High Barrier Properties of Butyl Rubber Composites Containing Liquid Rubber and Graphene Oxide
Abstract
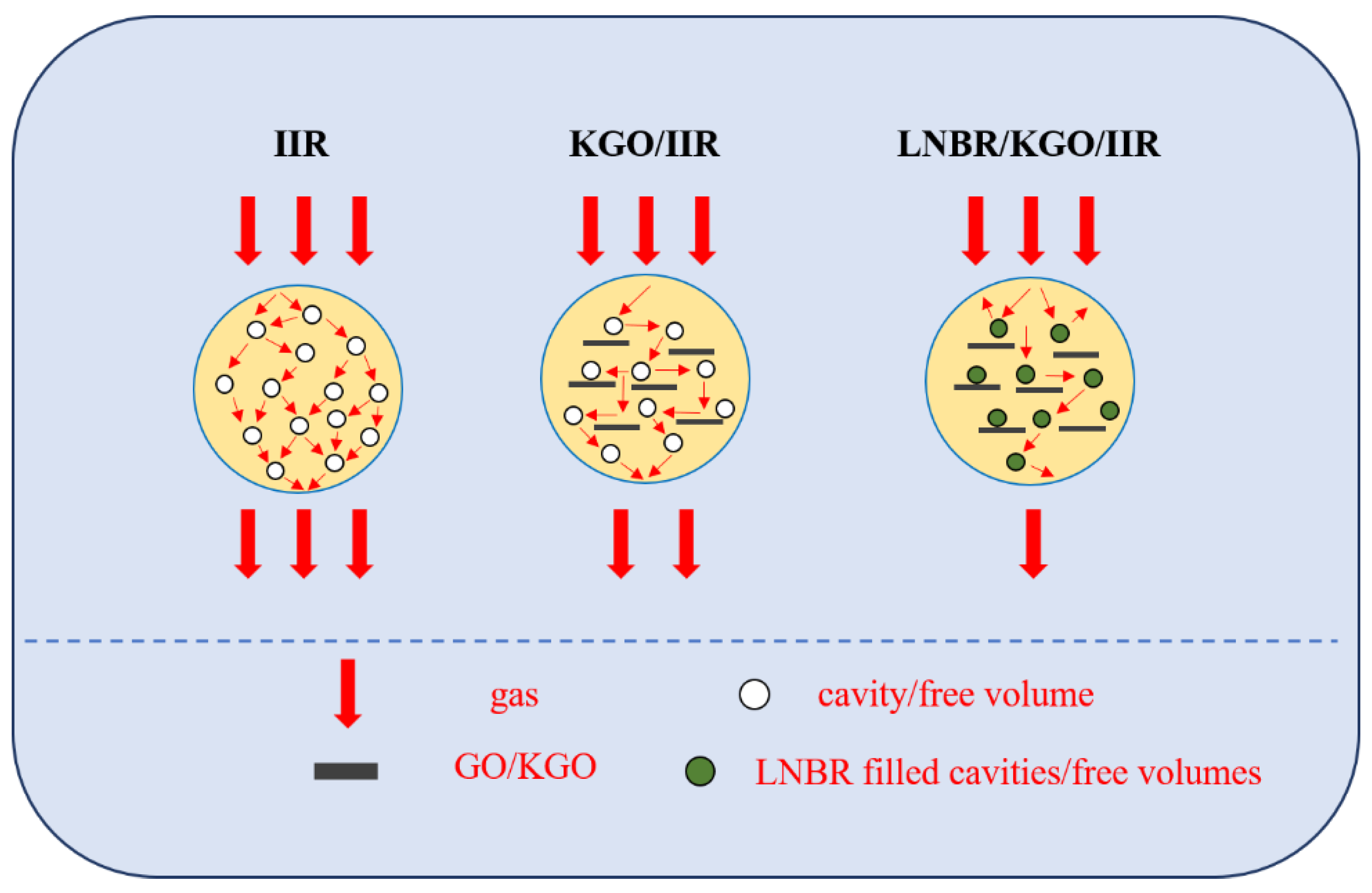
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of KH570 Modified GO
2.2.2. Preparation of LNBR/KGO/IIR Composites
2.3. Characterizations
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. X-ray Diffraction (XRD)
2.3.3. Thermal Analysis (TGA)
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. Rotorless Cure Meters
2.3.6. Nuclear Magnetic Resonance (NMR)
2.3.7. Rubber Processing Analyzer (RPA)
2.3.8. Dynamic Mechanical Thermal Analyzer (DMTA)
2.3.9. Tensile Test
2.3.10. Gas Permeability Measuring Apparatus
3. Results
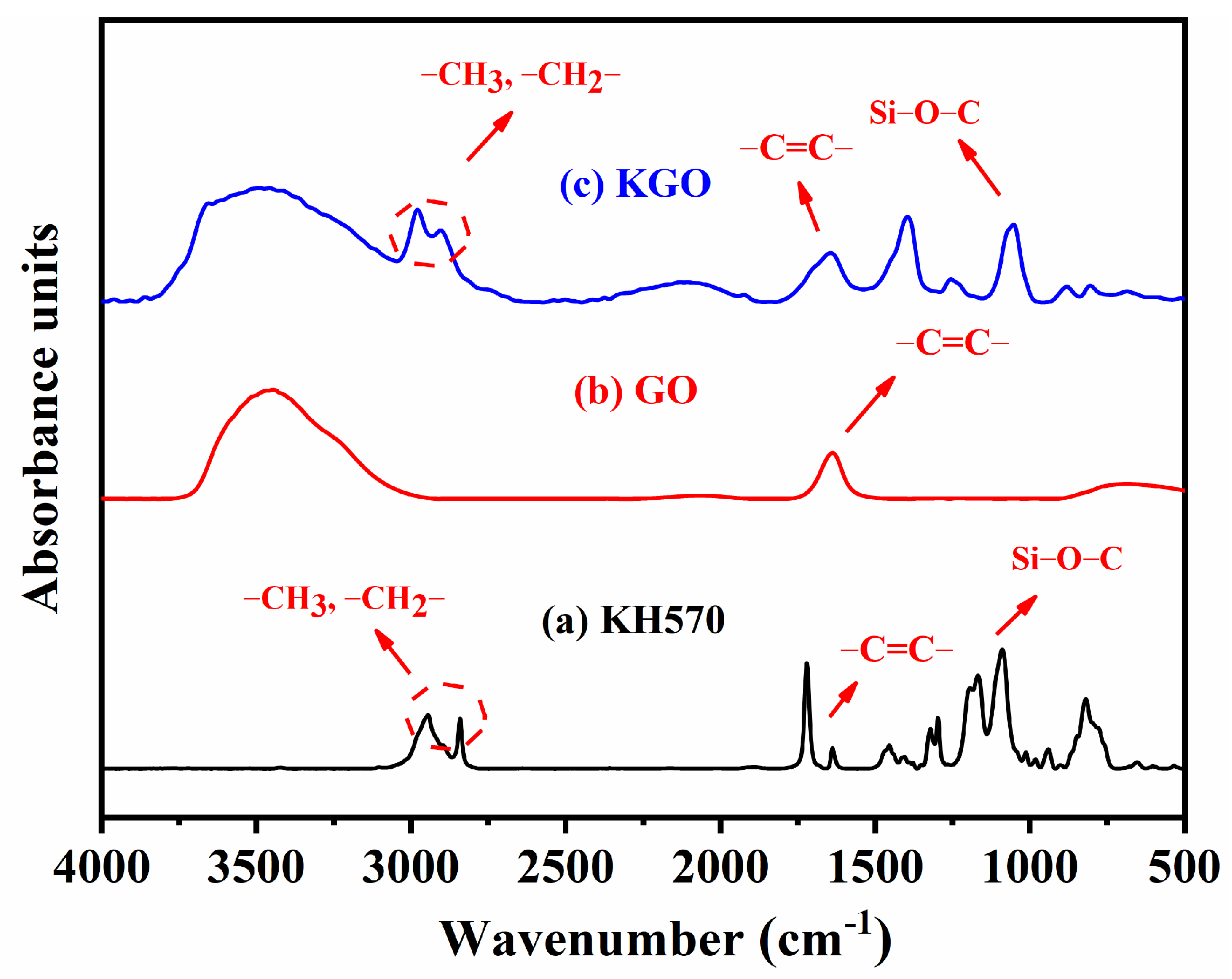
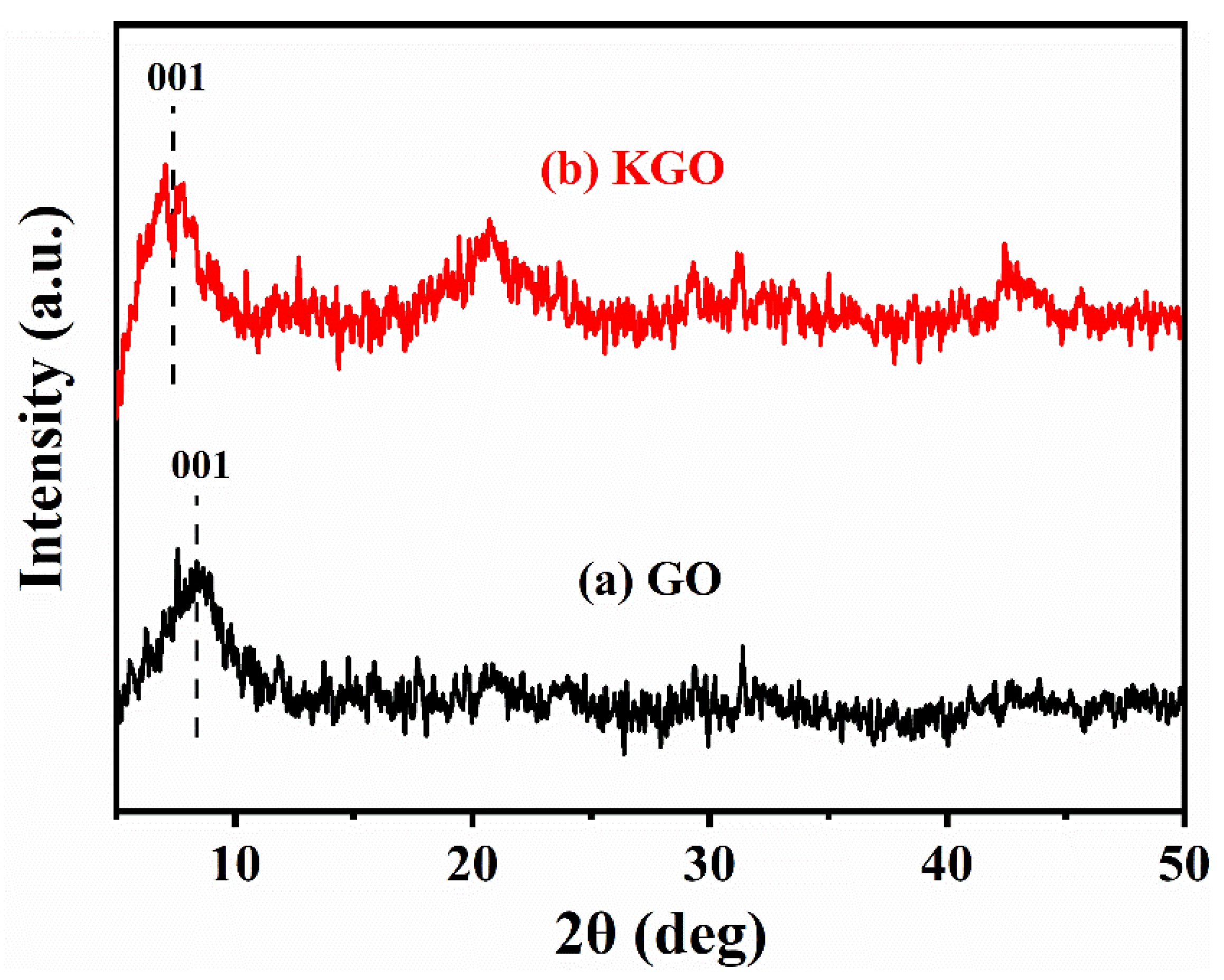
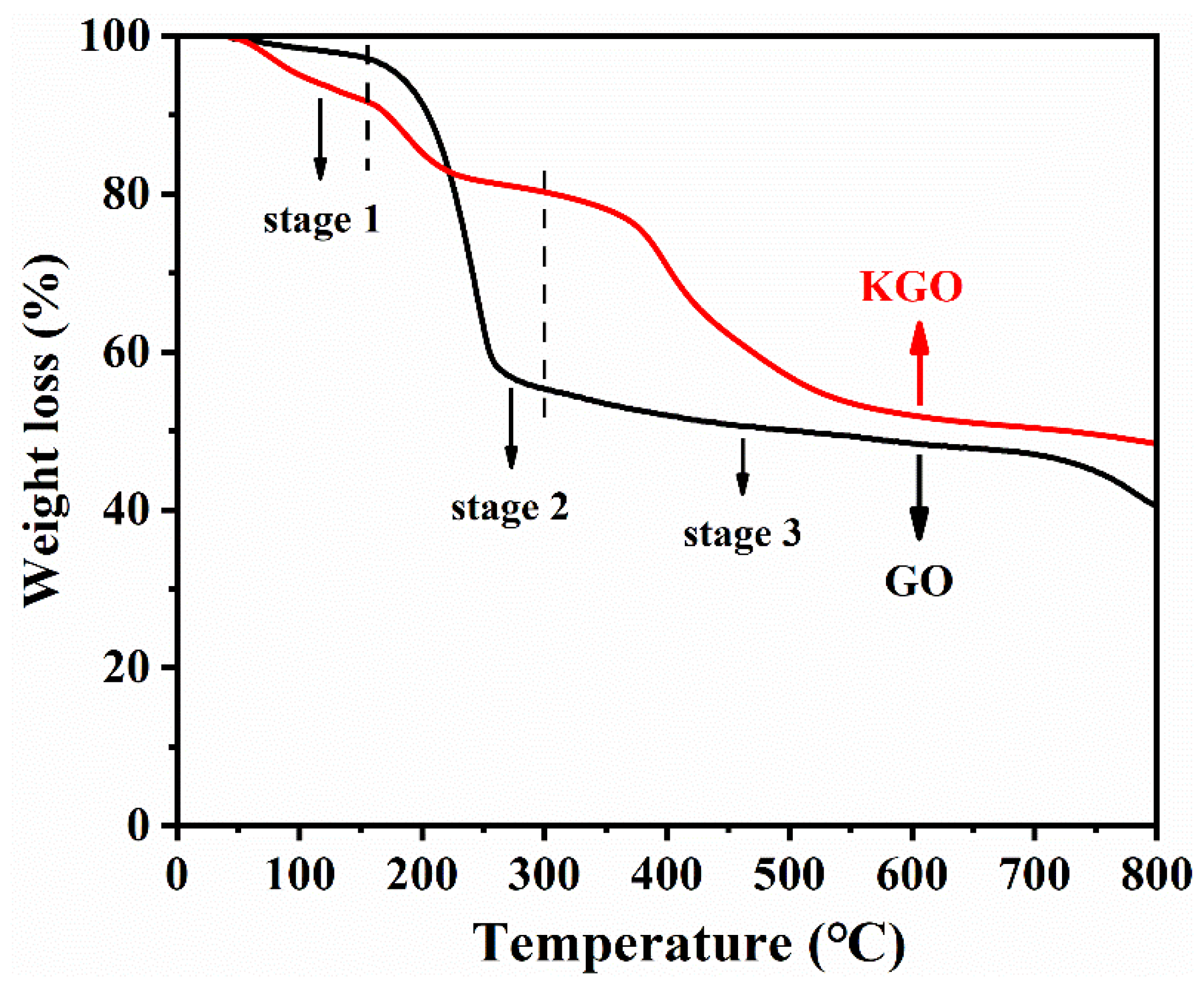
3.1. Characterization of KGO
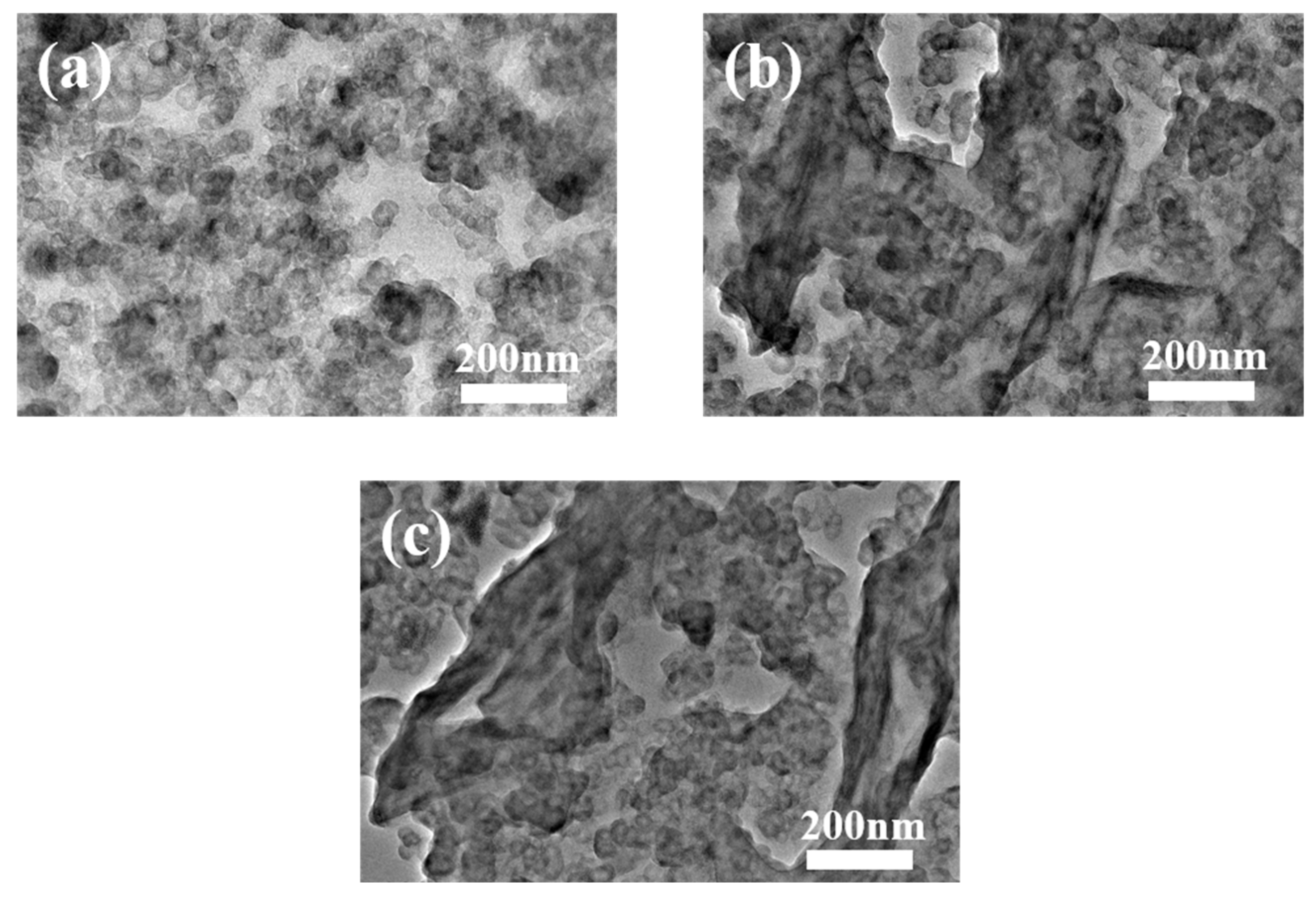
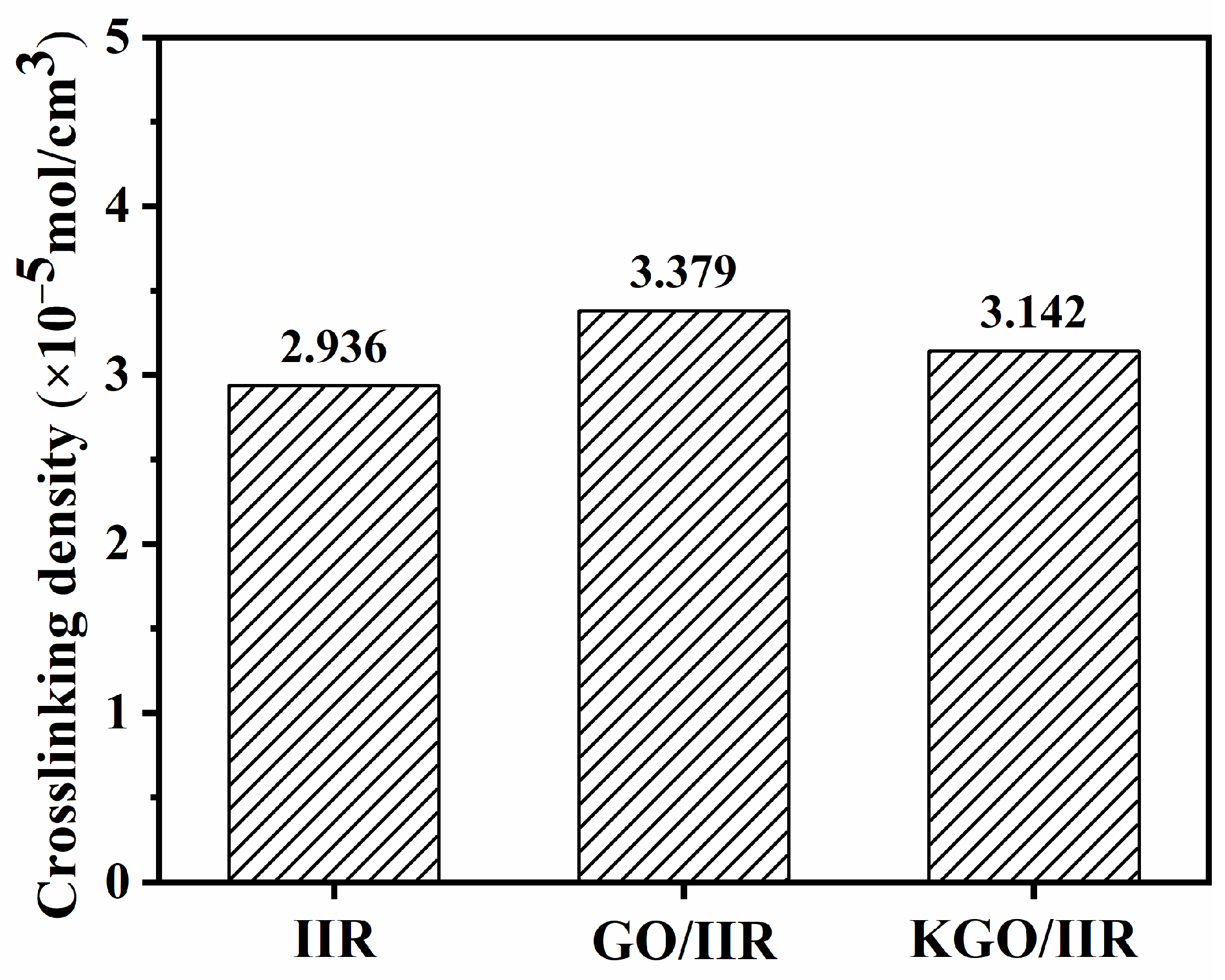
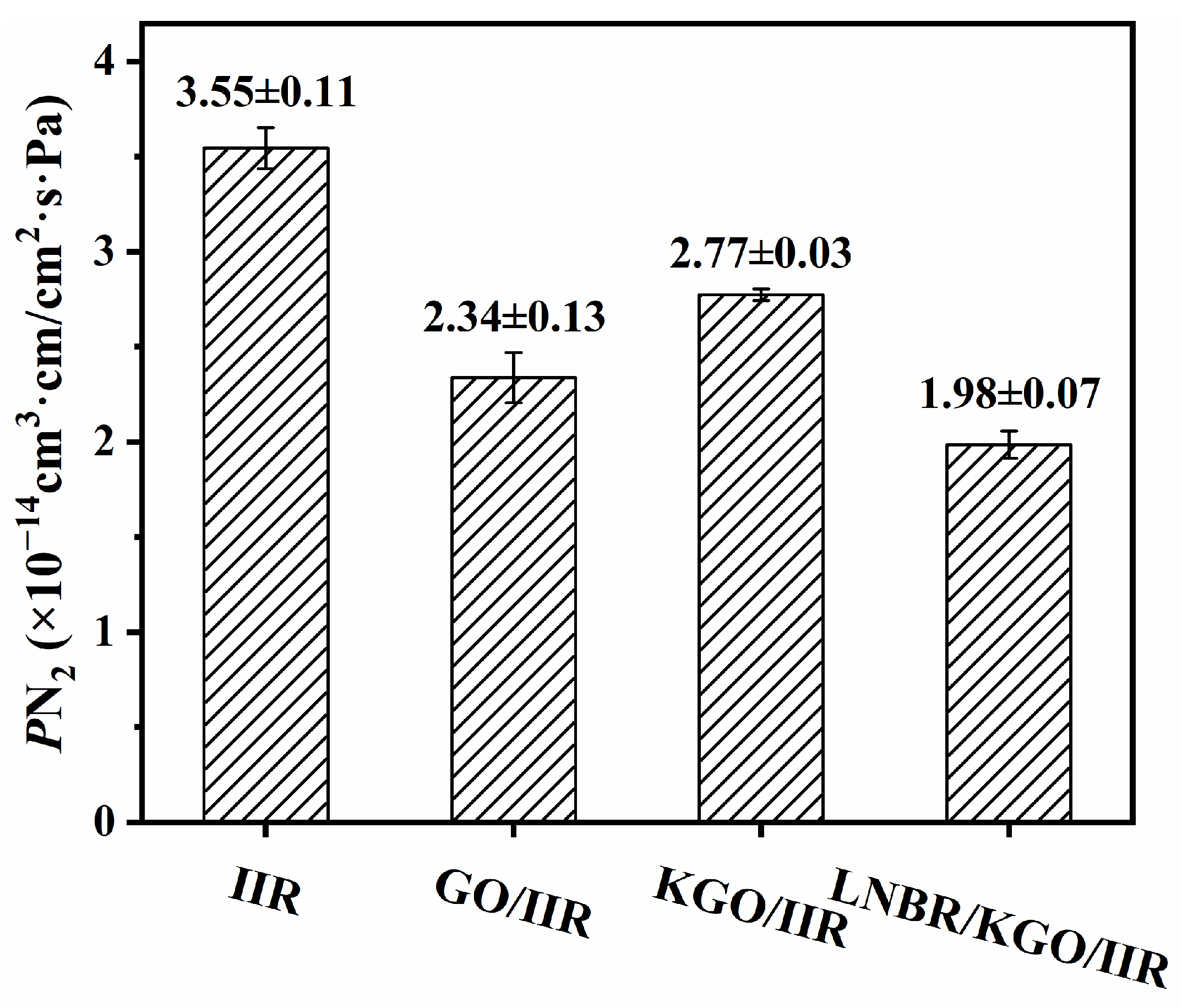
3.2. Characterizations of GO/CB/IIR Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dutta, A.; Chanda, J.; Bhandary, T.; Das, S.K.; Banerjee, S.S.; Ghosh, P.; Das Gupta, S.; Mukhopadhyay, R. Influence of organically modified clays on the barrier properties of bromo butyl rubber composites for tyre inner liner application. Polym.-Plast. Technol. 2022, 61, 2049–2062. [Google Scholar] [CrossRef]
- Li, T.; Su, Y.X.; Wang, D.Y.; Mao, Y.Y.; Wang, W.C.; Liu, L.; Wen, S.P. High antibacterial and barrier properties of natural rubber comprising of silver-loaded graphene oxide. Int. J. Biol. Macromol. 2022, 195, 449–455. [Google Scholar] [CrossRef]
