Optical Limiting Response of Porous Carbon Dispersions
Abstract
:1. Introduction
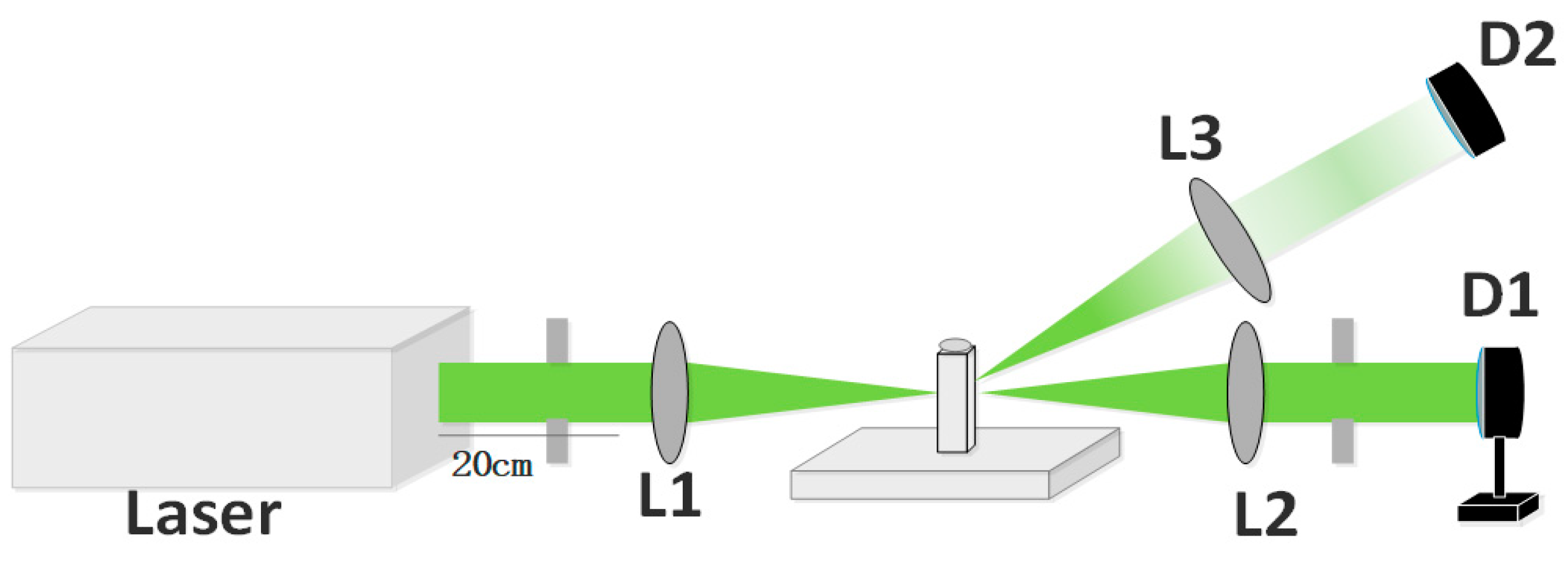
2. Experiment and Sample Preparation
3. Results and Discussion
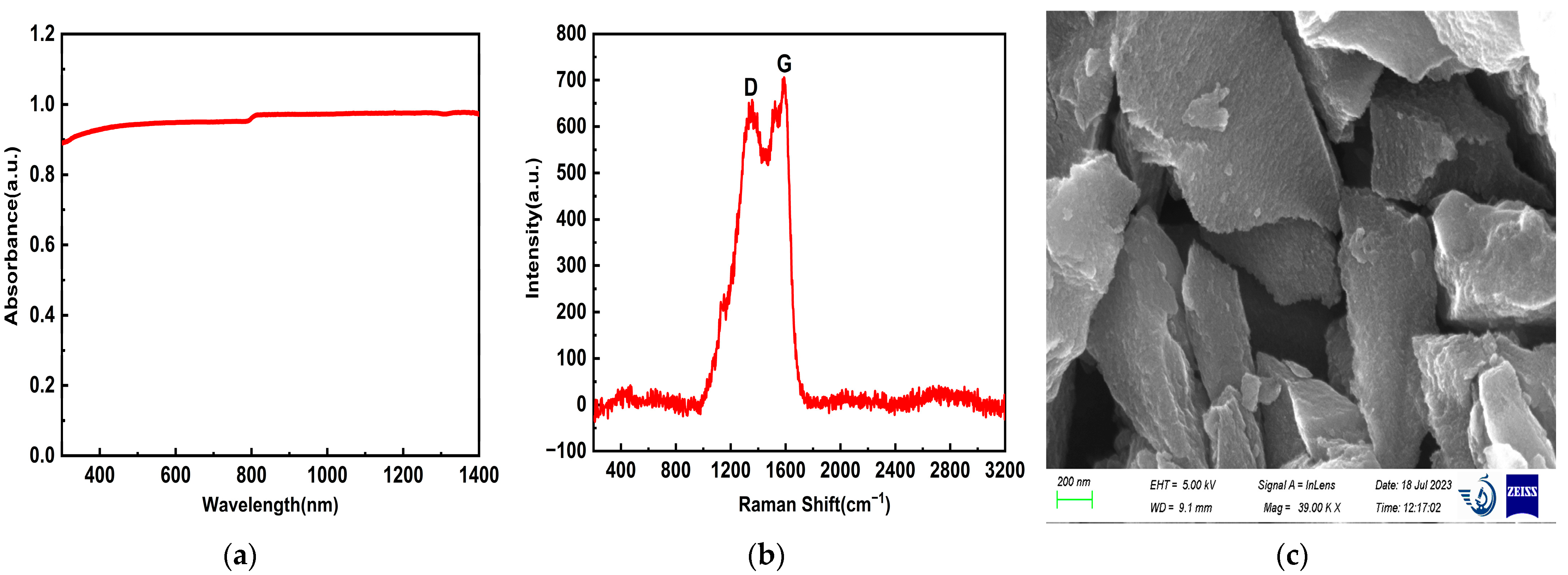
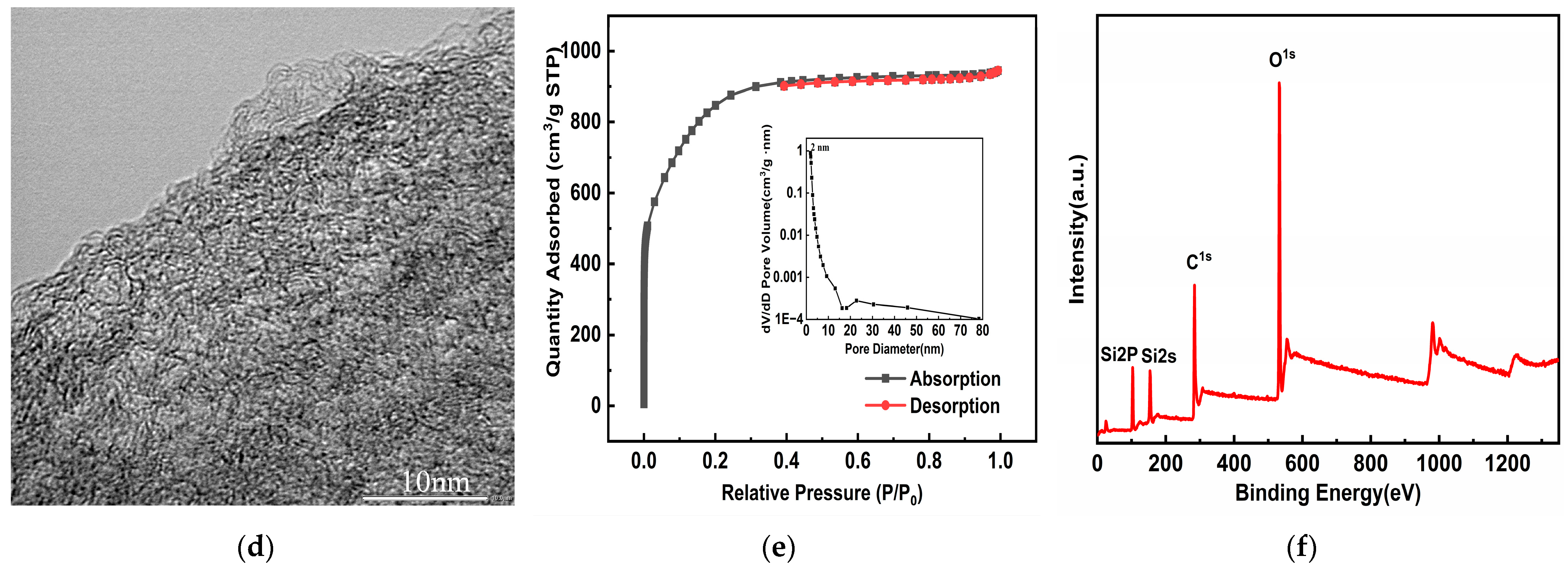
3.1. Characterization Results and Analysis
3.2. Characterization Results and Analysis OL Properties and Mechanisms of Porous Carbon Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Bai, T.; Dong, N.; Fan, F.; Zhang, S.; Zhuang, X.; Sun, J.; Zhang, B.; Zhang, X.; Wang, J.; et al. Graphene and its derivatives for laser protection. Prog. Mater. Sci. 2016, 84, 118–157. [Google Scholar] [CrossRef]
- Wang, J.; Fruchtl, D.; Sun, Z.; Coleman, J.N.; Blau, W.J. Control of Optical Limiting of Carbon Nanotube Dispersions by Changing Solvent Parameters. J. Phys. Chem. C 2010, 114, 6148–6156. [Google Scholar] [CrossRef]
- Vivien, L.; Lançon, P.; Riehl, D.; Hache, F.; Anglaret, E. Carbon nanotubes for optical limiting. Carbon 2002, 40, 1789–1797. