Synaptic Plasticity Modulation of Neuromorphic Transistors through Phosphorus Concentration in Phosphosilicate Glass Electrolyte Gate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Specifications
2.2. Synthesis of PSG Electrolyte Films
2.3. Fabrication of PSG Electrolyte-Based EDLT
2.4. Method of Characterizations
3. Results and Discussion
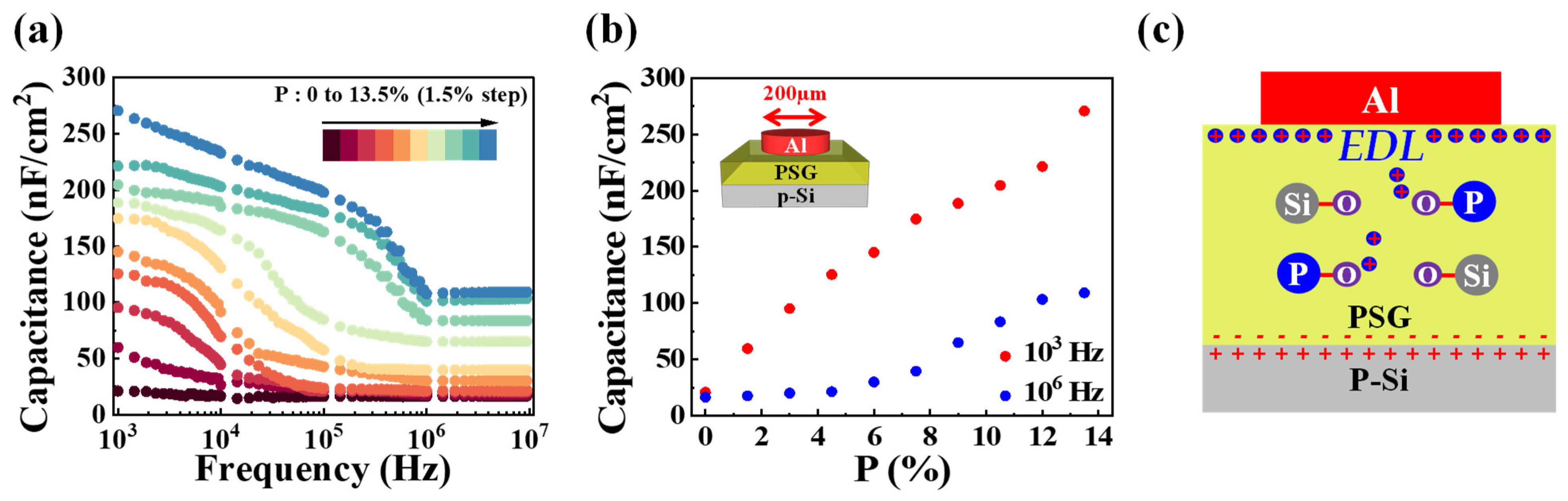
3.1. Electrical Characteristics of PSG-Based MOS Capacitors
3.2. EDL Operation of PSG Films for Synaptics
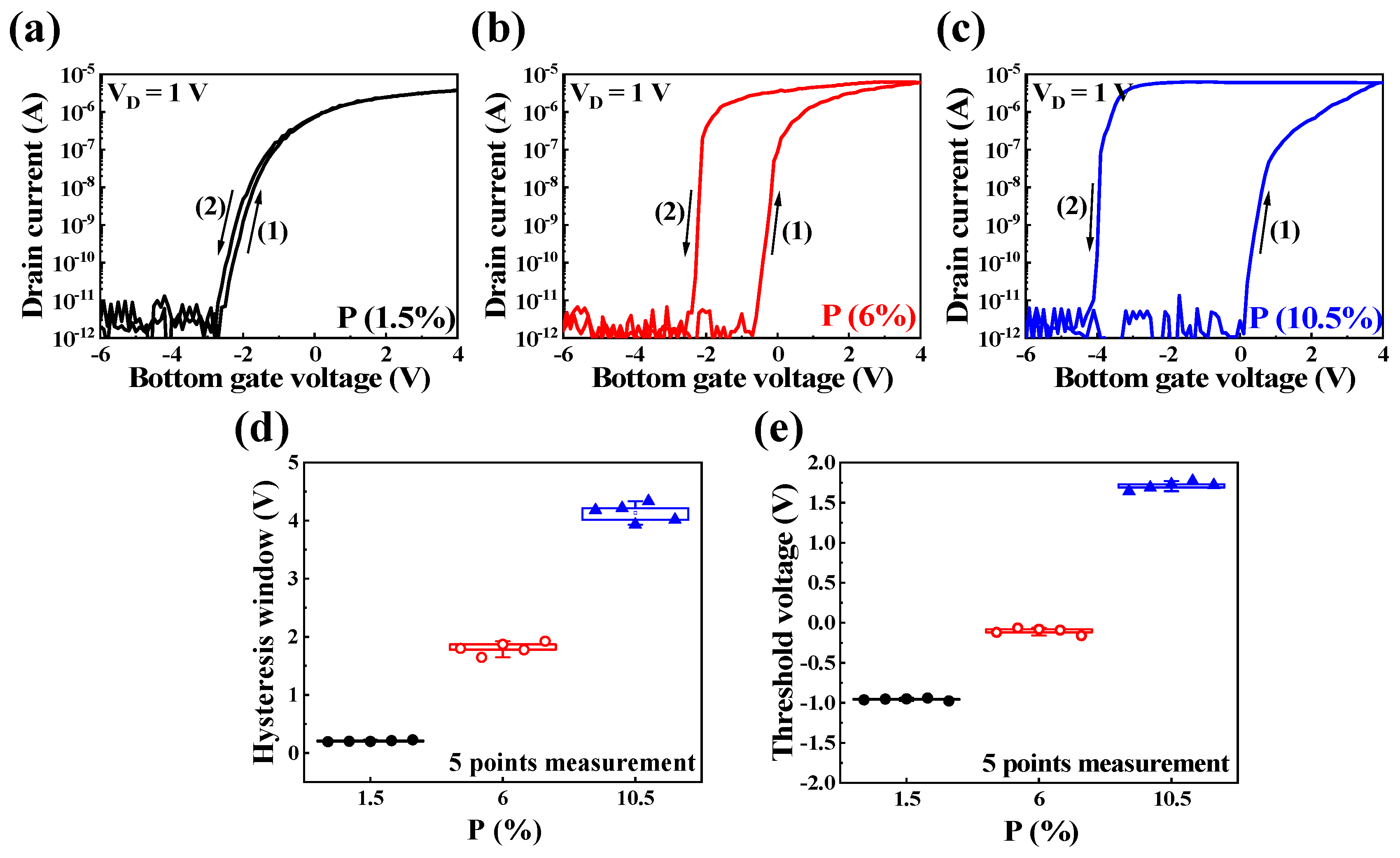
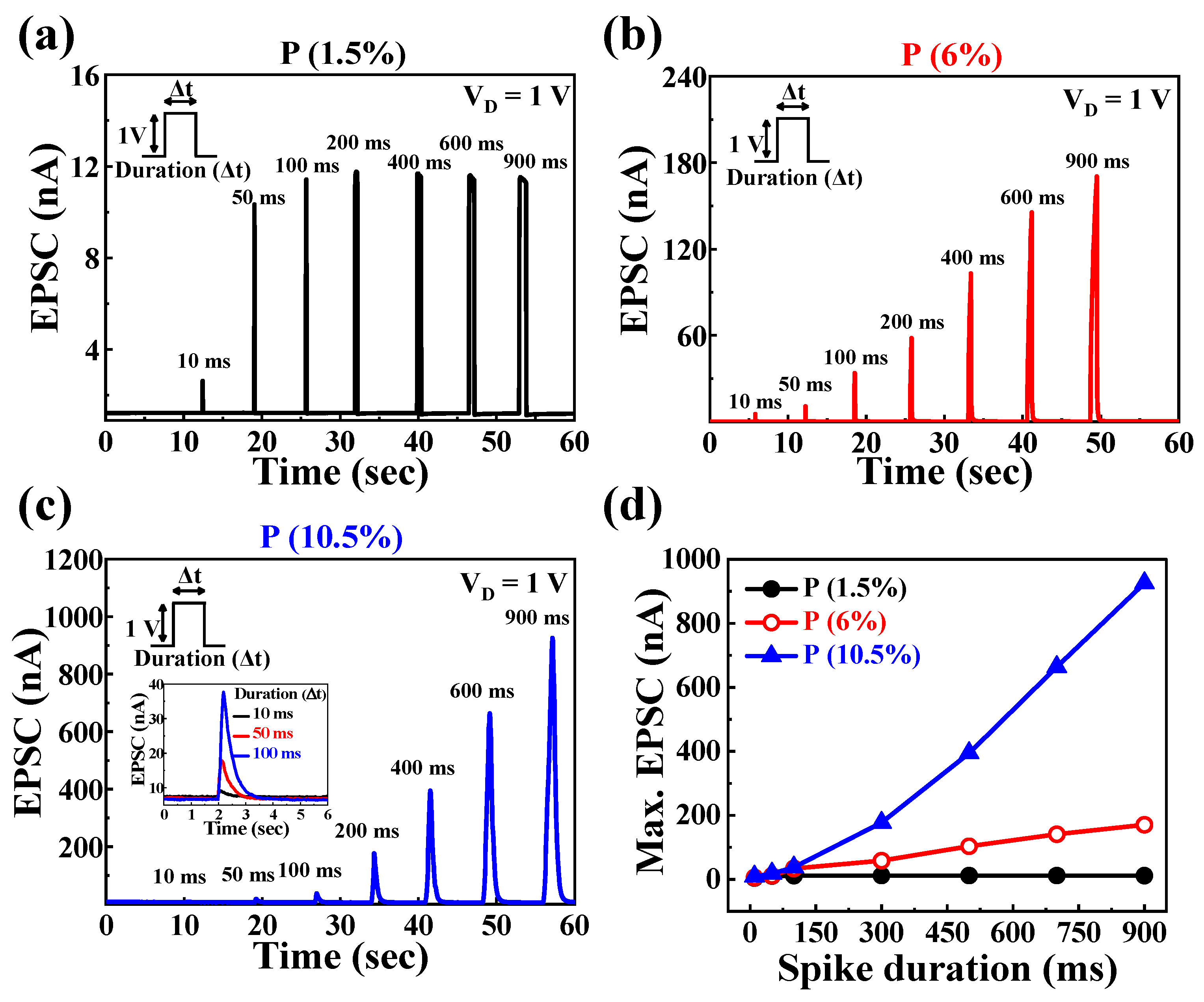
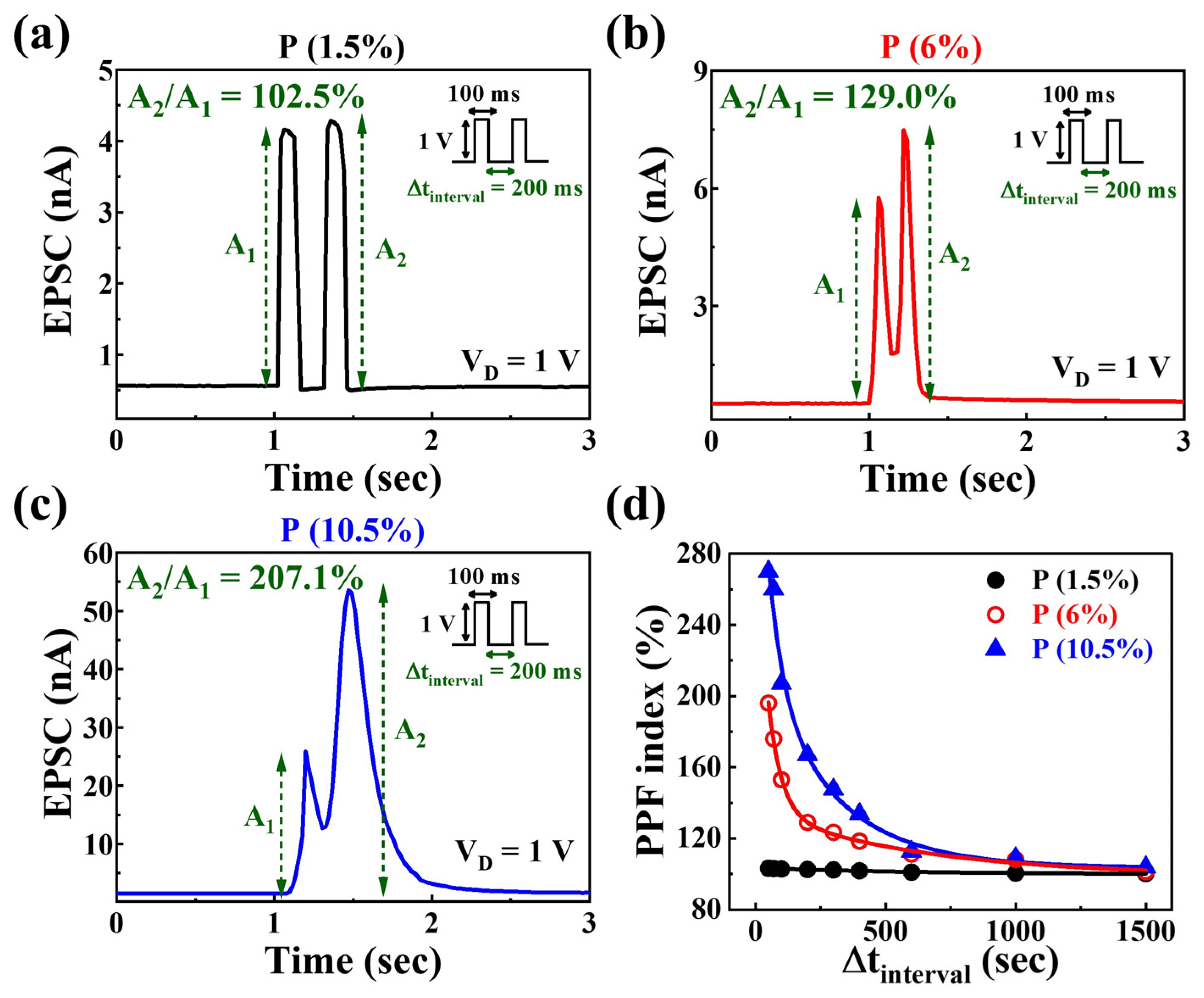
3.3. Synaptic Characteristics of P-Doped PSG-Based EDL Synaptic Transistors
3.4. MNIST ANN Simulation of Proposed Synaptic Transistors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94. [Google Scholar] [CrossRef]
- Chen, L.; Chen, P.; Lin, Z. Artificial intelligence in education: A review. IEEE Access 2020, 8, 75264–75278. [Google Scholar] [CrossRef]
- Abduljabbar, R.; Dia, H.; Liyanage, S.; Bagloee, S.A. Applications of artificial intelligence in transport: An overview. Sustainability 2019, 11, 189. [Google Scholar] [CrossRef]
- Ramos, C.; Augusto, J.C.; Shapiro, D. Ambient intelligence—The next step for artificial intelligence. IEEE Intell. Syst. 2008, 23, 15–18. [Google Scholar] [CrossRef]
- Huang, M.H.; Rust, R.; Maksimovic, V. The feeling economy: Managing in the next generation of artificial intelligence (AI). Calif. Manag. Rev. 2019, 61, 43–65. [Google Scholar] [CrossRef]
- Iannucci, R.A. Toward a dataflow/von Neumann hybrid architecture. Comput. Archit. News 1988, 16, 131–140. [Google Scholar] [CrossRef]
- Indiveri, G.; Liu, S.C. Memory and information processing in neuromorphic systems. Proc. IEEE. 2015, 103, 1379–1397. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.W.; Zhou, P. Two-dimensional materials for synaptic electronics and neuromorphic systems. Sci. Bull. 2019, 64, 1056–1066. [Google Scholar] [CrossRef]
