Bioactivity Enhancement of Plasma-Sprayed Hydroxyapatite Coatings through Non-Contact Corona Electrical Charging
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Corona Triode
2.2. Sample Preparation
2.3. Structural and Morphologic Characterization
2.4. In Vitro Biological Tests
2.4.1. Simulated Body Fluid (SBF) Immersion Tests
2.4.2. Osteoblasts—Sample Interaction Tests
Osteoblastic Proliferation
Morphology and Vinculin Expression
2.5. Photoelectron Emission Spectroscopy
3. Results and Discussion
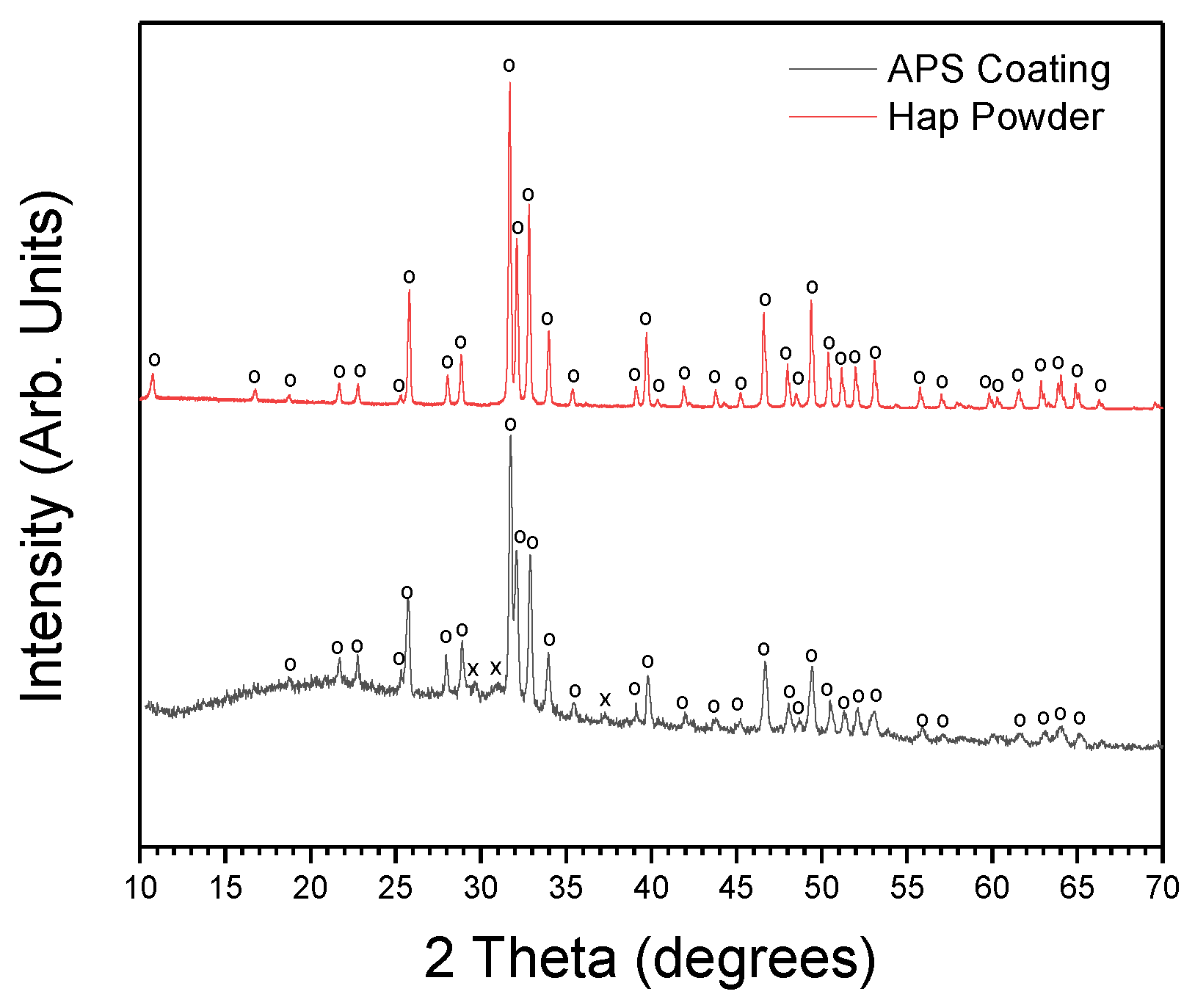
3.1. Structural and Morphological Analysis
3.2. Corona Triode Charging Experiments
3.3. In Vitro Biological Results
3.3.1. SBF Immersion Tests
3.3.2. Osteoblasts—Sample Interaction Tests
Osteoblastic Proliferation and Vinculin Expression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heimann, R.B.; Lehmann, H.D. Bioceramic Coatings for Medical Implants: Trends and Techniques; Wiley: Hoboken, NJ, USA, 2015; p. 496. [Google Scholar] [CrossRef]
- McCabe, A.; Pickford, M.; Shawcross, J. The history, technical specifications and efficacy of plasma spray coatings applied to joint replacement prostheses. Reconstr. Rev. 2016, 6, 4. [Google Scholar] [CrossRef]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Hutkin, E. Hip Replacements Are on the Rise. 2015. Available online: http://www.sandiegouniontribune.com/news/health/sdut-hip-replacements-increasing-2015mar10-story.html (accessed on 22 December 2022).
- Losina, E.; Katz, J.N. Total knee arthroplasty on the rise in younger patients: Are we sure that past performance will guarantee future success? Arthritis Rheum. 2012, 64, 339–341. [Google Scholar] [CrossRef]
- Schreurs, B.W.; Hannink, G. Total joint arthroplasty in younger patients: Heading for trouble? Lancet 2017, 389, 1374–1375. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.W.J.L.; Timmer, C.; Hannink, G.; Schreurs, B.W. Systematic review: Lack of evidence for the success of revision arthroplasty outcome in younger patients. Hip. Int. 2018, 28, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H. Osteoblasts, osteoclasts, and osteocytes: Unveilling their intimate-associated responses to applied orthodontic forces. Semin. Orthod. 2012, 18, 237–248. [Google Scholar] [CrossRef]
- Nakamura, S.; Kobayashi, T.; Nakamura, M.; Yamashita, K. Enhanced in vivo responses of osteoblasts in electrostatically activated zones by hydroxyapatite electrets. J. Mater. Sci.-Mater. M 2009, 20, 99–103. [Google Scholar] [CrossRef]
- Sagawa, H.; Itoh, S.; Wang, W.; Yamashita, K. Enhanced bone bonding of the hydroxyapatite/β-tricalcium phosphate composite by electrical polarization in rabbit long bone. Artif. Organs 2010, 34, 491–497. [Google Scholar] [CrossRef]
