Imaging of Endometriotic Lesions Using cRGD-MN Probe in a Mouse Model of Endometriosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Model of Endometriosis
2.2. RGD-Cy5.5-MN Synthesis and Characterization
2.3. Magnetic Resonance Imaging
2.4. Ex Vivo Bioluminescence Optical Imaging
2.5. Ex Vivo Epifluorescence Optical Imaging
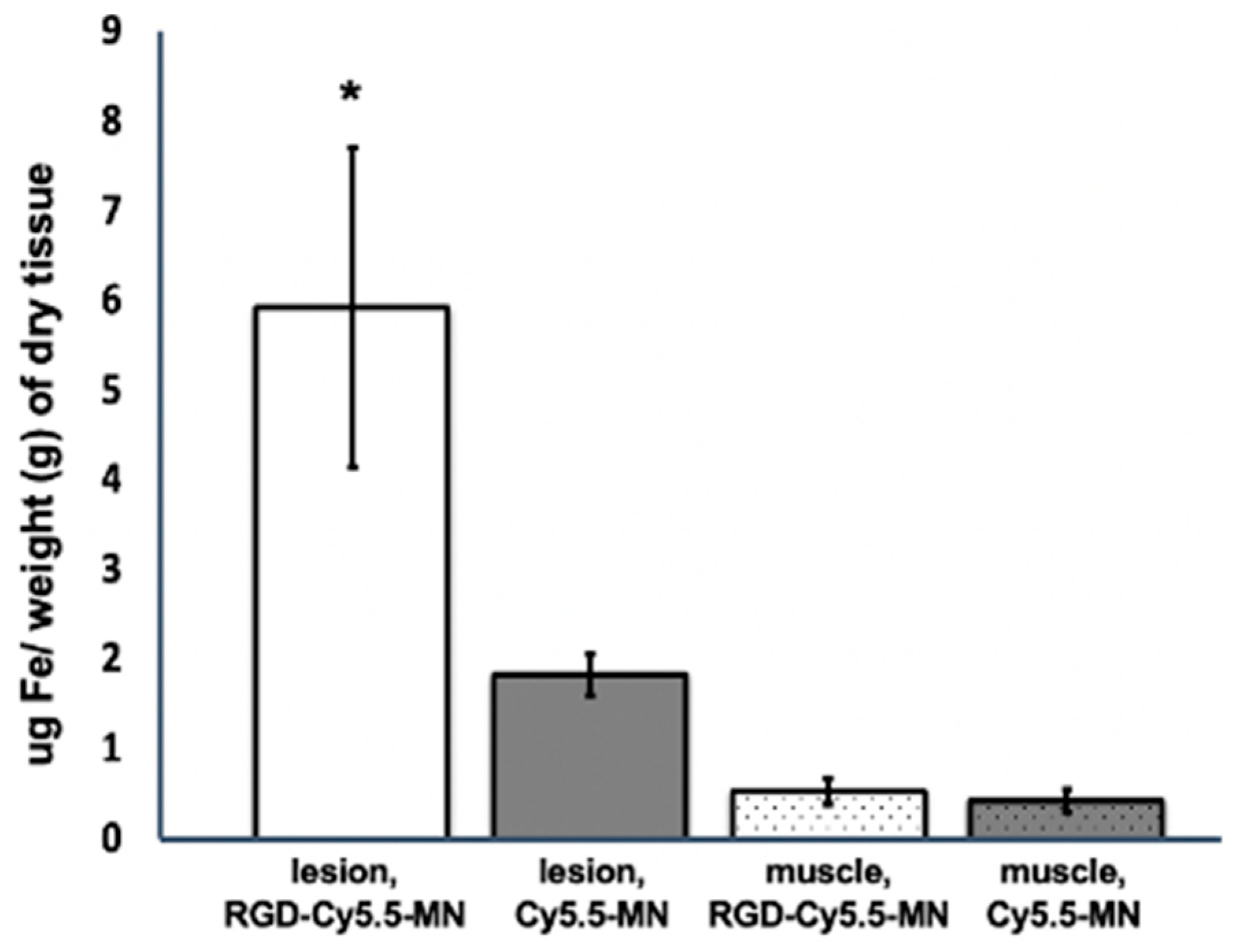
2.6. ICP-OES Analysis
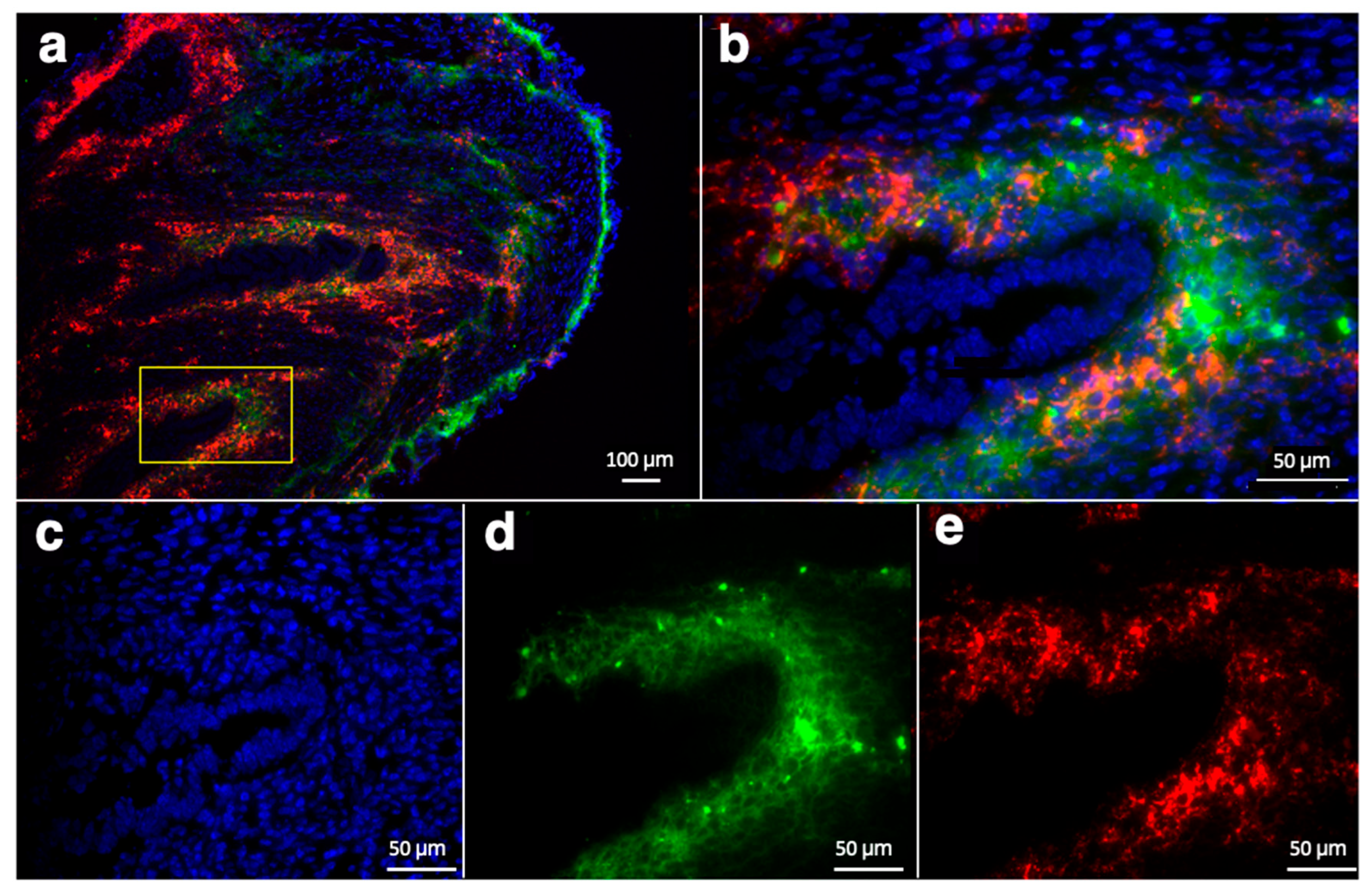
2.7. Histology and Immunostaining
2.8. Complete Blood Count, Blood Chemistry Analyses, and Histopathology
2.9. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of the Nanoparticle Probes
3.2. Magnetic Resonance Imaging of Endometriotic Lesions
3.3. RGD-Cy5.5-MN Biodistribution
3.4. Ex Vivo Analysis of RGD-Cy5.5-MN Accumulation and CD34 Expression
3.5. Biosafety Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malvezzi, H.; Marengo, E.B.; Podgaec, S.; Piccinato, C.d.A. Endometriosis: Current challenges in modeling a multifactorial disease of unknown etiology. J. Transl. Med. 2020, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef]
- Kodaman, P.H. Current strategies for endometriosis management. Obstet. Gynecol. Clin. 2015, 42, 87–101. [Google Scholar] [CrossRef]
- Prentice, A. Endometriosis. BMJ 2001, 323, 93–95. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef]
- Child, T.; Tan, S.L. Endometriosis-Aetiology, pathogenesis and treatment. Drugs 2001, 61, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Viceconte, R.; Baldi, A. New Insights in Pathogenesis of Endometriosis. Front. Med. 2022, 9, 879015. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, E.; Wessels, J.; Aas-Eng, M.; Abrao, M.; Condous, G.; Jurkovic, D.; Espada, M.; Exacoustos, C.; Ferrero, S.; Guerriero, S. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet. Gynecol. 2022, 60, 309–327. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Wykes, C.B.; Clark, T.J.; Khan, K.S. Accuracy of laparoscopy in the diagnosis of endometriosis: A systematic quantitative review. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 1204–1212. [Google Scholar] [CrossRef]
- Nilufer, R.; Karina, B.; Paraskevi, C.; Rebecca, D.; Genevieve, G.; Ayush, G.; Stuart, M.; Sally, M.; Yadav, S.; Andrew, S.J. Large-scale genome-wide association meta-analysis of endometriosis reveals 13 novel loci and genetically-associated comorbidity with other pain conditions. BioRxiv 2018, 406967. [Google Scholar] [CrossRef]
- May, K.; Conduit-Hulbert, S.; Villar, J.; Kirtley, S.; Kennedy, S.; Becker, C. Peripheral biomarkers of endometriosis: A systematic review. Hum. Reprod. Update 2010, 16, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Kiesel, L.; Sourouni, M. Diagnosis of endometriosis in the 21st century. Climacteric 2019, 22, 296–302. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, D009591. [Google Scholar] [CrossRef]
