In Vitro Toxicological Insights from the Biomedical Applications of Iron Carbide Nanoparticles in Tumor Theranostics: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
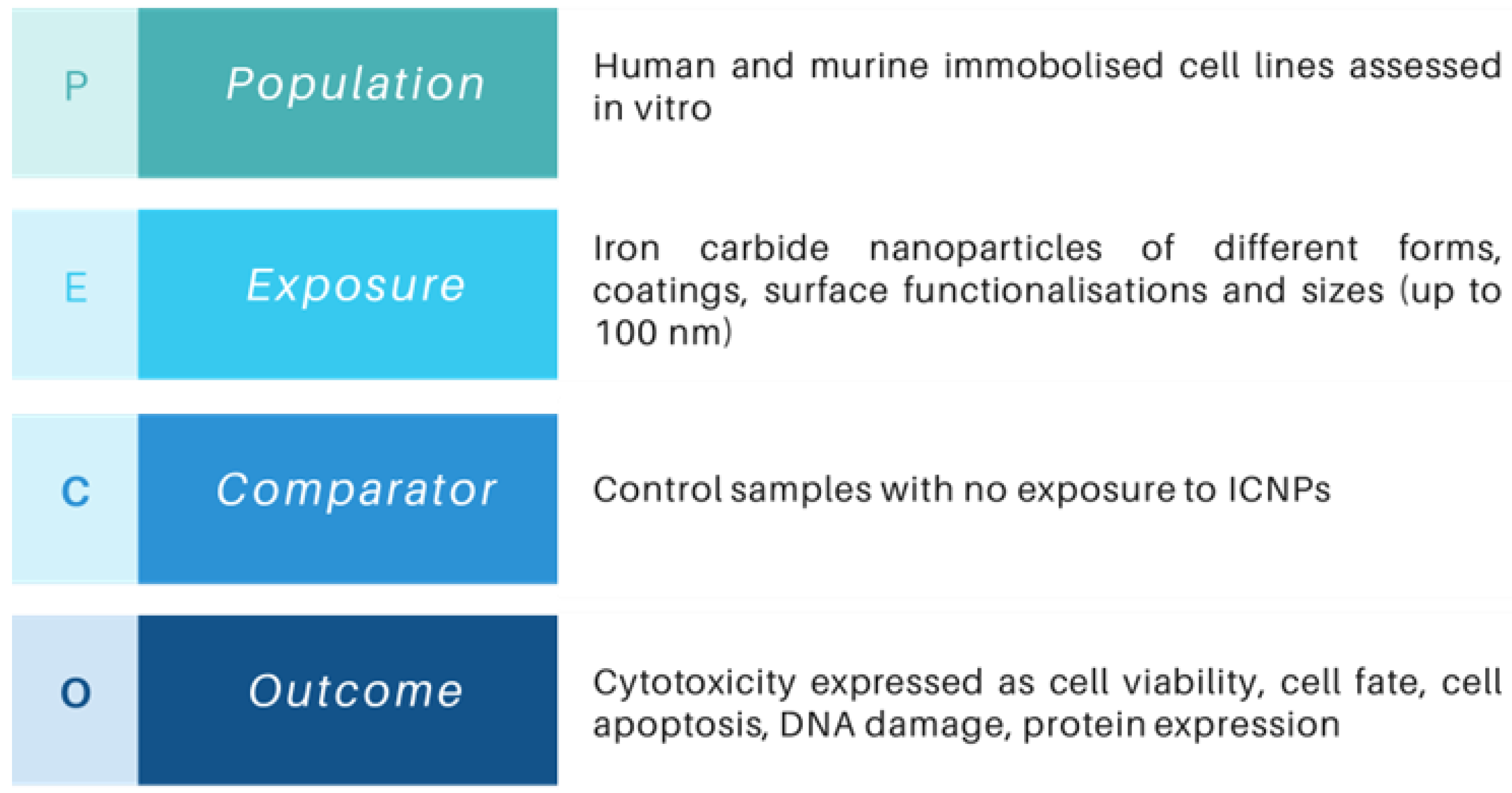
2.2. Eligibility Criteria
2.3. Search Strategy
- “Iron carbide” OR “FexCy” OR “Fe2C” OR “Fe3C” OR “Fe5C2” OR “Fe7C3”, AND
- cytotoxicity OR toxic OR “cell viability” OR adverse OR “in vitro”, AND
- biocompatibility OR biomedical OR nanomedicine OR theranostics OR mri OR “contrast agent” OR hyperthermia
2.4. Screening and Selection Process
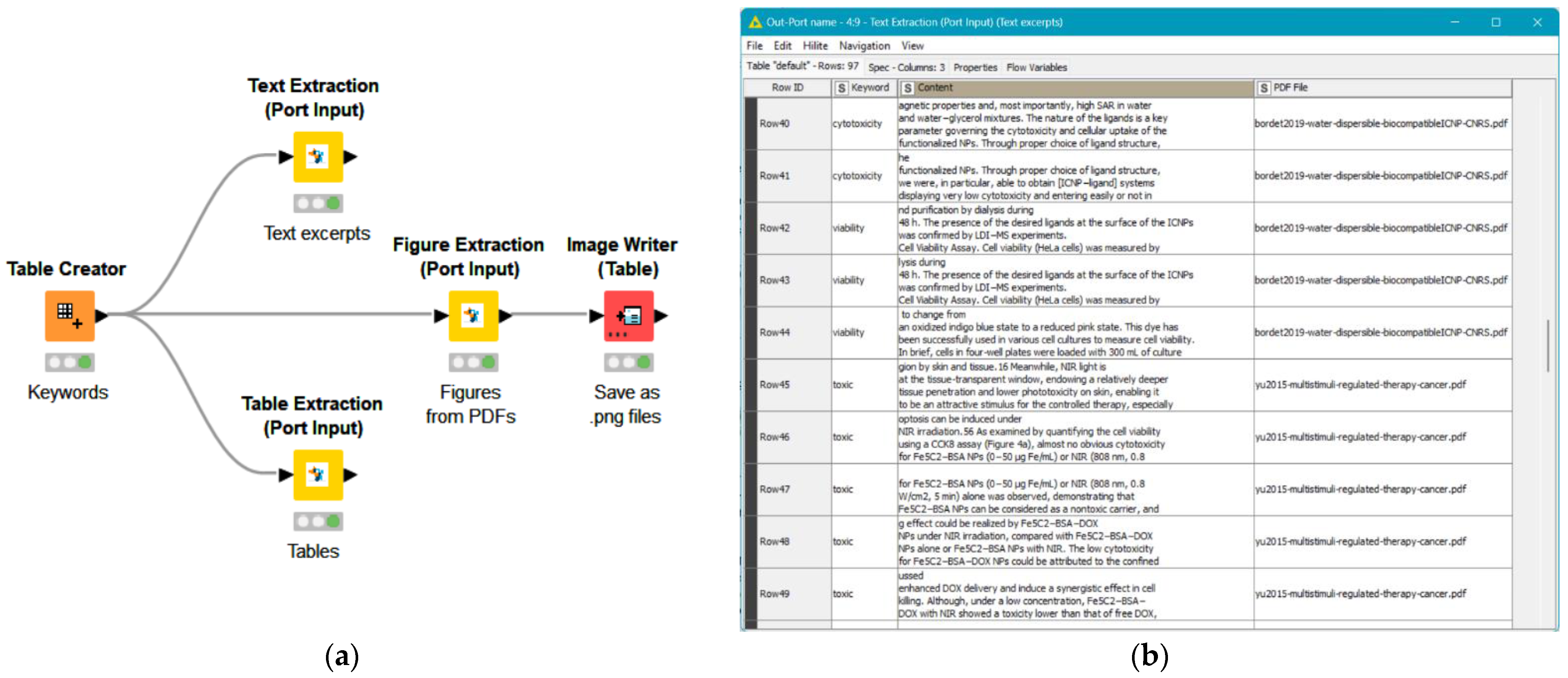
2.5. Data Extraction
2.6. Critical Appraisal
2.7. Statistical Analysis
3. Results
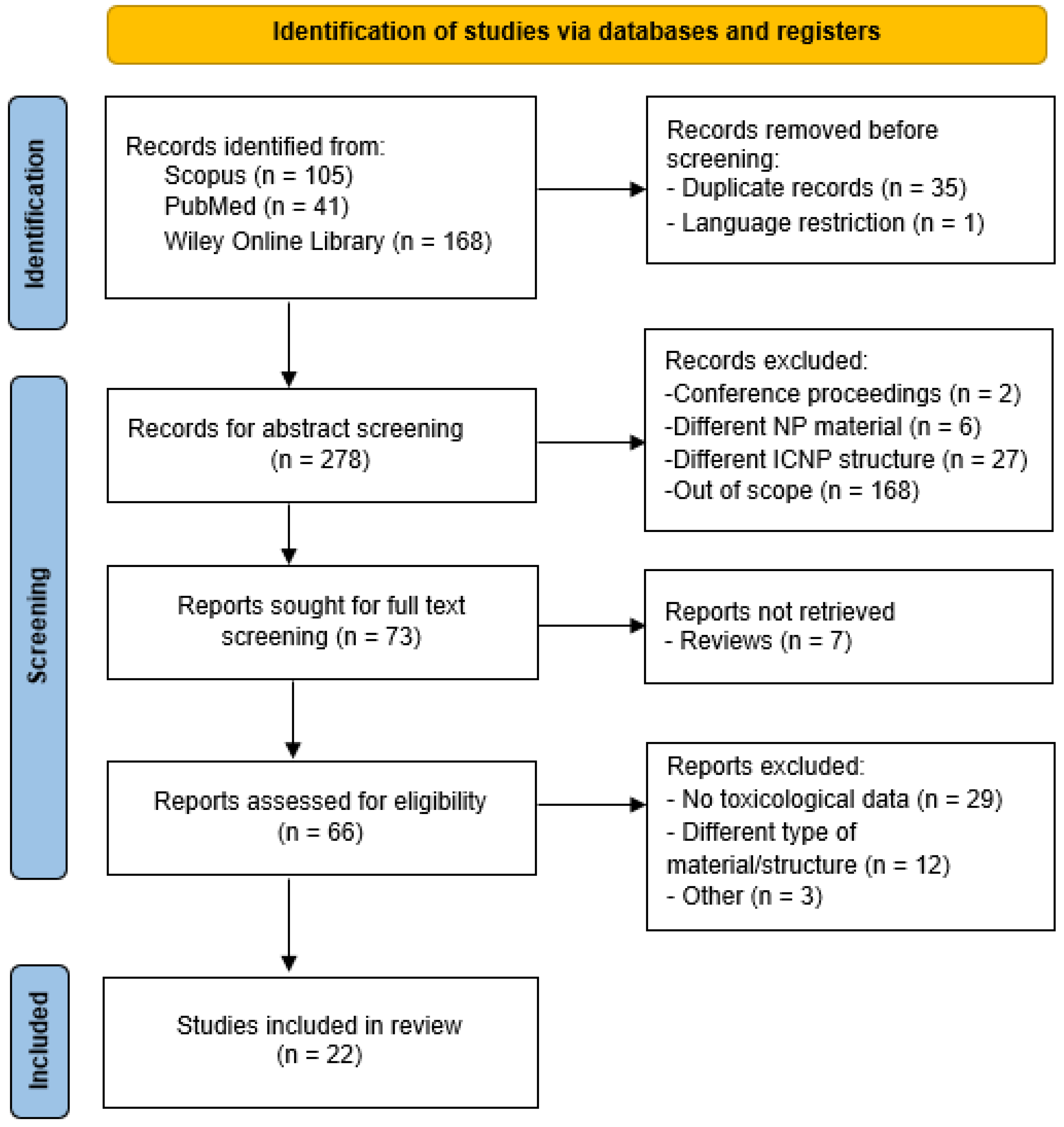
3.1. Literature Search
3.2. Characteristics of the Included Studies
3.3. Quality Assessment
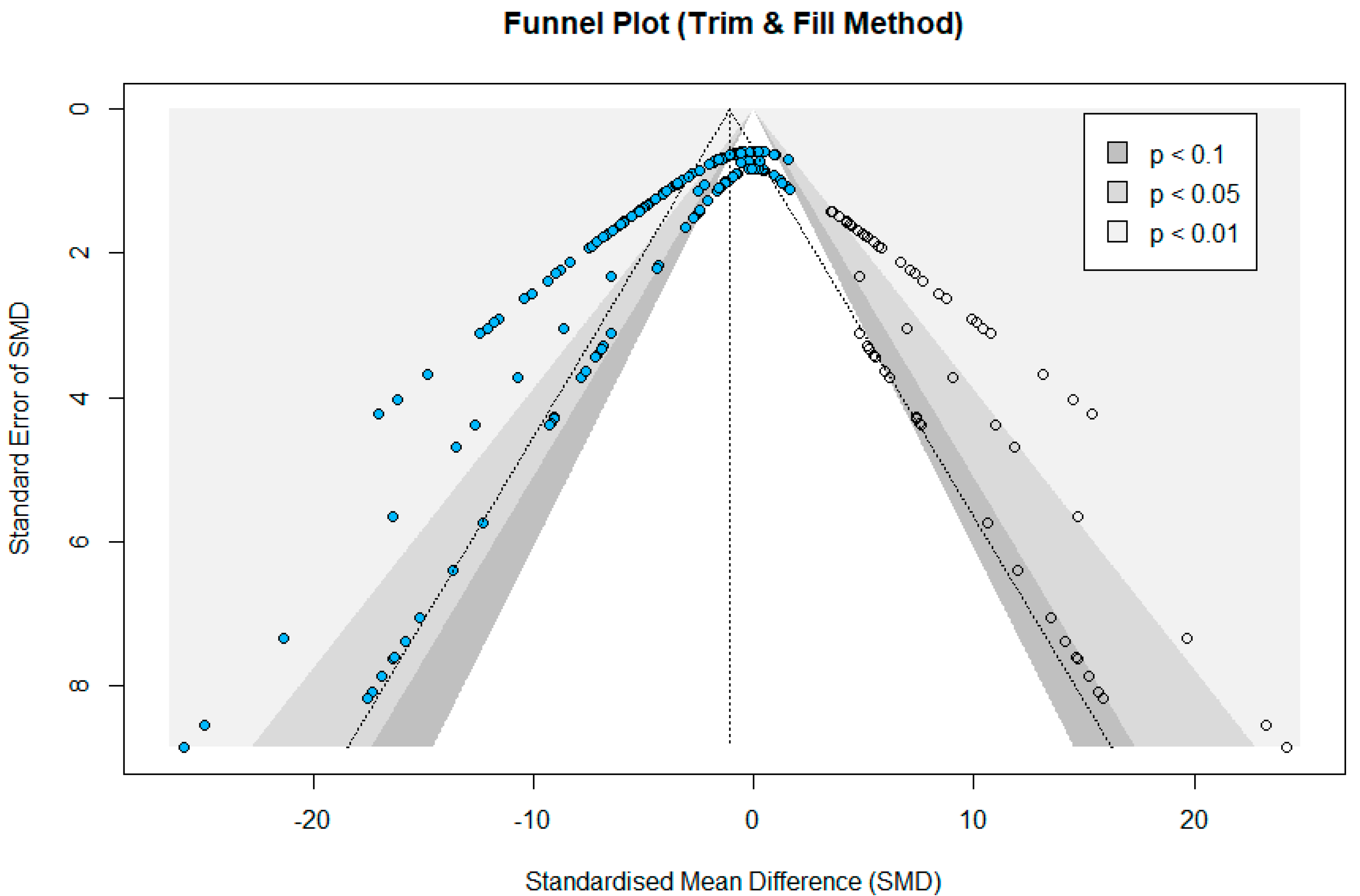
3.4. Meta-Analysis
4. Discussion
4.1. Primary Outcomes
4.2. Meta-Analysis Outcomes
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CDT | Chemodynamic therapy |
| CI | Confidence Interval |
| DOX | Doxorubicin |
| ES | Effect size |
| IC50 | Half-maximum inhibitory concentration |
| ICNP | Iron carbide nanoparticle |
| IONP | Iron oxide nanoparticles |
| LDH | Lactate dehydrogenase |
| MHT | Magnetic Hyperthermia |
| MNP | Magnetic nanoparticles |
| MRI | Magnetic Resonance Imaging |
| MeSH | Medical Subject Headings |
| Ms | Magnetization Saturation |
| NIR | Near-infrared |
| NM | Nanomaterial |
| NP | Nanoparticle |
| OSF | Open Science Framework |
| PAT | Photoacoustic Tomography |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PRISMA | Preferred reporting items for systematic review and meta-analyses |
| PTT | Photothermal therapy |
| ROS | Reactive Oxygen Species |
| SMD | Standardized Mean Difference |
| SR | Systematic Review |
| SRB | Sulforhodamine B |
| ZP | Zeta Potential |
References
- Tran, N.; Webster, T.J. Magnetic Nanoparticles: Biomedical Applications and Challenges. J. Mater. Chem. 2010, 20, 8760. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Current Investigations into Magnetic Nanoparticles for Biomedical Applications. J. Biomed. Mater. Res. A 2016, 104, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Kalubowilage, M.; Janik, K.; Bossmann, S.H. Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia. Appl. Sci. 2019, 9, 2927. [Google Scholar] [CrossRef]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, 1802309. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Martín, M.; Salazar, P.; Villalonga, R.; Campuzano, S.; Pingarrón, J.M.; González-Mora, J.L. Preparation of Core–Shell Fe3O4 @poly(Dopamine) Magnetic Nanoparticles for Biosensor Construction. J. Mater. Chem. B 2014, 2, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic Nanoparticles for Hyperthermia in Cancer Treatment: An Emerging Tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent Trends in Preparation and Biomedical Applications of Iron Oxide Nanoparticles. J. Nanobiotech. 2024, 22, 24. [Google Scholar] [CrossRef]
- Duong, H.T.K.; Abdibastami, A.; Gloag, L.; Barrera, L.; Gooding, J.J.; Tilley, R.D. A Guide to the Design of Magnetic Particle Imaging Tracers for Biomedical Applications. Nanoscale 2022, 14, 13890–13914. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive Cytotoxicity Studies of Superparamagnetic Iron Oxide Nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Syrovets, T.; Röcker, C.; Tron, K.; Ulrich Nienhaus, G.; Rasche, V.; Mailänder, V.; Landfester, K.; Simmet, T. Lysosomal Degradation of the Carboxydextran Shell of Coated Superparamagnetic Iron Oxide Nanoparticles and the Fate of Professional Phagocytes. Biomaterials 2010, 31, 9015–9022. [Google Scholar] [CrossRef]
- Kornberg, T.; Stueckle, T.; Antonini, J.; Rojanasakul, Y.; Castranova, V.; Yang, Y.; Wang, L. Potential Toxicity and Underlying Mechanisms Associated with Pulmonary Exposure to Iron Oxide Nanoparticles: Conflicting Literature and Unclear Risk. Nanomaterials 2017, 7, 307. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Fernández-Afonso, Y.; Guedes, G.; Guisasola, E.; Gutiérrez, L.; Cortajarena, A.L. Engineered Protein-Driven Synthesis of Tunable Iron Oxide Nanoparticles as T1 and T2 Magnetic Resonance Imaging Contrast Agents. Chem. Mater. 2022, 34, 10832–10841. [Google Scholar] [CrossRef]
- Zeng, L.; Gowda, B.H.J.; Ahmed, M.G.; Abourehab, M.A.S.; Chen, Z.-S.; Zhang, C.; Li, J.; Kesharwani, P. Advancements in Nanoparticle-Based Treatment Approaches for Skin Cancer Therapy. Mol. Cancer 2023, 22, 10. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y. New Types of Magnetic Nanoparticles for Stimuli-Responsive Theranostic Nanoplatforms. Adv. Sci. 2024, 11, 202305459. [Google Scholar] [CrossRef]