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Li, R.; Dong, H.; Zhang, L.; Lotya, M.; Blau, W.J. Nonlinear optical properties of graphene and carbon nanotube composites. In Carbon Nanotubes—Synthesis, Characterization, Applications; IntechOpen: Rijeka, Croatia, 2011; p. 397. [Google Scholar]
- Callaghan, J.; Blau, W.J.; Henari, F.Z. Picosecond reverse saturable absorption and optical limiting in fullerenes and their metal derivatives. J. Nonlinear Opt. Phys. Mater. 2000, 9, 505–521. [Google Scholar] [CrossRef]
- Song, Y.; Fang, G.; Wang, Y.; Liu, S.; Li, C.; Song, L.; Zhu, Y.; Hu, Q. Excited-state absorption and optical-limiting properties of organometallic fullerene–C 60 derivatives. Appl. Phys. Lett. 1999, 74, 332–334. [Google Scholar] [CrossRef]
- Henari, F.Z.; Blau, W.J.; Milgrom, L.R.; Yahioglu, G.; Phillips, D.; Lacey, J.A. Third-order optical non-linearity in Zn(II) complexes of 5,10,15,20-tetraarylethynyl-substituted porphyrins. Chem. Phys. Lett. 1997, 267, 229–233. [Google Scholar] [CrossRef]
- Mansour, K.; Soileau, M.J.; Van Stryland, E.W. Nonlinear optical properties of carbon-black suspensions (ink). J. Opt. Soc. Am. B 1992, 9, 1100–1109. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Q.; Pan, Q.; Hu, J.; Liu, R.; Song, G.; Zhu, H. High performance Pt (II) complex and its hybridized carbon quantum dots: Synthesis and the synergistic enhanced optical limiting property. Appl. Surf. Sci. 2022, 584, 152567. [Google Scholar] [CrossRef]
- Izard, N.; Billaud, P.; Riehl, D.; Anglaret, E. Influence of structure on the optical limiting properties of nanotubes. Opt. Lett. 2005, 30, 1509–1511. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Huang, L.; Goh, S.H.; Xu, G.; Ji, W. Size-dependent optical limiting behavior of multi-walled carbon nanotubes. Chem. Phys. Lett. 2002, 352, 328–333. [Google Scholar] [CrossRef]
- Wang, J.; Blau, W.J. Solvent Effect on Optical Limiting Properties of Single-Walled Carbon Nanotube Dispersions. J. Phys. Chem. C 2008, 112, 2298–2303. [Google Scholar] [CrossRef]
- Xiong, Y.; Yan, L.; Si, J.; Yi, W.; Ding, W.; Tan, W.; Liu, X.; Chen, F.; Hou, X. Cascaded optical limiter with low activating and high damage thresholds using single-layer graphene and single-walled carbon nanotubes. J. Appl. Phys. 2014, 115, 223. [Google Scholar] [CrossRef]