- Kinkel, K.; Frei, K.A.; Balleyguier, C.; Chapron, C. Diagnosis of endometriosis with imaging: A review. Eur. Radiol. 2006, 16, 285–298. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Savelli, L. Transvaginal sonography for the assessment of ovarian and pelvic endometriosis: How deep is our understanding? Ultrasound Obstet. Gynecol. 2009, 33, 497–501. [Google Scholar] [CrossRef]
- Exacoustos, C.; Manganaro, L.; Zupi, E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 655–681. [Google Scholar] [CrossRef]
- Samreen, J.N.; Bookwalter, C.A.; Burnett, T.L.; Feldman, M.; Sheedy, S.P.; Menias, C.; VanBuren, W.M.; Kabashi, A. MRI of endometriosis: A comprehensive review. Appl. Radiol. 2019, 48, 6–12. [Google Scholar] [CrossRef]
- Siegelman, E.S.; Oliver, E.R. MR imaging of endometriosis: Ten imaging pearls. Radiographics 2012, 32, 1675–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Sun, W.; Hu, Y.; Zhang, G.; Shen, M.; Shi, X. Hyaluronic acid-modified magnetic iron oxide nanoparticles for MR imaging of surgically induced endometriosis model in rats. PLoS ONE 2014, 9, e94718. [Google Scholar] [CrossRef]
- Bianek-Bodzak, A.; Szurowska, E.; Sawicki, S.; Liro, M. The importance and perspective of magnetic resonance imaging in the evaluation of endometriosis. BioMed Res. Int. 2013, 2013, 436589. [Google Scholar] [CrossRef] [PubMed]
- Bourgioti, C.; Preza, O.; Panourgias, E.; Chatoupis, K.; Antoniou, A.; Nikolaidou, M.; Moulopoulos, L. MR imaging of endometriosis: Spectrum of disease. Diagn. Interv. Imaging 2017, 98, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Morawski, A.M.; Lanza, G.A.; Wickline, S.A. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr. Opin. Biotechnol. 2005, 16, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Schwager, K.; Bootz, F.; Imesch, P.; Kaspar, M.; Trachsel, E.; Neri, D. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum. Reprod. 2011, 26, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Demessie, A.A.; Luo, A.; Taratula, O.R.; Moses, A.S.; Do, P.; Campos, L.; Jahangiri, Y.; Wyatt, C.R.; Albarqi, H.A. Targeted Nanoparticles with High Heating Efficiency for the Treatment of Endometriosis with Systemically Delivered Magnetic Hyperthermia. Small 2022, 18, 2107808. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Zhou, J.; Hou, W.; Wen, X.; Zhang, H.; Kong, F.; Luo, L.; Li, Q.; Du, Y. Specific photothermal ablation therapy of endometriosis by targeting delivery of gold nanospheres. Small 2017, 13, 1603270. [Google Scholar] [CrossRef]
- Moses, A.S.; Demessie, A.A.; Taratula, O.; Korzun, T.; Slayden, O.D.; Taratula, O. Nanomedicines for endometriosis: Lessons learned from cancer research. Small 2021, 17, 2004975. [Google Scholar] [CrossRef]