- Ma, W.; Zidan, M.A.; Lu, W.D. Neuromorphic computing with memristive devices. Sci. China Inf. Sci. 2018, 61, 060422. [Google Scholar] [CrossRef]
- Maind, S.B.; Wankar, P. Research paper on basic of artificial neural network. Int. J. Recent Innov. Trends Comput. Commun. 2014, 2, 96–100. [Google Scholar]
- Kuzum, D.; Yu, S.; Wong, H.P. Synaptic electronics: Materials, devices and applications. Nanotechnology 2013, 24, 382001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, G.; Xin, Z.; Fu, C.; Wan, Q.; Shan, F. Solution-processed, electrolyte-gated In2O3 flexible synaptic transistors for brain-inspired neuromorphic applications. ACS Appl. Mater. Interfaces 2019, 12, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Katz, B. Mechanisms of synaptic transmission. Rev. Mod. Phys. 1959, 31, 524. [Google Scholar] [CrossRef]
- Abbott, L.F.; Regehr, W.G. Synaptic computation. Nature 2004, 431, 796–803. [Google Scholar] [CrossRef]
- Wan, Q.; Sharbati, M.T.; Erickson, J.R.; Du, Y.; Xiong, F. Emerging artificial synaptic devices for neuromorphic computing. Adv. Mater. Technol. 2019, 4, 1900037. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Y.; Xu, W. Recent process of flexible transistor-structured memory. Small 2021, 17, 1905332. [Google Scholar] [CrossRef]
- Ling, H.; Koutsouras, D.A.; Kazemzadeh, S.; Van De Burgt, Y.; Yan, F.; Gkoupidenis, P. Electrolyte-gated transistors for synaptic electronics, neuromorphic computing, and adaptable biointerfacing. Appl. Phys. Rev. 2020, 7, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, S.; Savel’ev, S.E.; Jiang, H.; Midya, R.; Lin, P.; Hu, M.; Ge, N.; Strachan, J.P.; Li, Z.; et al. Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 2017, 16, 101–108. [Google Scholar] [CrossRef]
- Fu, Y.M.; Wan, C.J.; Zhu, L.Q.; Xiao, H.; Chen, X.D.; Wan, Q. Hodgkin–Huxley artificial synaptic membrane based on protonic/electronic hybrid neuromorphic transistors. Adv. Biosyst. 2018, 2, 1700198. [Google Scholar] [CrossRef]