- Yu, J.; Chen, F.; Gao, W.; Ju, Y.; Chu, X.; Che, S.; Sheng, F.; Hou, Y. Iron Carbide Nanoparticles: An Innovative Nanoplatform for Biomedical Applications. Nanoscale Horiz. 2017, 2, 81–88. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y. Iron Carbide Nanostructures: An Emerging Material for Tumor Theranostics. Acc. Mater. Res. 2022, 3, 89–99. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, P.; Lei, X.; Wang, X.; Zhao, N.; Yang, H. Iron Carbides and Nitrides: Ancient Materials with Novel Prospects. Chem.—A Eur. J. 2018, 24, 8922–8940. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpoor, F.; Delavari, H.H.; Shojaosadati, S.A. Porous versus Dense—Effect of Silica Coating on Contrast Enhancement of Iron Carbide Nanoparticles in T2-Weighted Magnetic Resonance Imaging. ChemistrySelect 2020, 5, 1135–1139. [Google Scholar] [CrossRef]
- Jin, X.; Yang, W.; Xu, Y.; Bian, K.; Zhang, B. Emerging Strategies of Activatable MR Imaging Probes and Their Advantages for Biomedical Applications. VIEW 2021, 2, 20200141. [Google Scholar] [CrossRef]
- Loizou, K.; Mourdikoudis, S.; Sergides, A.; Besenhard, M.O.; Sarafidis, C.; Higashimine, K.; Kalogirou, O.; Maenosono, S.; Thanh, N.T.K.; Gavriilidis, A. Rapid Millifluidic Synthesis of Stable High Magnetic Moment FexCy Nanoparticles for Hyperthermia. ACS Appl. Mater. Interfaces 2020, 12, 28520–28531. [Google Scholar] [CrossRef] [PubMed]
- Meffre, A.; Mehdaoui, B.; Kelsen, V.; Fazzini, P.F.; Carrey, J.; Lachaize, S.; Respaud, M.; Chaudret, B. A Simple Chemical Route toward Monodisperse Iron Carbide Nanoparticles Displaying Tunable Magnetic and Unprecedented Hyperthermia Properties. Nano Lett. 2012, 12, 4722–4728. [Google Scholar] [CrossRef] [PubMed]
- Bordet, A.; Soulantika, A.; Chaudret, B. Iron Carbide Nanoparticles, Method for Preparing Same and Use Thereof for Heat Generation. U.S. Patent 16/062,994, 13 February 2020. [Google Scholar]
- Gong, J.-Y.; Holt, M.G.; Hoet, P.H.M.; Ghosh, M. Neurotoxicity of Four Frequently Used Nanoparticles: A Systematic Review to Reveal the Missing Data. Arch. Toxicol. 2022, 96, 1141–1212. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Rolland, E.; Piovesan, J.; Puertas Gallardo, A.; Bremer-Hoffmann, S. Toxic Effects of Nanomaterials for Health Applications: How Automation Can Support a Systematic Review of the Literature? J. Appl. Toxicol. 