | 3.25 | 0.28 | |
| Aromatic | 1e | 2.93 | 0.99 | 3.11 | 0.94 | 3.16 | 0.53 |
| Aminoalcohol | Film | Photoluminescent Band | Origin | |||
|---|---|---|---|---|---|---|
| Center (nm) | FWHM (nm) | Area (a.u.) | % | |||
| Aliphatic | 1a | 570.0 | 51.2 | 6.94 | 10.5 | VO or VO+ |
| 627.3 | 84.2 | 33.92 | 51.0 | VO++ | ||
| 687.6 | 59.6 | 10.92 | 16.4 | Oi | ||
| 714.4 | 17.1 | 1.07 | 1.6 | Oi | ||
| 750.3 | 83.2 | 13.61 | 20.5 | Oi | ||
| 1b | 534.5 | 46.6 | 1.27 | 2.2 | VO | |
| 601.5 | 115.9 | 24.97 | 43.6 | VO+ | ||
| 680.3 | 57.5 | 1.26 | 2.2 | Oi | ||
| 710.5 | 28.5 | 2.78 | 4.8 | Oi | ||
| 742.1 | 99.2 | 27.04 | 47.2 | Oi | ||
| 1c | 593.3 | 56.8 | 2.78 | 4.9 | VO+ | |
| 593.3 | 125.7 | 12.45 | 22.2 | VO+ | ||
| 658.2 | 89.97 | 14.63 | 26.0 | VO++ | ||
| 724.9 | 277.6 | 21.58 | 38.4 | Oi | ||
| 763.4 | 69.4 | 4.76 | 8.5 | Oi | ||
| Aromatic | 1e | 574.0 | 89.1 | 14.67 | 20.7 | VO+ |
| 596.9 | 17.9 | 0.32 | 0.4 | VO+ | ||
| 628.3 | 41.8 | 2.55 | 3.6 | VO++ | ||
| 676.3 | 83.2 | 11.19 | 15.8 | Oi | ||
| 696.6 | 200.7 | 42.19 | 59.5 | Oi | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilà, A.; Gómez-Núñez, A.; Alcobé, X.; Palacios, S.; Puig Walz, T.; López, C. Influence of the Nature of Aminoalcohol on ZnO Films Formed by Sol-Gel Methods. Nanomaterials 2023, 13, 1057. https://doi.org/10.3390/nano13061057
Vilà A, Gómez-Núñez A, Alcobé X, Palacios S, Puig Walz T, López C. Influence of the Nature of Aminoalcohol on ZnO Films Formed by Sol-Gel Methods. Nanomaterials. 2023; 13(6):1057. https://doi.org/10.3390/nano13061057
Chicago/Turabian StyleVilà, Anna, Alberto Gómez-Núñez, Xavier Alcobé, Sergi Palacios, Teo Puig Walz, and Concepción López. 2023. "Influence of the Nature of Aminoalcohol on ZnO Films Formed by Sol-Gel Methods" Nanomaterials 13, no. 6: 1057. https://doi.org/10.3390/nano13061057