- Onen, M.; Emond, N.; Li, J.; Yildiz, B.; Del Alamo, J.A. CMOS-compatible protonic programmable resistor based on phosphosilicate glass electrolyte for analog deep learning. Nano Lett. 2021, 21, 6111–6116. [Google Scholar] [CrossRef]
- Song, M.K.; Kang, J.H.; Zhang, X.; Ji, W.; Ascoli, A.; Messaris, I.; Demirkol, A.S.; Dong, B.; Aggarwal, S.; Wan, W.; et al. Recent advances and future prospects for memristive materials, devices, and systems. ACS Nano 2023, 17, 11994–12039. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, G.; Hediger, S.; Bardet, M.; Favennec, L.; Zenasni, A.; Beynet, J.; Gourhant, O.; Jousseaume, V. Spin-coated and PECVD low dielectric constant porous organosilicate films studied by 1D and 2D solid-state NMR. Phys. Chem. Chem. Phys. 2009, 11, 9729–9737. [Google Scholar] [CrossRef]
- Larsson, O.; Said, E.; Berggren, M.; Crispin, X. Insulator polarization mechanisms in polyelectrolyte-gated organic field-effect transistors. Adv. Funct. Mater. 2009, 19, 3334–3341. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Sun, J.; Wu, G.D.; Zhang, H.L.; Wan, Q. Self-assembled dual in-plane gate thin-film transistors gated by nanogranular SiO2 proton conductors for logic applications. Nanoscale 2013, 5, 1980–1985. [Google Scholar] [CrossRef]
- Jin, Y.G.; Qiao, S.Z.; da Costa, J.D.; Wood, B.J.; Ladewig, B.P.; Lu, G.Q. Hydrolytically stable phosphorylated hybrid silicas for proton conduction. Adv. Funct. Mater. 2007, 17, 3304–3311. [Google Scholar] [CrossRef]
- Yoon, J. Nonvolatile memory functionality of ZnO nanowire transistors controlled by mobile protons. ACS Nano 2010, 5, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Shimotani, H.; Tsukazaki, A.; Ohtomo, A.; Kawasaki, M.; Iwasa, Y. Hydrogenation-induced surface polarity recognition and proton memory behavior at protic-ionic-liquid/oxide electric-double-layer interfaces. J. Am. Chem. Soc. 2010, 132, 6672–6678. [Google Scholar] [CrossRef] [PubMed]