2022, 42, 41–51. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Bian, Q.; Ning, J.; Yong, L.; Ou, T.; Song, Y.; Wei, S. Genotoxicity Evaluation of Titanium Dioxide Nanoparticles In Vivo and In Vitro: A Meta-Analysis. Toxics 2023, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Ling, C.; Xu, M.; Hu, M.; Wang, H.; Liu, J.; Song, G.; Liu, J. Oxidative Damage Induced by Nano-Titanium Dioxide in Rats and Mice: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2020, 194, 184–202. [Google Scholar] [CrossRef]
- Chang, H.; Wang, Q.; Meng, X.; Chen, X.; Deng, Y.; Li, L.; Yang, Y.; Song, G.; Jia, H. Effect of Titanium Dioxide Nanoparticles on Mammalian Cell Cycle In Vitro: A Systematic Review and Meta-Analysis. Chem. Res. Toxicol. 2022, 35, 1435–1456. [Google Scholar] [CrossRef]
- Janzadeh, A.; Behroozi, Z.; Saliminia, F.; Janzadeh, N.; Arzani, H.; Tanha, K.; Hamblin, M.R.; Ramezani, F. Neurotoxicity of Silver Nanoparticles in the Animal Brain: A Systematic Review and Meta-Analysis. Forensic Toxicol. 2022, 40, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The Size-Dependent Cytotoxicity of Amorphous Silica Nanoparticles: A Systematic Review of in Vitro Studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- van den Akker, O.R.; Peters, G.-J.Y.; Bakker, C.J.; Carlsson, R.; Coles, N.A.; Corker, K.S.; Feldman, G.; Moreau, D.; Nordström, T.; Pickering, J.S.; et al. Increasing the Transparency of Systematic Reviews: Presenting a Generalized Registration Form. Syst. Rev. 2023, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Afantitis, A.; Antoniou, M.; Melagraki, G.; Lynch, I. A Systematic Review for the Toxicological Impacts Associated with Biomedical Applications of Iron Carbide Nanoparticles in Tumour Theranostics. Available online: https://osf.io/84rv5 (accessed on 16 February 2024).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Santos, C.M.d.C.; Pimenta, C.A. de M.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- PubMed, National Library of Medicine, National Center for Biotechnology Information. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 16 February 2024).
- Scopus. Available online: https://www.scopus.com/ (accessed on 16 February 2024).
- Wiley Online Library. Available online: https://onlinelibrary.wiley.com/ (accessed on 16 February 2024).