- Kralickova, M.; Vetvicka, V. Role of angiogenesis in endometriosis. Pathol. Discov. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell. Physiol. 2019, 234, 19384–19392. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.S.; Han, S.J. Endometriosis-Associated Angiogenesis and Anti-angiogenic Therapy for Endometriosis. Front. Glob. Womens Health 2022, 3, 856316. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, Y.; Zhang, Y.; Gu, N.; Yang, B.; Shan, S.; Liu, N.; Ouyang, J.; Yang, Y.; Sun, F.; et al. Endometrial stromal cell ferroptosis promotes angiogenesis in endometriosis. Cell Death Discov. 2022, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.C.; Clark, R.A.; Cheresh, D.A. Requirement of vascular integrin αvβ3 for angiogenesis. Science 1994, 264, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Serini, G.; Valdembri, D.; Bussolino, F. Integrins and angiogenesis: A sticky business. Exp. Cell Res. 2006, 312, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, C.; Dormond, O.; Mariotti, A. Endothelial cell integrins and COX-2: Mediators and therapeutic targets of tumor angiogenesis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2004, 1654, 51–67. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-based strategies to target alpha (v) beta (3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Liu, F.; Zhou, Y.; Luo, L.; Qiao, H. RGD Peptide-Based Target Drug Delivery of Doxorubicin Nanomedicine. Drug Dev. Res. 2017, 78, 283–291. [Google Scholar] [CrossRef]
- Montet, X.; Funovics, M.; Montet-Abou, K.; Weissleder, R.; Josephson, L. Multivalent effects of RGD peptides obtained by nanoparticle display. J. Med. Chem. 2006, 49, 6087–6093. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Yang, J.; Wei, P.; Luo, Y.; Ding, L.; Sun, W.; Zhang, G.; Shi, X.; Shen, M. Facile synthesis of RGD peptide-modified iron oxide nanoparticles with ultrahigh relaxivity for targeted MR imaging of tumors. Biomater. Sci. 2015, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Seyednejad, H.; Laurent, S.; Atyabi, F.; Saei, A.A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging. Contrast Media Mol. Imaging 2015, 10, 329–355. [Google Scholar] [CrossRef]
- Yigit, M.V.; Moore, A.; Medarova, Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharm. Res. 2012, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef]
- Montet, X.; Montet-Abou, K.; Reynolds, F.; Weissleder, R.; Josephson, L. Nanoparticle imaging of integrins on tumor cells. Neoplasia 2006, 8, 214–222. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, T.H.; Shin, J.H.; Marquardt, R.M.; Muller, U.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.G.; Jeong, J.W. Loss of MIG-6 results in endometrial progesterone resistance via ERBB2. Nat. Commun. 2022, 13, 1101. [Google Scholar] [CrossRef]