- Zhang, C.F.; An, X.L.; Tang, Z.H.; Fang, S.F.; Guo, B.C.; Zhang, L.Q.; Liu, F.; Liu, J.J.; Chen, Z.Q. Creation of Tortuosity in Unfilled Rubber via Heterogeneous Cross-Linking toward Improved Barrier Property. Macromolecules 2021, 54, 11522–11532. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, D.Y.; Xu, Z.C.; Zhang, L.Q.; Liu, L.; Wen, S.P. High barrier properties against sulfur mustard of graphene oxide/butyl rubber composites. Compos. Sci. Technol. 2019, 170, 141–147. [Google Scholar] [CrossRef]
- Li, Y.J.; He, Q.; Zhang, H.; Liu, A.J.; He, Z.Y.; Li, Y.T.; Zhou, Y.L. Functionalised graphene oxide-bromobutyl rubber composites with segregated structure for enhanced gas barrier properties. Plast. Rubber Compos. 2022, 51, 363–371. [Google Scholar] [CrossRef]
- He, F.F.; Mensitieri, G.; Lavorgna, M.; de Luna, M.S.; Filippone, G.; Xia, H.S.; Esposito, R.; Scherillo, G. Tailoring gas permeation and dielectric properties of bromobutyl rubber—Graphene oxide nanocomposites by inducing an ordered nanofiller microstructure. Compos. Part B Eng. 2017, 116, 361–368. [Google Scholar] [CrossRef]
- Gruber, T.C.; Crossley, S.D.; Smith, A.P. Computational investigation of the effects of spherical filler morphology and loading on diffusion morphology and rubber permeability. Rubber Chem. Technol. 2013, 86, 175–189. [Google Scholar] [CrossRef]
- Raju, A.T.; Das, M.; Dash, B.; Dey, P.; Nair, S.; Naskar, K. Variation of air permeability in bromobutyl rubber/epoxidized natural rubber composites: Influence of structure of filler particle. Polym. Eng. Sci. 2022, 62, 718–729. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, B.; Qi, N.; Zhang, H.F.; Zhang, L.Q. Influence of fillers on free volume and gas barrier properties in styrene-butadiene rubber studied by positrons. Polymer 2005, 46, 719–724. [Google Scholar] [CrossRef]