- Gusenbauer, M.; Haddaway, N.R. Which Academic Search Systems Are Suitable for Systematic Reviews or Meta-analyses? Evaluating Retrieval Qualities of Google Scholar, PubMed, and 26 Other Resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef]
- Bramer, W.M.; De Jonge, G.B.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. A Systematic Approach to Searching: An Efficient and Complete Method to Develop Literature Searches. J. Med. Libr. Assoc. 2018, 106, 531. [Google Scholar] [CrossRef] [PubMed]
- Searching the Literature: A Guide to Comprehensive Searching in the Health Sciences: Precision vs. Sensitivity—Key to Effective Searching. University of Toronto Libraries. Available online: https://guides.library.utoronto.ca/c.php?g=577919&p=4304403 (accessed on 16 February 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Briscoe, S.; Bethel, A.; Rogers, M. Conduct and Reporting of Citation Searching in Cochrane Systematic Reviews: A Cross-sectional Study. Res. Synth. Methods 2020, 11, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a New Tool to Assess the Reliability of Toxicological Data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef]
- Klimisch, H.-J.; Andreae, M.; Tillmann, U. A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data. Regul. Toxicol. Pharmacol. 1997, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, version 6.4; Cochrane: London, UK, 2023. [Google Scholar]
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of Random Effects Meta-Analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-Enhanced Meta-Analysis Funnel Plots Help Distinguish Publication Bias from Other Causes of Asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Shi, L.; Lin, L. The Trim-and-Fill Method for Publication Bias: Practical Guidelines and Recommendations Based on a Large Database of Meta-Analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Urner, M.; Hasler, M.; Roth-Z’Graggen, B.; Aemisegger, C.; Baulig, W.; Athanassiou, E.K.; Regenass, S.; Stark, W.J.; Beck-Schimmer, B. Iron Core/Shell Nanoparticles as Magnetic Drug Carriers: Possible Interactions with the Vascular Compartment. Nanomedicine 2011, 6, 1199–1213. [Google Scholar] [CrossRef]
- Sharma, M.; Mantri, S.; Bahadur, D. Study of Carbon Encapsulated Iron Oxide/Iron Carbide Nanocomposite for Hyperthermia. J. Magn. Magn. Mater. 2012, 324, 3975–3980. [Google Scholar] [CrossRef]
- Schumacher, C.M.; Herrmann, I.K.; Bubenhofer, S.B.; Gschwind, S.; Hirt, A.; Beck-Schimmer, B.; Günther, D.; Stark, W.J. Quantitative Recovery of Magnetic Nanoparticles from Flowing Blood: Trace Analysis and the Role of Magnetization. Adv. Funct. Mater. 2013, 23, 4888–4896. [Google Scholar] [CrossRef]
- Tang, W.; Zhen, Z.; Yang, C.; Wang, L.; Cowger, T.; Chen, H.; Todd, T.; Hekmatyar, K.; Zhao, Q.; Hou, Y.; et al. Fe5C2 Nanoparticles with High MRI Contrast Enhancement for Tumor Imaging. Small 2014, 10, 1245–1249. [Google Scholar] [CrossRef]
- Yu, J.; Yang, C.; Li, J.; Ding, Y.; Zhang, L.; Yousaf, M.Z.; Lin, J.; Pang, R.; Wei, L.; Xu, L.; et al. Multifunctional Fe5C2 Nanoparticles: A Targeted Theranostic Platform for Magnetic Resonance Imaging and Photoacoustic Tomography-Guided Photothermal Therapy. Adv. Mater. 2014, 26, 4114–4120. [Google Scholar] [CrossRef] [PubMed]
- Izydorzak-Wozniak, M.; Leonowicz, M. Carbon Matrix Based Magnetic Nanocomposites for Potential Biomedical Applications. J. Nanosci. Nanotechnol. 2014, 14, 2258–2267. [Google Scholar] [CrossRef]
- Huang, G.; Hu, J.; Zhang, H.; Zhou, Z.; Chi, X.; Gao, J. Highly Magnetic Iron Carbide Nanoparticles as Effective T2 Contrast Agents. Nanoscale 2014, 6, 726–730. [Google Scholar] [CrossRef]
- Cowger, T.A.; Tang, W.; Zhen, Z.; Hu, K.; Rink, D.E.; Todd, T.J.; Wang, G.D.; Zhang, W.; Chen, H.; Xie, J. Casein-Coated Fe5C2 Nanoparticles with Superior r2 Relaxivity for Liver-Specific Magnetic Resonance Imaging. Theranostics 2015, 5, 1225–1232. [Google Scholar] [CrossRef]