- Yigit, M.; Ghosh, S.; Kumar, M.; Petkova, V.; Kavishwar, A.; Moore, A.; Medarova, Z. Context-dependent differences in miR-10b breast oncogenesis can be targeted for the prevention and arrest of lymph node metastasis. Oncogene 2013, 32, 1530–1538. [Google Scholar] [CrossRef]
- Yoo, B.; Kavishwar, A.; Ross, A.; Wang, P.; Tabassum, D.P.; Polyak, K.; Barteneva, N.; Petkova, V.; Pantazopoulos, P.; Tena, A. Combining miR-10b–targeted nanotherapy with low-dose doxorubicin elicits durable regressions of metastatic breast cancer. Cancer Res. 2015, 75, 4407–4415. [Google Scholar] [CrossRef]
- Shuhendler, A.J.; Prasad, P.; Leung, M.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. A novel solid lipid nanoparticle formulation for active targeting to tumor αvβ3 integrin receptors reveals cyclic RGD as a double-edged sword. Adv. Healthc. Mater. 2012, 1, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, T.D.; Dolgova, N.V.; Lewis, J.S.; Hamaker, K.; Clare-Salzler, M.J.; Keselowsky, B.G. Macrophage integrins modulate response to ultra-high molecular weight polyethylene particles and direct particle-induced osteolysis. Biomaterials 2017, 115, 128–140. [Google Scholar] [CrossRef]
- Schiffelers, R.M.; Koning, G.A.; ten Hagen, T.L.; Fens, M.H.; Schraa, A.J.; Janssen, A.P.; Kok, R.J.; Molema, G.; Storm, G. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J. Control. Release 2003, 91, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.C.; Silva, B.B.; Pinto, G.A.; Vassallo, J.; Moraes, N.G.; Santana, J.O.; Santos, L.G.; Carvasan, G.A.; Zeferino, L.C. CD34 as a marker for evaluating angiogenesis in cervical cancer. Pathol.-Res. Pract. 2005, 201, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; He, Q.; Gao, H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale 2015, 7, 9487–9496. [Google Scholar] [CrossRef]
- Colombo, F.; Durigutto, P.; De Maso, L.; Biffi, S.; Belmonte, B.; Tripodo, C.; Oliva, R.; Bardini, P.; Marini, G.M.; Terreno, E. Targeting CD34+ cells of the inflamed synovial endothelium by guided nanoparticles for the treatment of rheumatoid arthritis. J. Autoimmun. 2019, 103, 102288. [Google Scholar] [CrossRef]
- Boehm, O.; Zur, B.; Koch, A.; Tran, N.; Freyenhagen, R.; Hartmann, M.; Zacharowski, K. Clinical chemistry reference database for Wistar rats and C57/BL6 mice. Biol Chem. 2007, 388, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Vanhie, A.O.D.; Peterse, D.; Beckers, A.; Cuéllar, A.; Fassbender, A.; Meuleman, C.; Mestdagh, P.; D’Hooghe, T. Plasma miRNAs as biomarkers for endometriosis. Hum. Reprod. 