- Nakamura, S.; Kobayashi, T.; Yamashita, K. Numerical osteobonding evaluation of electrically polarized hydroxyapatite ceramics. J. Biomed. Mater. Res. A 2004, 68, 90–94. [Google Scholar] [CrossRef]
- Bodhak, S.; Bose, S.; Bandyopadhyay, A. Electrically polarized HAp-coated Ti: In vitro bone cell-material interactions. Acta Biomater. 2010, 6, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, N.; Nakamura, M.; Nagai, A.; Katayama, K.; Yamashita, K. Proton conduction related electrical dipole and space charge polarization in hydroxyapatite. J. Appl. Phys. 2012, 112, 074901. [Google Scholar] [CrossRef]
- Prezas, P.R.; Dekhtyar, Y.; Sorokins, H.; Costa, M.M.; Soares, M.J.; Graça, M.P.F. Electrical charging of bioceramics by corona discharge. J. Electrost. 2022, 115, 103664. [Google Scholar] [CrossRef]
- Giacometti, J.A.; Campos, J.S.C. Constant current corona triode with grid voltage control. Application to polymer foil charging. Rev. Sci. Instrum. 1990, 61, 1143–1150. [Google Scholar] [CrossRef]
- Giacometti, J.A.; Fedosov, S.; Costa, M.M. Corona charging of polymers: Recent advances on constant current charging. Braz. J. Phys. 1999, 29, 269–279. [Google Scholar] [CrossRef]
- Giacometti, J.A. Constant-current corona triode adapted and optimized for the characterization of thin dielectric films. Rev. Sci. Instrum. 2018, 89, 055109. [Google Scholar] [CrossRef]
- Giacometti, J.A.; Ribeiro, P.A.; Raposo, M.; Marat-Mendes, J.N.; Campos, J.S.C.; DeReggi, A.S. Study of poling behavior of biaxially stretched poly(vinylidene fluoride) films using the constant-current corona triode. J. Appl. Phys. 1995, 78, 5597. [Google Scholar] [CrossRef]
- Ferreira, G.F.L.; Figueiredo, M.T. Corona charging of electrets: Models and results. IEEE Trans. Dielectr. Electr. Insul. 1992, 27, 719–738. [Google Scholar] [CrossRef]
- Silva, C.C.; Valente, M.A.; Graça, M.P.F.; Sombra, A.S.B. Preparation and optical characterization of hydroxyapatite and ceramic systems with titanium and zirconium formed by dry high-energy mechanical alloying. Solid State Sci. 2004, 6, 1365–1374. [Google Scholar] [CrossRef]
- Silva, C.C.; Valente, M.A.; Graça, M.P.F.; Sombra, A.S.B. The modulus formalism used in the dielectric analysis of hydroxyapatite and calcium phosphate with titanium formed by dry ball milling. J. Non-Cryst. Solids 2005, 351, 2945–2950. [Google Scholar] [CrossRef]
- Silva, C.C.; Graça, M.P.F.; Valente, M.A.; Sombra, A.S.B. Crystallite size study of nanocrystalline hydroxyapatite and ceramic system with titanium oxide obtained by dry ball milling. J. Mat. Sci. 2007, 42, 3851–3855. [Google Scholar] [CrossRef]