- Jacobson, M.; Roth Z’graggen, B.; Graber, S.M.; Schumacher, C.M.; Stark, W.J.; Dumrese, C.; Mateos, J.M.; Aemisegger, C.; Ziegler, U.; Urner, M.; et al. Uptake of Ferromagnetic Carbon-Encapsulated Metal Nanoparticles in Endothelial Cells: Influence of Shear Stress and Endothelial Activation. Nanomedicine 2015, 10, 3537–3546. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ju, Y.; Zhao, L.; Chu, X.; Yang, W.; Tian, Y.; Sheng, F.; Lin, J.; Liu, F.; Dong, Y.; et al. Multistimuli-Regulated Photochemothermal Cancer Therapy Remotely Controlled via Fe5C2 Nanoparticles. ACS Nano 2016, 10, 159–169. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Beck-Schimmer, B.; Schumacher, C.M.; Gschwind, S.; Kaech, A.; Ziegler, U.; Clavien, P.-A.; Günther, D.; Stark, W.J.; Graf, R.; et al. In Vivo Risk Evaluation of Carbon-Coated Iron Carbide Nanoparticles Based on Short- and Long-Term Exposure Scenarios. Nanomedicine 2016, 11, 783–796. [Google Scholar] [CrossRef]
- Hasan, M.; Yang, W.; Ju, Y.; Chu, X.; Wang, Y.; Deng, Y.; Mahmood, N.; Hou, Y. Biocompatibility of Iron Carbide and Detection of Metals Ions Signaling Proteomic Analysis via HPLC/ESI-Orbitrap. Nano Res. 2017, 10, 1912–1923. [Google Scholar] [CrossRef]
- Hasan, M.; Mustafa, G.; Iqbal, J.; Ashfaq, M.; Mahmood, N. Quantitative Proteomic Analysis of HeLa Cells in Response to Biocompatible Fe2C@C Nanoparticles: 16O/18O-Labelling & HPLC-ESI-Orbit-Trap Profiling Approach. Toxicol. Res. 2018, 7, 84–92. [Google Scholar] [CrossRef]
- Feng, L.; Gai, S.; Dai, Y.; He, F.; Sun, C.; Yang, P.; Lv, R.; Niu, N.; An, G.; Lin, J. Controllable Generation of Free Radicals from Multifunctional Heat-Responsive Nanoplatform for Targeted Cancer Therapy. Chem. Mater. 2018, 30, 526–539. [Google Scholar] [CrossRef]
- Ahmadpoor, F.; Shojaosadati, S.A.; Delavari, H.H.; Christiansen, G.; Saber, R. Synthesis of Fe5C2@SiO2 Core@shell Nanoparticles as a Potential Candidate for Biomedical Application. Mater. Res. Express 2018, 5, 055038. [Google Scholar] [CrossRef]
- Feng, L.; Xie, R.; Wang, C.; Gai, S.; He, F.; Yang, D.; Yang, P.; Lin, J. Magnetic Targeting, Tumor Microenvironment-Responsive Intelligent Nanocatalysts for Enhanced Tumor Ablation. ACS Nano 2018, 12, 11000–11012. [Google Scholar] [CrossRef] [PubMed]
- Bordet, A.; Landis, R.F.; Lee, Y.; Tonga, G.Y.; Asensio, J.M.; Li, C.-H.; Fazzini, P.-F.; Soulantica, K.; Rotello, V.M.; Chaudret, B. Water-Dispersible and Biocompatible Iron Carbide Nanoparticles with High Specific Absorption Rate. ACS Nano 2019, 13, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, F.; Gao, W.; Yang, X.; Ju, Y.; Zhao, L.; Guo, W.; Xie, J.; Liang, X.; Tao, X.; et al. Magnetic Reactive Oxygen Species Nanoreactor for Switchable Magnetic Resonance Imaging Guided Cancer Therapy Based on PH-Sensitive Fe5C2@Fe3O4 Nanoparticles. ACS Nano 2019, 13, 10002–10014. [Google Scholar] [CrossRef]
- Gangwar, A.; Varghese, S.S.; Meena, S.S.; Viswanadh, M.K.; Neogi, K.; Muthu, M.S.; Prasad, N.K. Physical and in Vitro Evaluation of Ultra-Fine Cohenite Particles for the Prospective Magnetic Hyperthermia Application. J. Mater. Sci. Mater. Electron. 2020, 31, 10772–10782. [Google Scholar] [CrossRef]
- Sun, C.; Wang, W.; Sun, X.; Chu, W.; Yang, J.; Dai, J.; Ju, Y. An Intrinsically Thermogenic Nanozyme for Synergistic Antibacterial Therapy. Biomater. Sci. 2021, 9, 8323–8334. [Google Scholar] [CrossRef]
- Zhao, F.; Yu, J.; Gao, W.; Yang, X.; Liang, L.; Sun, X.; Su, D.; Ying, Y.; Li, W.; Li, J.; et al. H2O2-Independent Chemodynamic Therapy Initiated from Magnetic Iron Carbide Nanoparticle-Assisted Artemisinin Synergy. RSC Adv. 2021, 11, 37504–37513. [Google Scholar] [CrossRef]
- Mertdinç-Ülküseven, S.; Onbasli, K.; Çakır, E.; Morova, Y.; Balcı-Çağıran, Ö.; Acar, H.Y.; Sennaroğlu, A.; Lütfi Öveçoğlu, M.; Ağaoğulları, D. Magnetic Core/Shell Structures: A Case Study on the Synthesis and Phototoxicity/Cytotoxicity Tests of Multilayer Graphene Encapsulated Fe/Fe3C Nanoparticles. J. Alloys Compd. 2023, 968, 172145. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for Accurate EC50/IC50 Estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Keepers, Y.P.; Pizao, P.E.; Peters, G.J.; van Ark-Otte, J.; Winograd, B.; Pinedo, H.M. Comparison of the Sulforhodamine B Protein and Tetrazolium (MTT) Assays for in Vitro Chemosensitivity Testing. Eur. J. Cancer Clin. Oncol. 1991, 27, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Awashra, M.; Młynarz, P. The Toxicity of Nanoparticles and Their Interaction with Cells: An in Vitro Metabolomic Perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Graham, U.M.; Jacobs, G.; Yokel, R.A.; Davis, B.H.; Dozier, A.K.; Birch, M.E.; Tseng, M.T.; Oberdörster, G.; Elder, A.; DeLouise, L. From Dose to Response: In Vivo Nanoparticle Processing and Potential Toxicity. In Modelling the Toxicity of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–100. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Hasan, M.; Zafar, A.; Jabbar, M.; Tariq, T.; Manzoor, Y.; Ahmed, M.M.; Hassan, S.G.; Shu, X.; Mahmood, N. Trident Nano-Indexing the Proteomics Table: Next-Version Clustering of Iron Carbide NPs and Protein Corona. Molecules 2022, 27, 5754. [Google Scholar] [CrossRef] [PubMed]