- Guarcello, C.; Valenti, D.; Spagnolo, B.; Pierro, V.; Filatrella, G. Josephson-based threshold detector for Lévy-distributed current fluctuations. Phys. Rev. Appl. 2019, 11, 044078. [Google Scholar] [CrossRef]
- Valenti, D.; Denaro, G.; La Cognata, A.; Spagnolo, B.; Bonanno, A.; Basilone, G.; Aronica, S. Picophytoplankton Dynamics in Noisy Marine Environment. Acta Phys. Pol. B 2012, 43, 1227–1240. [Google Scholar] [CrossRef]
- Stassi, R.; Savasta, S.; Garziano, L.; Spagnolo, B.; Nori, F. Output field-quadrature measurements and squeezing in ultrastrong cavity-QED. New J. Phys. 2016, 18, 123005. [Google Scholar] [CrossRef]
- Parisi, G. Nobel lecture: Multiple equilibria. Rev. Mod. Phys. 2023, 95, 030501. [Google Scholar] [CrossRef]
- Ribeiro, J.; Kaniewski, J.; Helsen, J.; Wehner, S. Device independence for two-party cryptography and position verification with memoryless devices. Phys. Rev. A 2018, 97, 062307. [Google Scholar] [CrossRef]
- Guarcello, C.; Valenti, D.; Augello, G.; Spagnolo, B. The role of non-Gaussian sources in the transient dynamics of long Josephson junctions. Acta Phys. Pol. B 2013, 44, 997–1005. [Google Scholar] [CrossRef]
- Ushakov, Y.V.; Dubkov, A.A.; Spagnolo, B. Regularity of spike trains and harmony perception in a model of the auditory system. Phys. Rev. Lett. 2011, 107, 108103. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Carollo, A.; Spagnolo, B. Stabilizing effect of driving and dissipation on quantum metastable states. Phys. Rev. A 2018, 97, 042109. [Google Scholar] [CrossRef]
- Bándy, E.; Rencz, M. The effect of heat treatment on spin-on oxide glasses in solar cell application. In Proceedings of the 19th International Workshop on Thermal Investigations of ICs and Systems (THERMINIC), Berlin, Germany, 25–27 September 2013. [Google Scholar]