- Wang, L.M.; Dou, Y.B.; Wang, J.J.; Han, J.B.; Liu, L.; Wei, M. Layer-by-layer assembly of layered double hydroxide/rubber multilayer films with excellent gas barrier property. Compos. Part A Appl. Sci. Manuf. 2017, 102, 314–321. [Google Scholar] [CrossRef]
- Cui, Y.B.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Paduvilan, J.K.; Velayudhan, P.; Amanulla, A.; Maria, H.J.; Saiter-Fourcin, A.; Thomas, S. Assessment of Graphene Oxide and Nanoclay Based Hybrid Filler in Chlorobutyl-Natural Rubber Blend for Advanced Gas Barrier Applications. Nanomaterials 2021, 11, 1098. [Google Scholar] [CrossRef]
- Roilo, D.; Patil, P.N.; Brusa, R.S.; Miotello, A.; Aghion, S.; Ferragut, R.; Checchetto, R. Polymer rigidification in graphene based nanocomposites: Gas barrier effects and free volume reduction. Polymer 2017, 121, 17–25. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Lin, S.Y.; Tsai, H.C. Butyl Rubber Nanocomposites with Monolayer MoS2 Additives: Structural Characteristics, Enhanced Mechanical, and Gas Barrier Properties. Polymers 2018, 10, 238. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Fu, Y.J.; An, Q.F.; Lo, S.C.; Zhong, Y.Z.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Enhancing polymer/graphene oxide gas barrier film properties by introducing new crystals. Carbon 2014, 75, 443–451. [Google Scholar] [CrossRef]