- Sun, X.; Yan, L.; Chen, T.; Yu, Y.; Matsuo, S. Effect of solvent surface tension on optical limiting properties of graphene dispersions. Laser Phys. 2015, 25, 035901. [Google Scholar] [CrossRef]
- Wang, J.; Hernandez, Y.; Lotya, M.; Coleman, J.N.; Blau, W.J. Broadband Nonlinear Optical Response of Graphene Dispersions; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chen, Y.; Lin, Y.; Liu, Y.; Doyle, J.; He, N.; Zhuang, X.; Bai, J.; Blau, W.J. Carbon nanotube-based functional materials for optical limiting. J. Nanosci. Nanotechnol. 2007, 7, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Chen, Y.; Chen, P.; Chen, X.; Wu, X.; Pu, X.; Zeng, Y.; Zhu, Z. Mesoporous CoO nanocubes @ continuous 3D porous carbon skeleton of rose-based electrode for high-performance supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 11839–11845. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.M.; Lu GQ, M. Carbon–sulfur composites for Li–S batteries: Status and prospects. J. Mater. Chem. A 2013, 1, 9382–9394. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent progress in metal-organic framework-based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Zhang, B.; Zhang, J. Bifunctional Oxygen Electrocatalysis of N, S-Codoped Porous Carbon with Interspersed Hollow CoO Nanoparticles for Rechargeable Zn–Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 16720–16728. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Hollins, R.C. Materials for optical limiters. Curr. Opin. Solid State Mater. Sci. 1999, 4, 189–196. [Google Scholar] [CrossRef]
- Mysyk, R.; Gao, Q.; Raymundo-Piñero, E.; Béguin, F. Microporous carbons finely-tuned by cyclic high-pressure low-temperature oxidation and their use in electrochemical capacitors. Carbon 2012, 50, 3367–3374. [Google Scholar] [CrossRef]
- Tian, B.; Wang, Y.H.; Hao, M.Q.; Shu, J.; Wang, Y.T.; Xu, T.J. Achieving of high utilization of reduced graphene Oxide-TiO2 nanoparticle composites via oxygen bonds for enhanced optical limiting performance. J. Lumin. Interdiscip. J. Res. Excit. State Process. Condens. Matter 2022, 244, 118696. [Google Scholar] [CrossRef]