- Partikel, K.; Korte, R.; Mulac, D.; Humpf, H.-U.; Langer, K. Serum Type and Concentration Both Affect the Protein-Corona Composition of PLGA Nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Faid, A.H.; Shouman, S.A.; Badr, Y.A.; Sharaky, M. Enhanced Cytotoxic Effect of Doxorubicin Conjugated Gold Nanoparticles on Breast Cancer Model. BMC Chem. 2022, 16, 90. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Paez, A. Gray Literature: An Important Resource in Systematic Reviews. J. Evid. Based Med. 2017, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]

| a/a | Reference | Metabolic Activity | Cell Uptake | LDH Release | Cytokine Analysis | Fluorescent Live/Dead | ROS Detection | Other |
|---|---|---|---|---|---|---|---|---|
| 1 | Herrmann, 2011 [57] | √ * | √ | √ | ||||
| 2 | Sharma, 2012 [58] | √ | ||||||
| 3 | Schumacher, 2013 [59] | √ | √ | |||||
| 4 | Tang, 2013 [60] | √ | √ * | √ * | ||||
| 5 | Yu, 2014 [61] | √ | √ * | |||||
| 6 | Izydorzak-Wozniak, 2014 [62] | √ ** | √ | |||||
| 7 | Huang, 2014 [63] | √ | ||||||
| 8 | Cowger, 2015 [64] | √ | √ | |||||
| 9 | Jacobson, 2015 [65] | √ ** | √ | √ ** | ||||
| 10 | Yu, 2015 [66] | √ | ||||||
| 11 | Herrmann, 2016 [67] | √ *** | ||||||
| 12 | Hasan, 2017a [68] | √ | ||||||
| 13 | Hasan, 2017b [69] | √ *** | ||||||
| 14 | Feng, 2017 [70] | √ | √ * | √ * | √ | |||
| 15 | Ahmadpoor, 2018 [71] | √ | ||||||
| 16 | Feng, 2018 [72] | √ | √ * | √ * | √ | |||
| 17 | Bordet, 2019 [73] | √ | √ | |||||
| 18 | Yu, 2019 [74] | √ | √ * | |||||
| 19 | Gangwar, 2020 [75] | √ | √ * | |||||
| 20 | Sun, 2021 [76] | √ | √ | |||||
| 21 | Zhao, 2021 [77] | √ | √ * | √ *** | √ | |||
| 22 | Ülküseven, 2023 [78] | √ | √ |
| Ref. | Iron Carbide | Shell | Ligand- Conjugate | Size (nm) | Exposure (h) | Organism | NIR | Dose Range (μg/mL) | Cell Viability Range (%) |
|---|---|---|---|---|---|---|---|---|---|
| [58] | Fe3C | Carbon | - | 92 | 24 | H/M | No | 0–500 | 100–81.7% (H), 100–81.9% (M) |
| [60] | Fe5C2 | Fe3O4 | DSPE-PEG-COOH | 22 | 24 | H | No | 0–25 | 102–80.5% |
| [61] | Fe5C2 | Carbon | Zher2:342 | 20 | 24 | H/M | No | 1–1000 | 105–91.8% (H), 103–91% (M) |
| Fe5C2 | Carbon | Zher2:342 | 20 | 24 | H | Yes | 1–1000 | 70–19.5% | |
| Fe5C2 | Carbon | PEG | 20 | 24 | H | Yes | 1–1000 | 102–44.6% | |
| [63] | Fe5C2 | Carbon | ST | 20 | 24 | H | No | 0–100 | 100–82.6% |
| [64] | Fe5C2 | - | DSPE-PEG-COOH (PL) | 5 | 4 | M | No | 0–100 | 100–84.9% |
| Fe5C2 | - | DSPE-PEG-COOH (PL) | 14 | 4 | M | No | 0–100 | 100–82.1% | |
| Fe5C2 | - | DSPE-PEG-COOH (PL) | 22 | 4 | M | No | 0–100 | 100–86% | |
| Fe5C2 | - | ZDS | 22 | 4 | M | No | 0–100 | 100–87.2% | |
| Fe5C2 | - | Casein | 22 | 4 | M | No | 0–100 | 100–88.5% | |
| [66,70] | Fe5C2 | Carbon | BSA-DOX | 20 | 24 | H/M | No | 0.05–50 | 100–71.5% (H), 102–63.8% (M) |
| Fe5C2 | Carbon | BSA-DOX | 20 | 24 | H | Yes | 0.05–50 | 95.9–5% | |
| Fe5C2 | Carbon | BSA | 20 | 24 | H | No | 0.05–50 | 101–91.7% | |
| Fe5C2 | Carbon | BSA | 20 | 24 | H | Yes | 0.05–50 | 97.7–41.5% | |
| [68] | Fe2C | - | PA | 16 | 24 | H | No | 0–400 | 92.1–91.3% |
| Fe2C | - | - | 16 | 24 | H | No | 0–400 | 91.7–16.2% | |
| [71] | Fe5C2 | SiO2 | - | 58 | 24 | H | No | 0–500 | 100–35.2% |
| [72] | Fe5C2 | MnO2 | GOD | 22.1 | 24 | H | No | 0–800 | 100–26% |
| Fe5C2 | MnO2 | - | 22.1 | 24 | H | No | 0–800 | 101–98% | |
| [73] | Fe2.2C | - | DOP-TEG-C6 | 15 | 4 | H | No | 10–2000 | 95.3–1.7% |
| Fe2.2C | - | DOP-TEG-C6 | 15 | 24 | H | No | 10–500 | 64.8–2 | |
| Fe2.2C | - | DOP-TEG-COOH | 15 | 4 | H | No | 10–2000 | 104.5–85.5% | |
| Fe2.2C | - | DOP-TEG-COOH | 15 | 24 | H | No | 10–500 | 105.4–59.4% | |
| Fe2.2C | - | DOP-TEG-Zwitter | 15 | 4 | H | No | 10–2000 | 95.4–93.2% | |
| Fe2.2C | - | DOP-TEG-Zwitter | 15 | 24 | H | No | 10–500 | 95.4–62.3% | |