- Liu, D.-M.; Troczynski, T.; Tseng, W.J. Water-based sol-gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–17302001. [Google Scholar] [CrossRef]
- Le, H.R.; Chen, K.Y.; Wang, C.A. Effect of pH and temperature on the morphology and phases of co-precipitated hydroxyapatite. J. Sol.-Gel. Sci. Tech. 2012, 61, 592–599. [Google Scholar] [CrossRef]
- Akmene, R.J.; Balodis, A.J.; Dekhtyar, Y.; Markelova, G.N.; Matvejevs, J.V. Exoelectron emission spectrometer complete set of surface local investigation. Phys. Chem. Mech. Surf. 1993, 8, 125–128. [Google Scholar]
- Dunne, C.F.; Twomey, B.; O’Neill, L.; Stanton, K.T. Co-blasting of titanium surfaces with an abrasive and hydroxyapatite to produce bioactive coatings: Substrate and coating characterization. J. Biomater. Appl. 2014, 28, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Ardhaoui, M.; Benyounis, K.; Looney, L.; Stokes, J.T. Plasma sprayed hydroxyapatite coatings: Understanding process relationships using design of experiment analysis. Surf. Coat. Technol. 2015, 283, 29–36. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Ferreira da Silva, M.G.; Sombra, A.S.B.; Valente, M.A. Electrical characterization of SiO2:LiNbO3 glass and glass-ceramics using dc conductivity, TSDC measurements and dielectric spectroscopy. J. Non-Cryst. Solids 2007, 353, 4390–4394. [Google Scholar] [CrossRef]
- Oliveira, O.N., Jr.; Ferreira, G.F.L. Electron transport in corona charged 12 μm teflon FEP with saturable deep traps. Appl. Phys. A 1987, 42, 213–217. [Google Scholar] [CrossRef]
- Sessler, G.M. Poling and properties of polarization of ferroelectric polymers and composites. Key Eng. Mater. 1994, 92–93, 249–274. [Google Scholar] [CrossRef]
- Das, A.; Pamu, D. A comprehensive review on electrical properties of hydroxyapatite based ceramic composites. Mater. Sci. Eng. C 2019, 101, 539–563. [Google Scholar] [CrossRef]
- Gross, B.; Giacometti, J.A.; Ferreira, G.F.L. Charge storage and transport in electron-irradiated and corona-charged dielectrics. IEEE Trans. Nucl. Sci. 1981, 28, 4153–4522. [Google Scholar] [CrossRef]
- Prezas, P.R.; Melo, B.M.G.; Costa, L.C.; Valente, M.A.; Lança, M.C.; Ventura, J.M.G.; Pinto, L.F.V.; Graça, M.P.F. TSDC and impedance spectroscopy measurements on hydroxyapatite, β-tricalcium phosphate and hydroxyapatite/β-tricalcium phosphate biphasic bioceramics. Appl. Surf. Sci. 2017, 424 Pt 1, 28–38. [Google Scholar] [CrossRef]