- Yang, S.Q.; Wu, H.; Li, C.H.; Xiong, Y.; Guo, S.Y. Constructing Oriented Two-Dimensional Large-Sized Modified Graphene Oxide Barrier Walls in Brominated Butyl Rubber to Achieve Excellent Gas Barrier Properties. ACS Appl. Mater. Interfaces 2020, 12, 3976–3983. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, J.T.; Sun, Y.Y.; Zhang, T.; Wang, L.; Wang, J.; Liang, Y.R.; Hao, M.Z.; Fu, Q. Green preparation and enhanced gas barrier property of rubber nanocomposite film based on graphene oxide-induced chemical crosslinking. Polymer 2021, 225, 123756. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Thomas, R.; Dufresne, A.; Thomas, S.; Hassan, A. Functionalized Liquid Natural Rubber and Liquid Epoxidized Natural Rubber: A Promising Green Toughening Agent for Polyester. J. Appl. Polym. Sci. 2015, 132, 41292. [Google Scholar] [CrossRef]
- Wen, S.P.; Zhang, R.; Xu, Z.C.; Zheng, L.; Liu, L. Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites. Materials 2020, 13, 5416. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Wang, Q.Q.; Xu, P.W.; Yang, W.J.; Hoch, M.; Ma, P.M. Toughening of epoxy resin with balanced performance by in situ constructing interfacial bonding and elastic segments into epoxy network. Mater. Chem. Phys. 2022, 277, 125529. [Google Scholar] [CrossRef]
- Jaafar, C.N.A.; Zainol, I.; Ishak, N.S.; Ilyas, R.A.; Sapuan, S.M. Effects of the liquid natural rubber (LNR) on mechanical properties and microstructure of epoxy/silica/kenaf hybrid composite for potential automotive applications. J. Mater. Res. Technol. 2021, 12, 1026–1038. [Google Scholar] [CrossRef]
- Vertuccio, L.; Guadagno, L.; Spinelli, G.; Russo, S.; Iannuzzo, G. Effect of carbon nanotube and functionalized liquid rubber on mechanical and electrical properties of epoxy adhesives for aircraft structures. Compos. Part B Eng. 2017, 129, 1–10. [Google Scholar] [CrossRef]