2019, 34, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, I.; Bravo, M.; Deschaume, O.; Cybulski, P.; Bartic, C.; Hofkens, J.; Uji-i, H.; Fortuni, B.; Rocha, S. Versatile and Robust Method for Antibody Conjugation to Nanoparticles with High Targeting Efficiency. Pharmaceutics 2021, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.S.; Taratula, O.R.; Lee, H.; Luo, F.; Grenz, T.; Korzun, T.; Lorenz, A.S.; Sabei, F.Y.; Bracha, S.; Alani, A.W. Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis. Small 2020, 16, 1906936. [Google Scholar] [CrossRef]
- Regidor, P.; Vogel, C.; Regidor, M.; Schindler, A.; Winterhager, E. Expression pattern of integrin adhesion molecules in endometriosis and human endometrium. Hum. Reprod. Update 1998, 4, 710–718. [Google Scholar] [CrossRef]
- Klemmt, P.A.; Carver, J.G.; Koninckx, P.; McVeigh, E.J.; Mardon, H.J. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: Towards a mechanistic model for endometriosis progression. Hum. Reprod. 2007, 22, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Dai, Y.; Xu, W.; Shi, L.; Jin, X.; Li, C.; Zhou, F.; Pan, Y.; Zhang, Y.; Lin, X. Hypoxia promotes ectopic adhesion ability of endometrial stromal cells via TGF-β 1/Smad signaling in endometriosis. Endocrinology 2018, 159, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Simón-Gracia, L.; Kiisholts, K.; Petrikaitė, V.; Tobi, A.; Saare, M.; Lingasamy, P.; Peters, M.; Salumets, A.; Teesalu, T. Homing Peptide-Based Targeting of Tenascin-C and Fibronectin in Endometriosis. Nanomaterials 2021, 11, 3257. [Google Scholar] [CrossRef]
- Rangger, C.; Helbok, A.; von Guggenberg, E.; Sosabowski, J.; Radolf, T.; Prassl, R.; Andreae, F.; Thurner, G.C.; Haubner, R.; Decristoforo, C. Influence of PEGylation and RGD loading on the targeting properties of radiolabeled liposomal nanoparticles. Int. J. Nanomed. 2012, 7, 5889. [Google Scholar] [CrossRef] [PubMed]
- Grote, K.; Salguero, G.; Ballmaier, M.; Dangers, M.; Drexler, H.; Schieffer, B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: Potential role in angiogenesis and endothelial regeneration. Blood J. Am. Soc. Hematol. 2007, 110, 877–885. [Google Scholar] [CrossRef]
- Velikyan, I.; Lindhe, O. Preparation and evaluation of a (68)Ga-labeled RGD-containing octapeptide for noninvasive imaging of angiogenesis: Biodistribution in non-human primate. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 15–31. [Google Scholar]
- Joshi, N.R.; Kohan-Ghadr, H.R.; Roqueiro, D.S.; Yoo, J.Y.; Fru, K.; Hestermann, E.; Yuan, L.; Ho, S.M.; Jeong, J.W.; Young, S.L.; et al. Genetic and epigenetic changes in the eutopic endometrium of women with endometriosis: Association with decreased endometrial alphavbeta3 integrin expression. Mol. Hum. Reprod. 2021, 27, gaab018. [Google Scholar] [CrossRef]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Medarova, Z.; Pham, W.; Farrar, C.; Petkova, V.; Moore, A. In vivo imaging of siRNA delivery and silencing in tumors. Nat. Med. 2007, 13, 372–377. [Google Scholar] [CrossRef]