- Seah, M.P.; Dench, W.A. Quantitative electron spectroscopy of surfaces: A standard data base for electron inelastic mean free paths in solids. Surf. Interface Anal. 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayed hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef]
- Gil, F.J.; Padrós, A.; Manero, J.M.; Aparicio, C.; Nilsson, M.; Planell, J.A. Growth of bioactive surfaces on titanium and its alloys for orthopaedic and dental implants. Mater. Sci. Eng. C 2002, 22, 53–60. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Kim, H.-M.; Himeno, T.; Kawashita, M.; Kokubo, T.; Nakamura, T. The mechanism of biomineralization of bone-like apatite on synthetic hydroxyapatite: An in vitro assessment. J. R. Soc. Interface 2004, 1, 17–22. [Google Scholar] [CrossRef]
- Wang, D.; Chen, C.; He, T.; Lei, T. Hydroxyapatite coating on Ti6Al4V alloy by a sol-gel method. J. Mater. Sci. Mater. Med. 2008, 19, 2281–2286. [Google Scholar] [CrossRef]
- Xuhui, Z.; Lingfang, Y.; Yu, Z.; Jinpinh, X. Hydroxyapatite coatings on titanium prepared by electrodeposition in a modified simulated body fluid. Chin. J. Chem. Eng. 2009, 17, 667–671. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, Y.; Ma, Y.; Fan, K.; Yang, W.; Yin, G. Nano-hydroxyapatite reinforced polyphenylene sulfide biocomposite with superior cytocompatibility and in vivo osteogenesis as a novel orthopedic implant. R. Soc. Chem. 2017, 7, 559–573. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Cho, S.; Cho, K. Porous hollow hydroxyapatite microspheres synthesized synthesized by spray pyrolysis using a microalga template: Preparation, drug delivery, and bioactivity. R. Soc. Chem. 2016, 6, 43041–43048. [Google Scholar] [CrossRef]







| −1 nA | −3.5 nA | |
|---|---|---|
| Work function (eV) | 4.83 | 4.76 |
| Standard deviation (eV) | 0.04 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prezas, P.R.; Soares, M.J.; Borges, J.P.; Silva, J.C.; Oliveira, F.J.; Graça, M.P.F. Bioactivity Enhancement of Plasma-Sprayed Hydroxyapatite Coatings through Non-Contact Corona Electrical Charging. Nanomaterials 2023, 13, 1058. https://doi.org/10.3390/nano13061058
Prezas PR, Soares MJ, Borges JP, Silva JC, Oliveira FJ, Graça MPF. Bioactivity Enhancement of Plasma-Sprayed Hydroxyapatite Coatings through Non-Contact Corona Electrical Charging. Nanomaterials. 2023; 13(6):1058. https://doi.org/10.3390/nano13061058
Chicago/Turabian StylePrezas, Pedro R., Manuel J. Soares, João P. Borges, Jorge C. Silva, Filipe J. Oliveira, and Manuel Pedro F. Graça. 2023. "Bioactivity Enhancement of Plasma-Sprayed Hydroxyapatite Coatings through Non-Contact Corona Electrical Charging" Nanomaterials 13, no. 6: 1058. https://doi.org/10.3390/nano13061058