- Bhusari, D.; Li, J.; Jayachandran, P.J.; Moore, C.; Kohl, P.A. Development of P-doped SiO2 as proton exchange membrane for microfuel cells. Electrochem. Solid-State Lett. 2005, 8, A588. [Google Scholar] [CrossRef]
- Lim, B.W.; Suh, M.C. Simple fabrication of a three-dimensional porous polymer film as a diffuser for organic light emitting diodes. Nanoscale 2014, 6, 14446–14452. [Google Scholar] [CrossRef]
- Murarka, S.P.; Li, S.C.; Guo, X.S.; Lanford, W.A. The capacitance-voltage characteristics and hydrogen concentration in phospho-silicate glass films: Relation to phosphorus concentration and annealing effects. J. Appl. Phys. 1992, 72, 4208–4213. [Google Scholar] [CrossRef]
- Ahsan, M.R.; Mortuza, M.G. Infrared spectra of xCaO (1 − x − z) SiO2zP2O5 glasses. J. Non. Cryst. Solids. 2005, 351, 2333–2340. [Google Scholar] [CrossRef]
- Jones, J.R.; Hench, L.L. Factors affecting the structure and properties of bioactive foam scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 68, 36–44. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Fujino, K.; Nishimoto, Y.; Tokumasu, N.; Maeda, K. Doped silicon oxide deposition by atmospheric pressure and low temperature chemical vapor deposition using tetraethoxysilane and ozone. J. Electrochem. Soc. 1991, 138, 3019. [Google Scholar] [CrossRef]
- Yuen, C.W.M.; Ku, S.K.A.; Choi, P.S.R.; Kan, C.W.; Tsang, S.Y. Determining functional groups of commercially available ink-jet printing reactive dyes using infrared spectroscopy. Res. J. Text. Appar. 2005, 9, 26–38. [Google Scholar] [CrossRef]
- Li, H.; Jin, D.; Kong, X.; Tu, H.; Yu, Q.; Jiang, F. High proton-conducting monolithic phosphosilicate glass membranes. Microporous Mesoporous Mater. 2011, 138, 63–67. [Google Scholar] [CrossRef]
- Fu, Y.M.; Wan, C.J.; Yu, F.; Xiao, H.; Tao, J.; Guo, Y.B.; Gao, W.T.; Zhu, L.Q. Electrolyte gated oxide pseudodiode for inhibitory synapse applications. Adv. Electron. Mater. 2018, 4, 1800371. [Google Scholar] [CrossRef]