- Pradhan, P.; Podila, R.; Molli, M.; Kaniyoor, A.; Muthukumar, V.S.; Sai, S.S.S.; Ramaprabhu, S.; Rao, A. Optical limiting and nonlinear optical properties of gold-decorated graphene nanocomposites. Opt. Mater. 2015, 39, 182–187. [Google Scholar] [CrossRef]
- Manjunatha, K.B.; Ravindra, R.; Antony, A.; Poornesh, P. Third-order nonlinear optical studies of carbon nanotubes developed by floating catalyst technique. Opt. Mater. 2020, 109, 110315. [Google Scholar] [CrossRef]
- Sanusi, K.; Amuhaya, E.K.; Nyokong, T. Enhanced optical limiting behavior of an indium phthalocyanine–single-walled carbon nanotube composite: An investigation of the effects of solvents. J. Phys. Chem. C 2014, 118, 7057–7069. [Google Scholar] [CrossRef]
- Zhang, H.T.; Pan, P.; Wang, M.R.; Yang, Z.C.; Liu, J.L.; Wei, Z. Enhanced optical limiting of dispersible MWCNTs/TiO2 nanocomposite. Opt. Laser Technol. 2015, 67, 44–49. [Google Scholar] [CrossRef]
- Dengler, S.; Muller, O.; Hege, C.; Eberle, B. Nonlinear optical effects in colloidal carbon nanohorns—A new optical limiting material. J. Phys. D: Appl. Phys. 2016, 49, 365501. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Blau, W.J. Carbon nanotubes and nanotube composites for nonlinear optical devices. J. Mater. Chem. 2009, 19, 7425–7443. [Google Scholar] [CrossRef]