- Gruendken, M.; Velencoso, M.M.; Hirata, K.; Blume, A. Structure-propery relationship of low molecular weight ‘liquid’ polymers in blends of sulfur cured SSBR-rich compounds. Polym. Test. 2020, 87, 106558. [Google Scholar] [CrossRef]
- Zeng, M.F.; Sun, X.D.; Yao, X.D.; Wang, Y.; Zhang, M.Z.; Wang, B.Y.; Qi, C.Z. Free-volume properties and toughening behavior of cyanate ester resin/carboxyl-randomized liquid butadiene-acrylonitrile rubber composites. J. Mater. Sci. 2009, 44, 4270–4278. [Google Scholar] [CrossRef]
- Yin, X.Y.; Xie, Z.P.; Liu, Q.; Yuan, X.B.; Hou, X.; Zhao, J. Synergistic toughening of epoxy resin by CTBN and CM-β-CD. J. Appl. Polym. Sci. 2021, 138, e51248. [Google Scholar] [CrossRef]
- Woraphutthaporn, S.; Pattananuwat, P.; Hayichelaeh, C.; Kobayashi, T.; Boonkerd, K. Enhancing the properties of graphene oxide/natural rubber nanocomposite-based strain sensor modified by amino-functionalized silanes. Polym. Adv. Technol. 2022, 33, 3386–3398. [Google Scholar] [CrossRef]
- Obaid, A.N.; Al-Bermany, E. Performance of functionalized graphene oxide to improve anti-corrosion of epoxy coating on 2024-T3 aluminium alloy. Mater. Chem. Phys. 2023, 305, 127849. [Google Scholar] [CrossRef]
- Zheng, L.; Jerrams, S.; Xu, Z.C.; Zhang, L.Q.; Liu, L.; Wen, S.P. Enhanced gas barrier properties of graphene oxide/rubber composites with strong interfaces constructed by graphene oxide and sulfur. Chem. Eng. J. 2020, 383, 123100. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Mazinani, S. Influences of sodium dodecyl sulfate on vulcanization kinetics and mechanical performance of EPDM/graphene oxide nanocomposites. RSC Adv. 2015, 5, 46694–46704. [Google Scholar] [CrossRef]