| [74] | Fe5C2 | Fe3O4 | DSPE-PEG | 20 | 24 | H/M | No | 0–400 | 100–72.6% (H), 100–56.8% (M) |
| Fe5C2 | Carbon | DSPE-PEG | 20 | 24 | M | No | 0–400 | 100–78.1% | |
| [75] | Fe3C | - | Pluronic acid F127 | 6 | 24 | H | No | 0–300 | 100–76% |
| Fe3C | - | Pluronic acid F127 | 6 | 48 | H | No | 0–300 | 100–73.1% | |
| Fe3C | - | - | 6 | 48 | H | No | 0–300 | 100–73.7% | |
| [76,77] | Fe2C | Fe3O4 | DSPE-PEG | 14 | 24 | H/M | No | 0–400 | 100–59.9% (H), 100–62.8% (M) |
| [78] | Fe3C | Carbon | PAA | 45 | 24 | H/M | No | 0–200 | 100–73.7% (H), 100–87% (M) |
| Fe3C | Carbon | PAA | 45 | 48 | H/M | No | 0–200 | 100–47% (H), 100–84.4% (M) | |
| Fe3C | Carbon | PAA | 45 | 24 | H | Yes | 0–200 | 100–6.4% |
| Organism | Cell Line | Source Organ/Tissue | Cell Type | Health Status | Age | Utilized in Studies |
|---|---|---|---|---|---|---|
| Human | A549 | Lung | Epithelial | C | A | [75] |
| HEK-293T | Kidney | Epithelial | N | E | [68,74] | |
| HeLa | Cervix | Epithelial | C | A | [58,61,63,68,72,73,74,77,78] | |
| MCF-7 | Breast | Epithelial | C | A | [58,71,78] | |
| MDA-MB-231 | Breast | Epithelial | C | A | [77] | |
| SK-BR-3 | Breast | Epithelial | C | A | [61] | |
| SK-OV-3 | Ovaries | Epithelial | C | A | [61,66] | |
| U87MG | Brain | Glioblastoma | C | A | [60] | |
| Murine | 4T1 | Mammary Glands | Epithelial | C | A | [74,77] |
| L929 | Adipose | Fibroblast | N | E | [58,74,78] | |
| NIH-3T3 | Embryos | Fibroblast | N | E | [61,66] | |
| RAW 264.7 | Blood | Macrophage | N | A | [61,64,66] |
| a/a | Reference | Complies with Essential Criteria (4) | Overall Score (Max 17) | Klimisch Category a |
|---|---|---|---|---|
| 1 | Sharma, 2012 [58] | √ | 14 | I b |
| 2 | Tang, 2013 [60] | √ | 15 | I |
| 3 | Yu, 2014 [61] | √ | 17 | I |
| 4 | Huang, 2014 [63] | √ | 16 | I |
| 5 | Cowger, 2015 [64] | √ | 13 | II b |
| 6 | Yu, 2015 [66] | √ | 15 | I |
| 7 | Hasan, 2017 [68] | √ | 16 | I |
| 8 | Ahmadpoor, 2018 [71] | √ | 16 | I |
| 9 | Feng, 2018 [72] | √ | 14 | I |
| 10 | Bordet, 2019 [73] | √ | 12 | II b |
| 11 | Yu, 2019 [74] | √ | 17 | I |
| 12 | Gangwar, 2020 [75] | √ | 16 | I |
| 13 | Sun, Zhao 2021 [76,77] | √ | 15 | I |
| 14 | Ülküseven, 2023 [78] | √ | 17 | I |
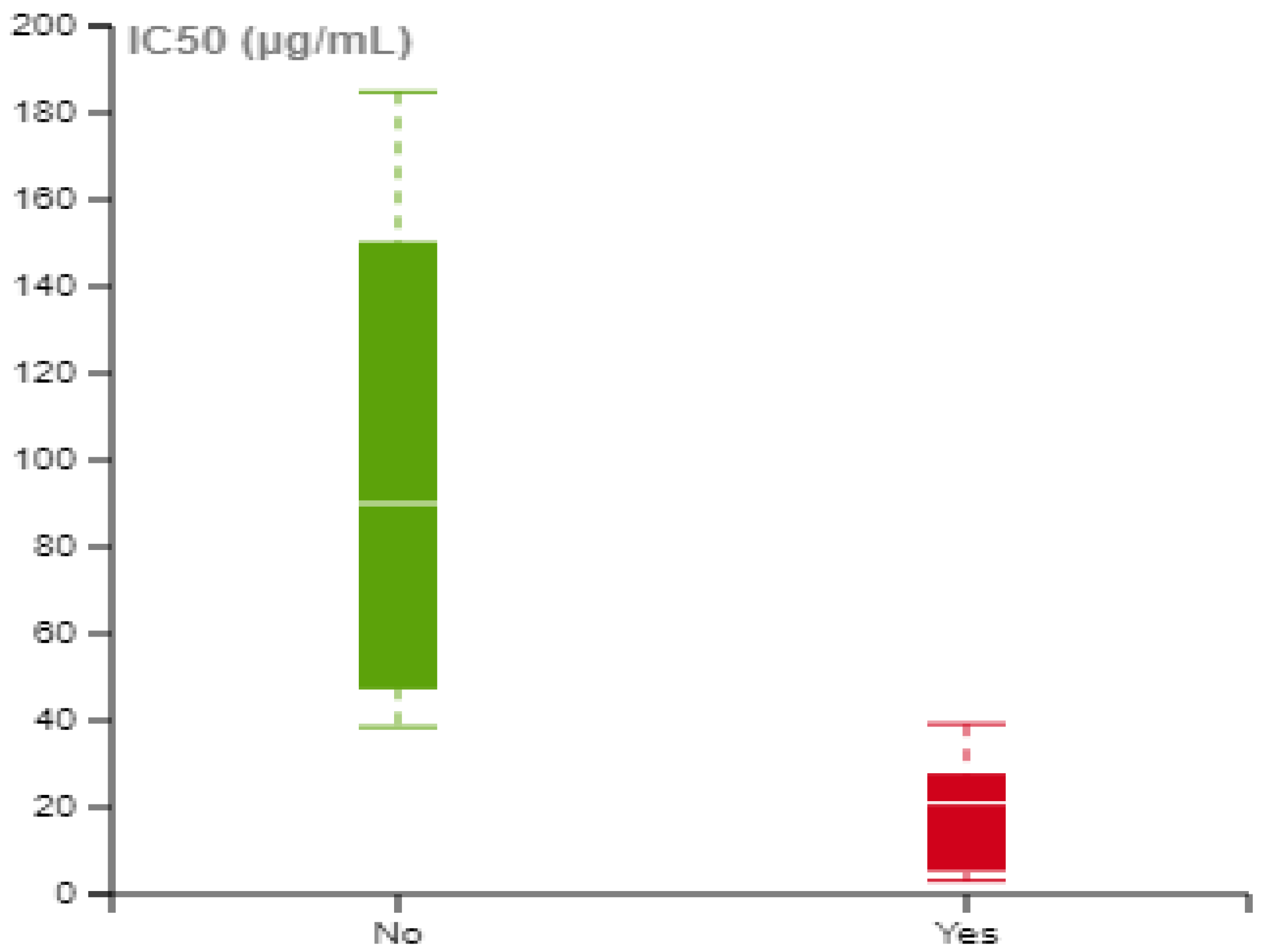
| Subgroup | Number of Experiments | Heterogeneity I2% | SMD (95% CI) | Overall p-Value a |
|---|---|---|---|---|
| Organism/Species | 0.2604 | |||
| Human | 115 | 75.0% | −2.791 [−3.383; −2.199] | |
| Murine | 57 | 80.6% | −2.288 [−2.849; −1.225] | |
| Health Status | 0.167 | |||
| Normal Cells | 44 | 78.9% | −2.037 [−2.849; −1.125] | |
| Cancer Cells | 128 | 76.4% | −2.711 [−3.214; −2.208] | |
| ICNPs Ligand | <0.001 | |||
| PEG-based | 52 | 79.4% | −4.062 [−4.835; −3.289] | |
| Protein-based | 52 | 80.6% | −2.280 [−3.092; −1.469] | |
| Other | 36 | 36.7% | −0.572 [−0.914; −0.229] | |
| None | 32 | 60.7% | −0.594 [−0.997; −0.191] | |
| Particle Size | 0.533 | |||
| <20 nm | 53 | 73.7% | −2.761 [−3.577; 1.947] | |
| ≥20 nm | 119 | 78.5% | −2.456 [−2.965; −1.947] | |
| Concentration Range | <0.001 | |||
| <10 μg/mL | 29 | 72.4% | −0.651 [−1.209; −0.093] | |
| 10–50 μg/mL | 46 | 72.9% | −1.987 [−2.713; −1.261] | |
| 50–100 μg/mL | 23 | 53.4% | −1.185 [−1.589; −0.781] | |
| 100–250 μg/mL | 33 | 66.0% | −3.358 [−4.166; −2.551] | |
| >250 μg/mL | 41 | 82.5% | −5.337 [−6.768; −3.906] | |
| Overall | 172 | 77.1% | −2.531 [−2.959; −2.102] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniou, M.; Melagraki, G.; Lynch, I.; Afantitis, A. In Vitro Toxicological Insights from the Biomedical Applications of Iron Carbide Nanoparticles in Tumor Theranostics: A Systematic Review and Meta-Analysis. Nanomaterials 2024, 14, 734. https://doi.org/10.3390/nano14090734
Antoniou M, Melagraki G, Lynch I, Afantitis A. In Vitro Toxicological Insights from the Biomedical Applications of Iron Carbide Nanoparticles in Tumor Theranostics: A Systematic Review and Meta-Analysis. Nanomaterials. 2024; 14(9):734. https://doi.org/10.3390/nano14090734
Chicago/Turabian StyleAntoniou, Maria, Georgia Melagraki, Iseult Lynch, and Antreas Afantitis. 2024. "In Vitro Toxicological Insights from the Biomedical Applications of Iron Carbide Nanoparticles in Tumor Theranostics: A Systematic Review and Meta-Analysis" Nanomaterials 14, no. 9: 734. https://doi.org/10.3390/nano14090734