- Xiong, Y.; Si, J.; Yan, L.; Song, H.; Yi, W.; Hou, X. The influence of nonlinear scattering light distributions on the optical limiting properties of carbon nanotubes. Laser Phys. Lett. 2014, 11, 115904. [Google Scholar] [CrossRef]

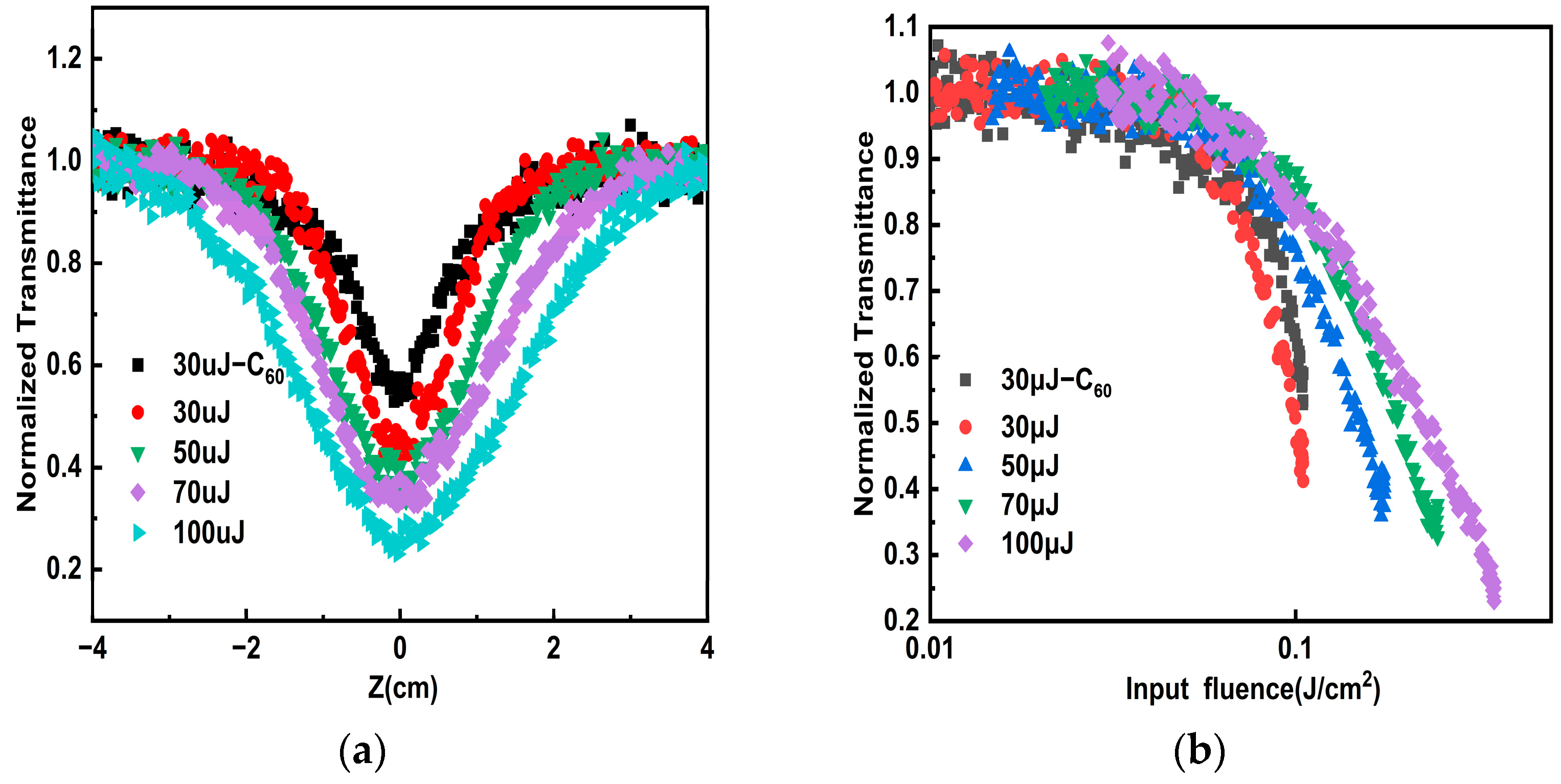

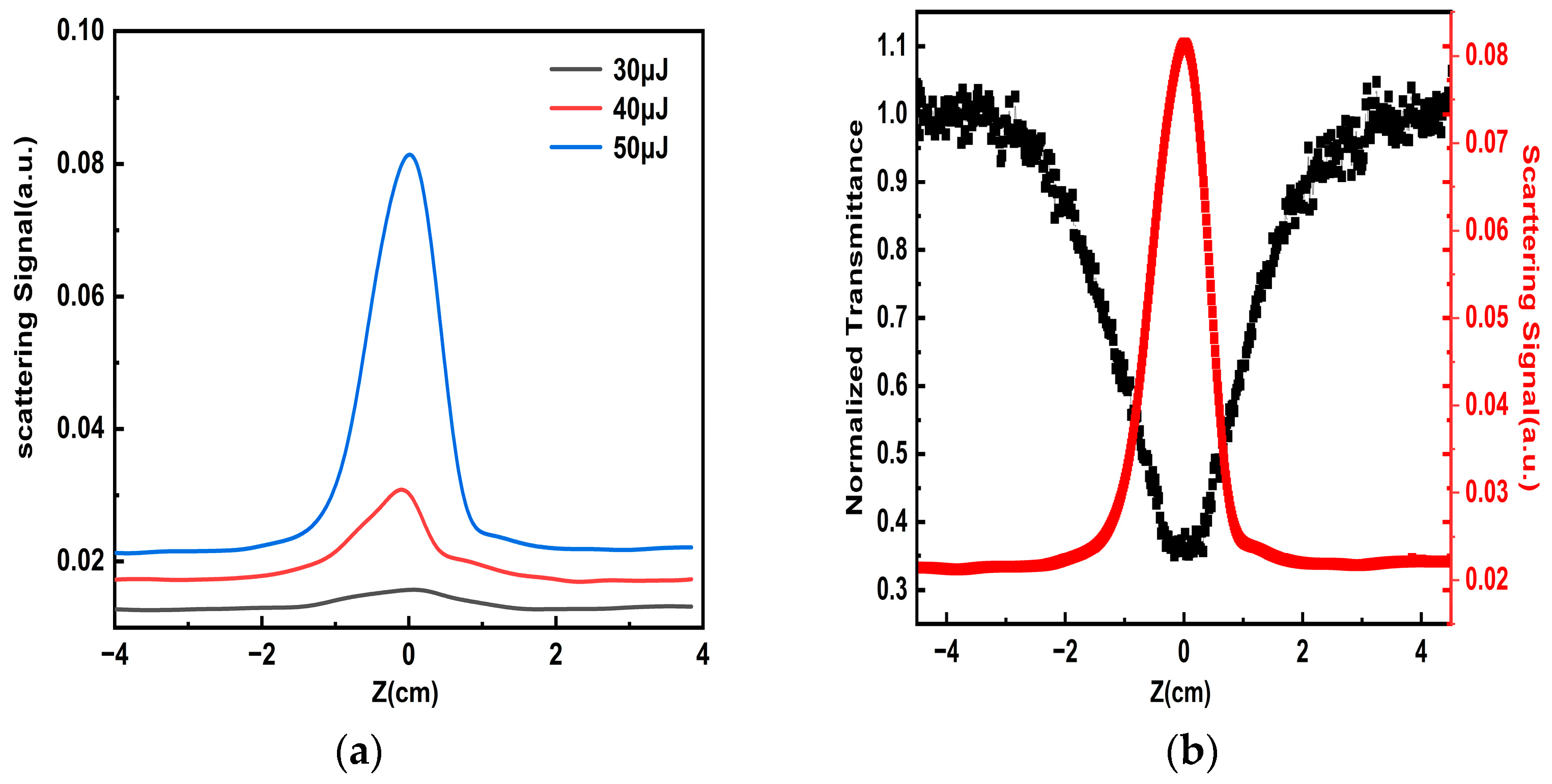
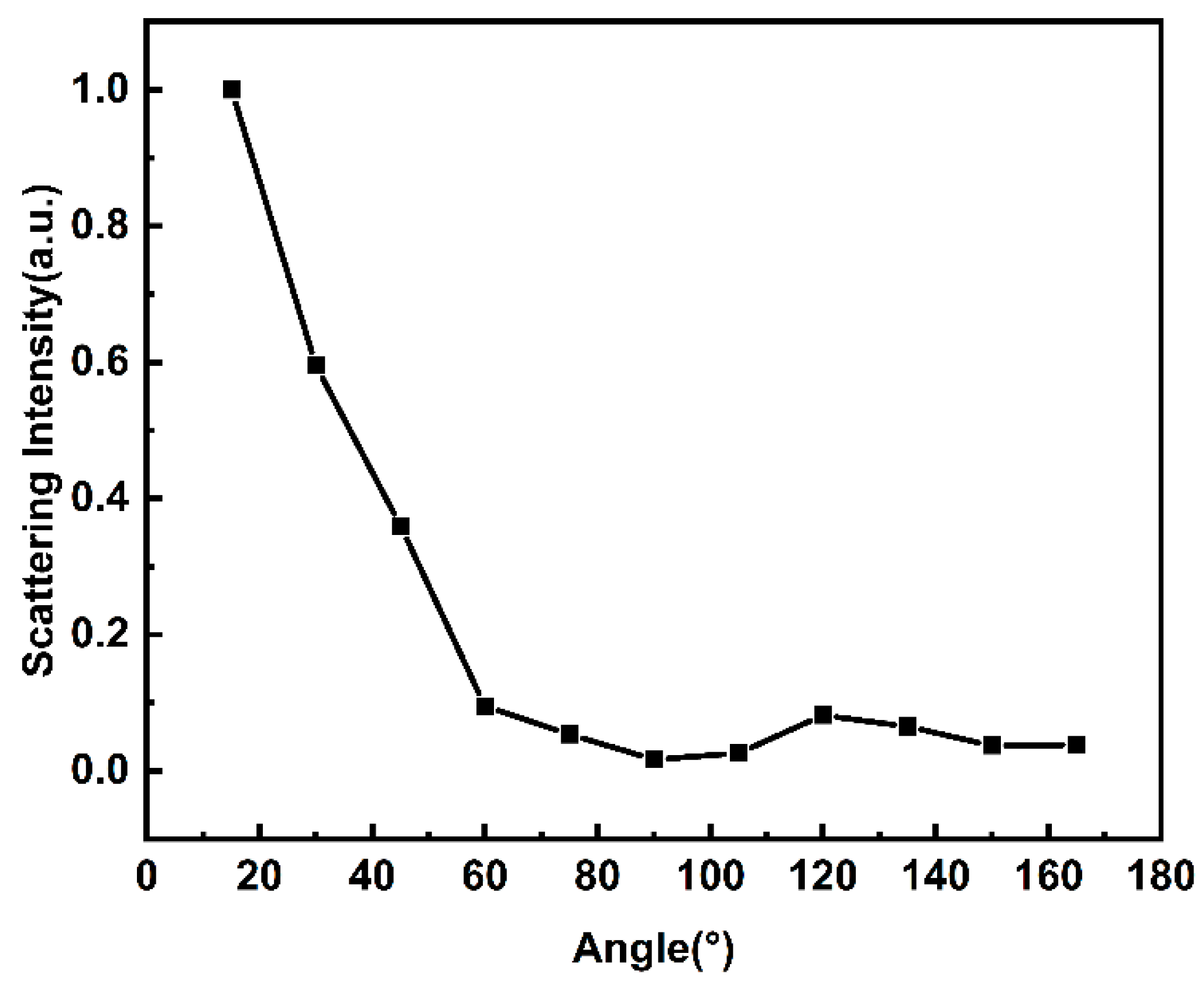
| Sample | Pulse Energy (μJ) | The Minimum Value of Normalized Transmittance | OL Threshold |
|---|---|---|---|
| C60 | 30 | 51.2% | 0.13 J/cm2 |
| Porous carbon | 30 | 38.8% | 0.11 J/cm2 |
| Porous carbon | 50 | 35.7% | 0.15 J/cm2 |
| Porous carbon | 70 | 32.7% | 0.19 J/cm2 |
| Porous carbon | 100 | 22.9% | 0.22 J/cm2 |
| Pulse Energy (μJ) | The Minimum Value of Normalized Transmittance | OL Threshold |
|---|---|---|
| 20 | 74.7% | |
| 30 | 66.4% | |
| 50 | 55.1% | |
| 70 | 49.8% | 0.25 J/cm2 |
| 100 | 40.2% | 0.32 J/cm2 |
| Sample | Normalized Transmittance at the Focal Point | Optical Limiting Threshold | Reference |
|---|---|---|---|