- Yadav, A.S.; Radharani, N.N.V.; Gorain, M.; Bulbule, A.; Shetti, D.; Roy, G.; Baby, T.; Kundu, G.C. RGD functionalized chitosan nanoparticle mediated targeted delivery of raloxifene selectively suppresses angiogenesis and tumor growth in breast cancer. Nanoscale 2020, 12, 10664–10684. [Google Scholar] [CrossRef]
- Yuan, Z.; He, H.; Zou, J.; Wang, H.; Chen, Y.; Chen, Y.; Lan, M.; Zhao, Y.; Gao, F. Polydopamine-coated ferric oxide nanoparticles for R848 delivery for photothermal immunotherapy in breast cancer. Int. J. Pharm. 2023, 644, 123249. [Google Scholar] [CrossRef]
- Harel, Z. Dysmenorrhea in adolescents and young adults: Etiology and management. J. Pediatr. Adolesc. Gynecol. 2006, 19, 363–371. [Google Scholar] [CrossRef]
- Tirado-González, I.; Barrientos, G.; Tariverdian, N.; Arck, P.C.; García, M.G.; Klapp, B.F.; Blois, S.M. Endometriosis research: Animal models for the study of a complex disease. J. Reprod. Immunol. 2010, 86, 141–147. [Google Scholar] [CrossRef]
- Braundmeier, A.G.; Fazleabas, A.T. The non-human primate model of endometriosis: Research and implications for fecundity. Mol. Hum. Reprod. 2009, 15, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Harirchian, P.; Gashaw, I.; Lipskind, S.T.; Braundmeier, A.G.; Hastings, J.M.; Olson, M.R.; Fazleabas, A.T. Lesion kinetics in a non-human primate model of endometriosis. Hum. Reprod. 2012, 27, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Donnez, O.; Van Langendonckt, A.; Defrere, S.; Colette, S.; Van Kerk, O.; Dehoux, J.P.; Squifflet, J.; Donnez, J. Induction of endometriotic nodules in an experimental baboon model mimicking human deep nodular lesions. Fertil. Steril. 2013, 99, 783–789.e3. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talebloo, N.; Bernal, M.A.O.; Kenyon, E.; Mallett, C.L.; Mondal, S.K.; Fazleabas, A.; Moore, A. Imaging of Endometriotic Lesions Using cRGD-MN Probe in a Mouse Model of Endometriosis. Nanomaterials 2024, 14, 319. https://doi.org/10.3390/nano14030319
Talebloo N, Bernal MAO, Kenyon E, Mallett CL, Mondal SK, Fazleabas A, Moore A. Imaging of Endometriotic Lesions Using cRGD-MN Probe in a Mouse Model of Endometriosis. Nanomaterials. 2024; 14(3):319. https://doi.org/10.3390/nano14030319
Chicago/Turabian StyleTalebloo, Nazanin, M. Ariadna Ochoa Bernal, Elizabeth Kenyon, Christiane L. Mallett, Sujan Kumar Mondal, Asgerally Fazleabas, and Anna Moore. 2024. "Imaging of Endometriotic Lesions Using cRGD-MN Probe in a Mouse Model of Endometriosis" Nanomaterials 14, no. 3: 319. https://doi.org/10.3390/nano14030319