- Aswathy, T.R.; Dash, B.; Dey, P.; Nair, S.; Naskar, K. Synergistic effect of graphene with graphene oxide, nanoclay, and nanosilica in enhancing the mechanical and barrier properties of bromobutyl rubber/epoxidized natural rubber composites. J. Appl. Polym. Sci. 2021, 138, e50746. [Google Scholar] [CrossRef]
- Wu, J.; He, Q.; Li, Y.J.; Liu, D.L.; Zhou, Y.L. Facile and Eco-Friendly Preparation of GO/BIIR Composite for Gas Barrier Ap-plications. Nano 2019, 14, 28–36. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zhang, L.Q.; Feng, W.J.; Guo, B.C.; Liu, F.; Jia, D.M. Rational Design of Graphene Surface Chemistry for High-Performance Rubber/Graphene Composites. Macromolecules 2014, 47, 8663–8673. [Google Scholar] [CrossRef]
- Yan, N.; Buonocore, G.; Lavorgna, M.; Kaciulis, S.; Balijepalli, S.K.; Zhan, Y.; Xia, H.; Ambrosio, L. The role of reduced graphene oxide on chemical, mechanical and barrier properties of natural rubber composites. Compos. Sci. Technol. 2014, 102, 74–81. [Google Scholar] [CrossRef]
- Mensah, B.; Onwona-Agyeman, B.; Efavi, J.K.; Ofor, R.A.; Zigah, M.; Koranteng, J.; Karikari, M.; Frank Nsaful, F.; Addo, D.A. Investigating the Effect of Curing Activators on the Cure Kinetics of Acrylonitrile-Butadiene Rubber Filled with Graphene Oxide and Reduced Graphene Oxides Nanocomposites. Int. J. Polym. Sci. 2023, 2023, 6387898. [Google Scholar] [CrossRef]
- Wu, J.R.; Xing, W.; Huang, G.S.; Li, H.; Tang, M.; Wu, S.; Liuet, Y. Vulcanization kinetics of graphene/natural rubber nanocomposites. Polymer 2013, 54, 3314–3323. [Google Scholar] [CrossRef]