| Porous carbon at 532 nm | 42.7% | 0.11 J/cm2 | This paper |
| Porous carbon at 1064 nm | 49.8% | 0.25 J/cm2 | This paper |
| N-CD-Pt at 532 nm | 30.1% | 0.62 J/cm2 | [11] |
| rGO-TiO2 at 1030 nm | 29.7% | 1.5 J/cm2 | [24] |
| Au-graphene nanocomposites at 532 nm | 23.2% | 0.4 J/cm2 | [25] |
| CNT’s at 532 nm | 37.9% | 3 J/cm2 | [26] |
| indium phthalocyanine/SWCNTs at 532 nm | 24.9% | 0.21 J/cm2 | [27] |
| MWCNTs/TiO2 at 532 nm | 19% | 0.22 J/cm2 | [28] |
| CNH at 532 nm | 43% | 1.8 J/cm2 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, B.; Zhao, X.; Yan, L.; Yang, L.; Zhang, Y.; Lin, T.; Si, J. Optical Limiting Response of Porous Carbon Dispersions. Nanomaterials 2024, 14, 533. https://doi.org/10.3390/nano14060533
Gao B, Zhao X, Yan L, Yang L, Zhang Y, Lin T, Si J. Optical Limiting Response of Porous Carbon Dispersions. Nanomaterials. 2024; 14(6):533. https://doi.org/10.3390/nano14060533
Chicago/Turabian StyleGao, Bo, Xuhui Zhao, Lihe Yan, Lijiao Yang, Yue Zhang, Tao Lin, and Jinhai Si. 2024. "Optical Limiting Response of Porous Carbon Dispersions" Nanomaterials 14, no. 6: 533. https://doi.org/10.3390/nano14060533