- Matsuda, A.; Kanzaki, T.; Kotani, Y.; Tatsumisago, M.; Minami, T. Proton conductivity and structure of phosphosilicate gels derived from tetraethoxysilane and phosphoric acid or triethylphosphate. Solid State Ion. 2001, 139, 113–119. [Google Scholar] [CrossRef]
- Beom, K.; Yang, P.; Park, D.; Kim, H.J.; Lee, H.H.; Kang, C.J.; Yoon, T.S. Single-and double-gate synaptic transistor with TaOx gate insulator and IGZO channel layer. Nanotechnology 2018, 30, 025203. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, Y.; Wang, Y.; Zhang, J.; Fang, L.; Jin, S.; Shao, Y.; Huang, J. Recent advances in transistor-based artificial synapses. Adv. Funct. Mater. 2019, 29, 1903700. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Shi, Y.; Wan, Q. Solution-processed chitosan-gated IZO-based transistors for mimicking synaptic plasticity. IEEE Electron Device Lett. 2014, 35, 280–282. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Pei, Y. Planar multi-gate artificial synaptic transistor with solution-processed AlOx solid electric double layer dielectric and InOx channel. Coatings 2023, 13, 719. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, J.; Xie, D.; Liu, B.; Yang, J.; He, J. Proton–electron-coupled MoS2 synaptic transistors with a natural renewable biopolymer neurotransmitter for brain-inspired neuromorphic learning. J. Mater. Chem. C 2019, 7, 682–691. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Zhu, L.Q.; Guo, Y.B.; Long, T.Y.; Yu, F.; Xiao, H.; Lu, H.L. Threshold-tunable, spike-rate-dependent plasticity originating from interfacial proton gating for pattern learning and memory. ACS Appl. Mater. Interfaces 2020, 12, 7833–7839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Q.; Wan, C.J.; Guo, L.Q.; Shi, Y.; Wan, Q. Artificial synapse network on inorganic proton conductor for neuromorphic systems. Nat. Commun. 2014, 5, 3158. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, Y.; Nie, S.; Liu, R.; Wan, Q. Electric-double-layer transistors for synaptic devices and neuromorphic systems. J. Mater. Chem. C 2018, 6, 5336–5352. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, H.; Cho, W.J. Biocompatible casein electrolyte-based electric-double-layer for artificial synaptic transistors. Nanomaterials 2022, 12, 2596. [Google Scholar] [CrossRef]