- Jose, J.; Susamma, A.P. Studies on natural rubber nanocomposites by incorporating amine functionalised graphene oxide. Plast. Rubber Compos. 2021, 50, 35–47. [Google Scholar] [CrossRef]
- Raef, M.; Razzaghi-Kashani, M. The role of interface in gas barrier properties of styrene butadiene rubber-reduced graphene oxide composites. Polymer 2019, 182, 121816. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Wu, D.; Hakkarainen, M. Immobilized Graphene Oxide Nanosheets as Thin but Strong Nanointerfaces in Biocomposites. ACS Sustain. Chem. Eng. 2016, 4, 2211–2222. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.Q.; Peng, J.; Liu, L. Constructing a segregated graphene network in rubber composites towards improved electrically conductive and barrier properties. Compos. Sci. Technol. 2016, 131, 40–47. [Google Scholar] [CrossRef]
- Scherillo, G.; Lavorgna, M.; Buonocore, G.G.; Zhan, Y.H.; Xiaet, H.S.; Giuseppe Mensitieri, G.; Ambrosio, L. Tailoring Assembly of Reduced Graphene Oxide Nanosheets to Control Gas Barrier Properties of Natural Rubber Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 2230–2234. [Google Scholar] [CrossRef] [PubMed]


| Samples | IIR | GO/IIR | KGO/IIR | LNBR/KGO/IIR |
|---|---|---|---|---|
| IIR | 70 | 70 | 70 | 70 |
| IIR Latex | 30 | 30 | 30 | 30 |
| GO | - | 1.5 | 1.5 | 1.5 |
| KH570 | - | - | 3 | 3 |
| N330 | 40 | 40 | 40 | 40 |
| ZnO | 4 | 4 | 4 | 4 |
| SA | 2 | 2 | 2 | 2 |
| accelerant DM | 1.5 | 1.5 | 1.5 | 1.5 |
| accelerant TT | 0.8 | 0.8 | 0.8 | 0.8 |
| S | 2 | 2 | 2 | 2 |
| LNBR | - | - | - | 10 |