- Park, K.W.; Cho, W.J. Binary-synaptic plasticity in ambipolar Ni-silicide Schottky barrier poly-Si thin film transistors using chitosan electric double layer. Nanomaterials 2022, 12, 3063. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Cho, W.J. CMOS-compatible synaptic transistor gated by chitosan electrolyte-Ta2O5 hybrid electric double layer. Sci. Rep. 2020, 10, 15561. [Google Scholar] [CrossRef]
- Yang, C.S.; Shang, D.S.; Liu, N.; Fuller, E.J.; Agrawal, S.; Talin, A.A.; Li, Y.Q.; Shen, B.G.; Sun, Y. All-solid-state synaptic transistor with ultralow conductance for neuromorphic computing. Adv. Funct. Mater. 2018, 28, 1804170. [Google Scholar] [CrossRef]
- Jang, J.W.; Park, S.; Burr, G.W.; Hwang, H.; Jeong, Y.H. Optimization of conductance change in Pr1−xCaxMnO3-Based synaptic devices for neuromorphic systems. IEEE Electron Device Lett. 2015, 36, 457–459. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mah, D.-G.; Park, H.; Cho, W.-J. Synaptic Plasticity Modulation of Neuromorphic Transistors through Phosphorus Concentration in Phosphosilicate Glass Electrolyte Gate. Nanomaterials 2024, 14, 203. https://doi.org/10.3390/nano14020203
Mah D-G, Park H, Cho W-J. Synaptic Plasticity Modulation of Neuromorphic Transistors through Phosphorus Concentration in Phosphosilicate Glass Electrolyte Gate. Nanomaterials. 2024; 14(2):203. https://doi.org/10.3390/nano14020203
Chicago/Turabian StyleMah, Dong-Gyun, Hamin Park, and Won-Ju Cho. 2024. "Synaptic Plasticity Modulation of Neuromorphic Transistors through Phosphorus Concentration in Phosphosilicate Glass Electrolyte Gate" Nanomaterials 14, no. 2: 203. https://doi.org/10.3390/nano14020203