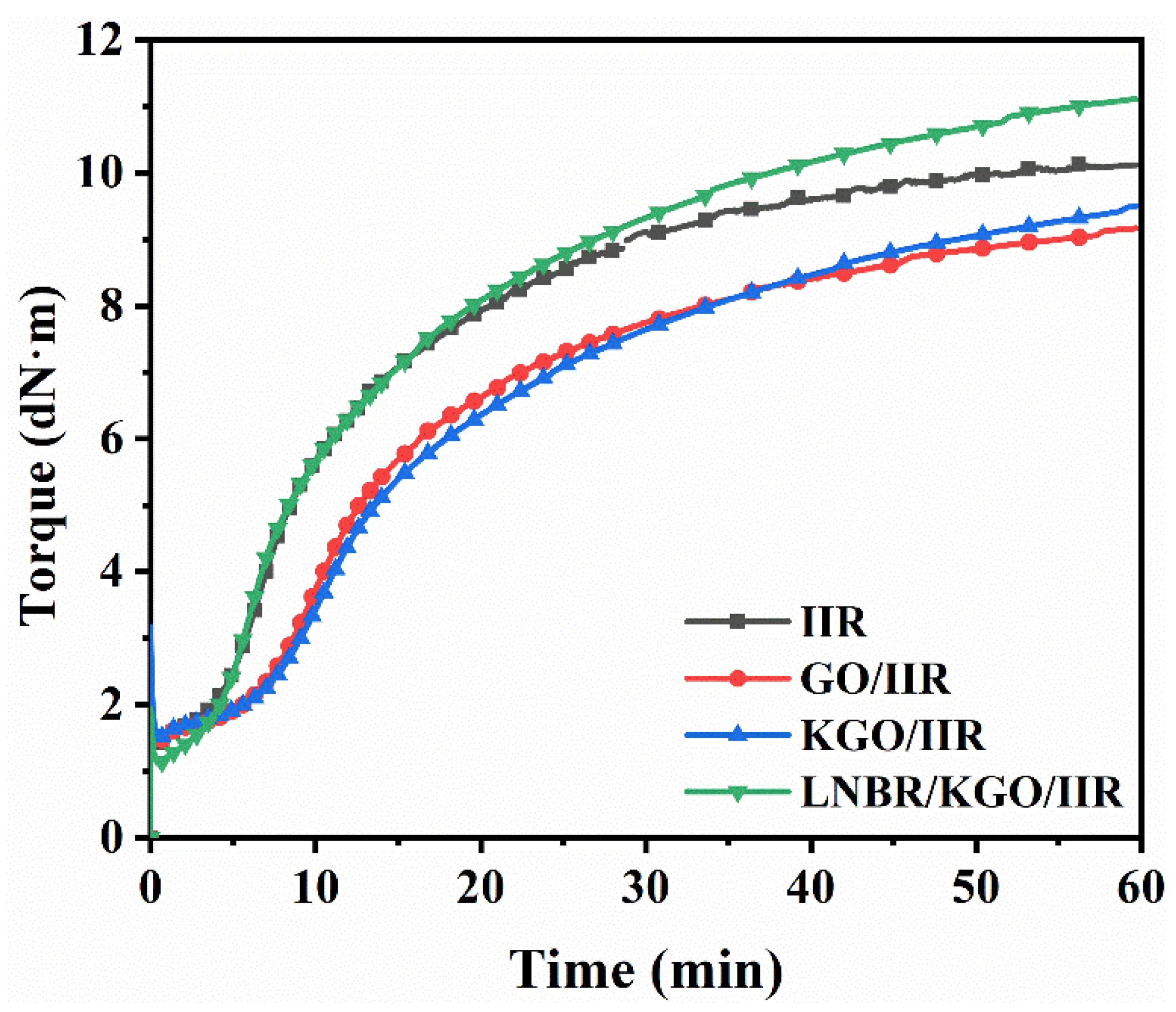
| Samples | MH (dN·m) | ML (dN·m) | ΔM (dN·m) | T10 (min) | T90 (min) |
|---|---|---|---|---|---|
| IIR | 9.93 | 1.36 | 8.57 | 4.5 | 28.6 |
| GO/IIR | 8.99 | 1.43 | 7.56 | 6.6 | 39.8 |
| KGO/IIR | 9.31 | 1.48 | 7.83 | 7.2 | 43.0 |
| LNBR/KGO/IIR | 10.89 | 1.10 | 9.79 | 4.4 | 39.2 |
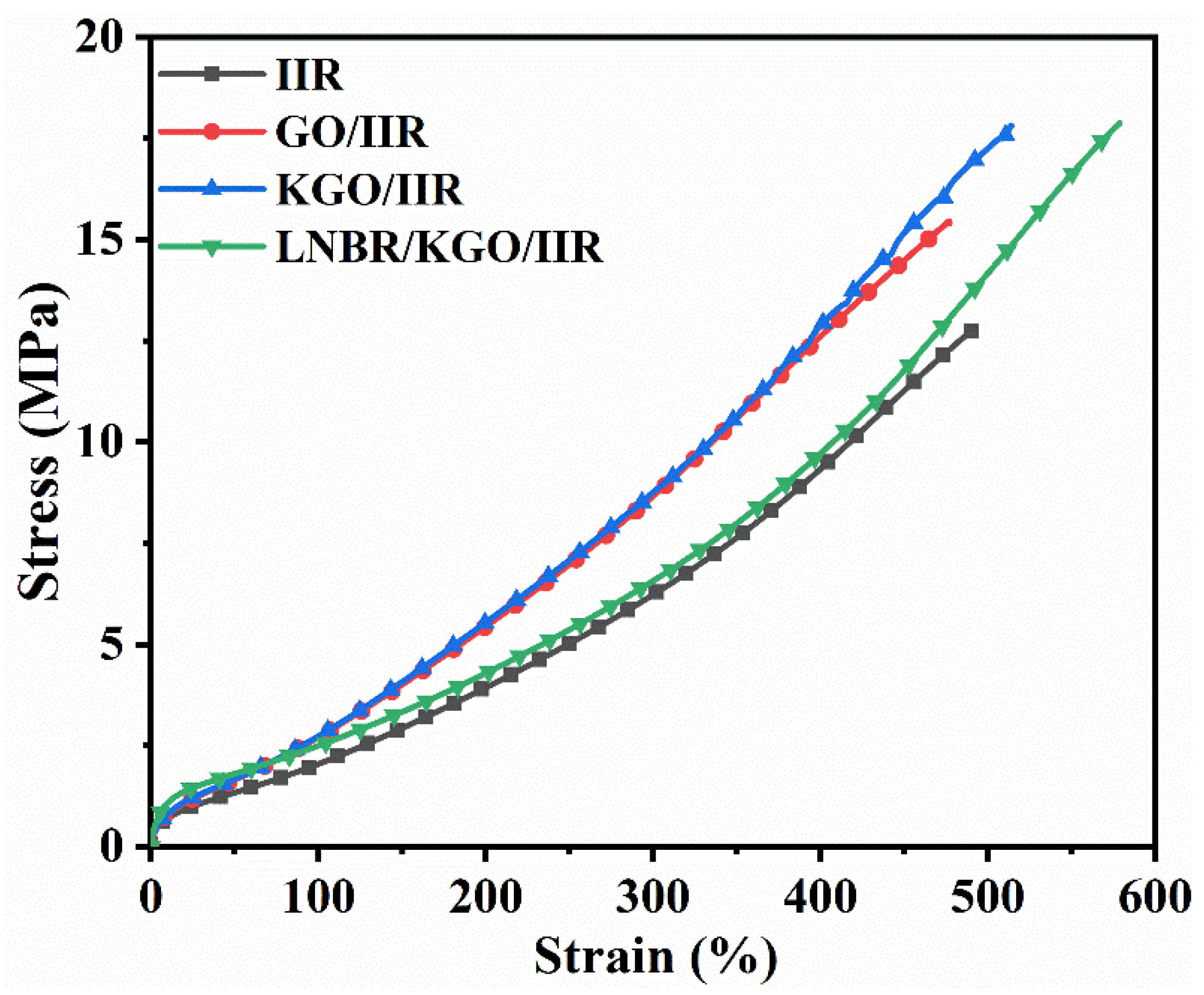
| Samples | Shore a Hardness | 100% Modulus (MPa) | 300% Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Tear Strength (kN/m) |
|---|---|---|---|---|---|---|
| IIR | 62 ± 1 | 2.0 ± 0.1 | 6.2 ± 0.2 | 12.8 ± 0.4 | 491 ± 11 | 34.5 ± 1.3 |
| GO/IIR | 64 ± 1 | 2.7 ± 0.2 | 8.6 ± 0.3 | 15.4 ± 0.5 | 477 ± 14 | 38.1 ± 1.5 |
| KGO/IIR | 65 ± 1 | 2.7 ± 0.1 | 8.9 ± 0.4 | 17.8 ± 0.5 | 514 ± 17 | 39.6 ± 1.7 |
| LNBR/KGO/IIR | 61 ± 1 | 2.5 ± 0.1 | 6.5 ± 0.2 | 17.8 ± 0.6 | 578 ± 16 | 46.2 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, Z.; Hu, S.; Huang, X.; Jerrams, S.; Hu, S.; Liu, L.; Wen, S. High Barrier Properties of Butyl Rubber Composites Containing Liquid Rubber and Graphene Oxide. Nanomaterials 2024, 14, 534. https://doi.org/10.3390/nano14060534
Li J, Yang Z, Hu S, Huang X, Jerrams S, Hu S, Liu L, Wen S. High Barrier Properties of Butyl Rubber Composites Containing Liquid Rubber and Graphene Oxide. Nanomaterials. 2024; 14(6):534. https://doi.org/10.3390/nano14060534
Chicago/Turabian StyleLi, Jiaye, Zhanghao Yang, Shanjun Hu, Xianhong Huang, Stephen Jerrams, Shui Hu, Li Liu, and Shipeng Wen. 2024. "High Barrier Properties of Butyl Rubber Composites Containing Liquid Rubber and Graphene Oxide" Nanomaterials 14, no. 6: 534. https://doi.org/10.3390/nano14060534





