A Systematic Genotoxicity Assessment of a Suite of Metal Oxide Nanoparticles Reveals Their DNA Damaging and Clastogenic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. MONPs, MOMPs, and Dissolved Metal Analogs
2.2. Primary Particle Size Analysis of MONPs by Transmission Electron Microscopy (TEM)
2.3. Specific Surface Area (SSA) Measurement for MONPs and MOMPs
2.4. MONPs and MOMPs Exposure Suspensions
2.5. MONPs Characterization by Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS)
2.6. Cell Culture
2.7. Cell Viability Assay to Determine the Experimental Conditions for the Comet Assay
2.8. CometChip Assay
2.9. In Vitro MicroFlow® MN Assay
2.10. Correlation Analysis
2.11. Benchmark Concentration Modeling (BMC)
3. Results
3.1. MONPs Characterization
3.2. Cell Viability and Relative Survival
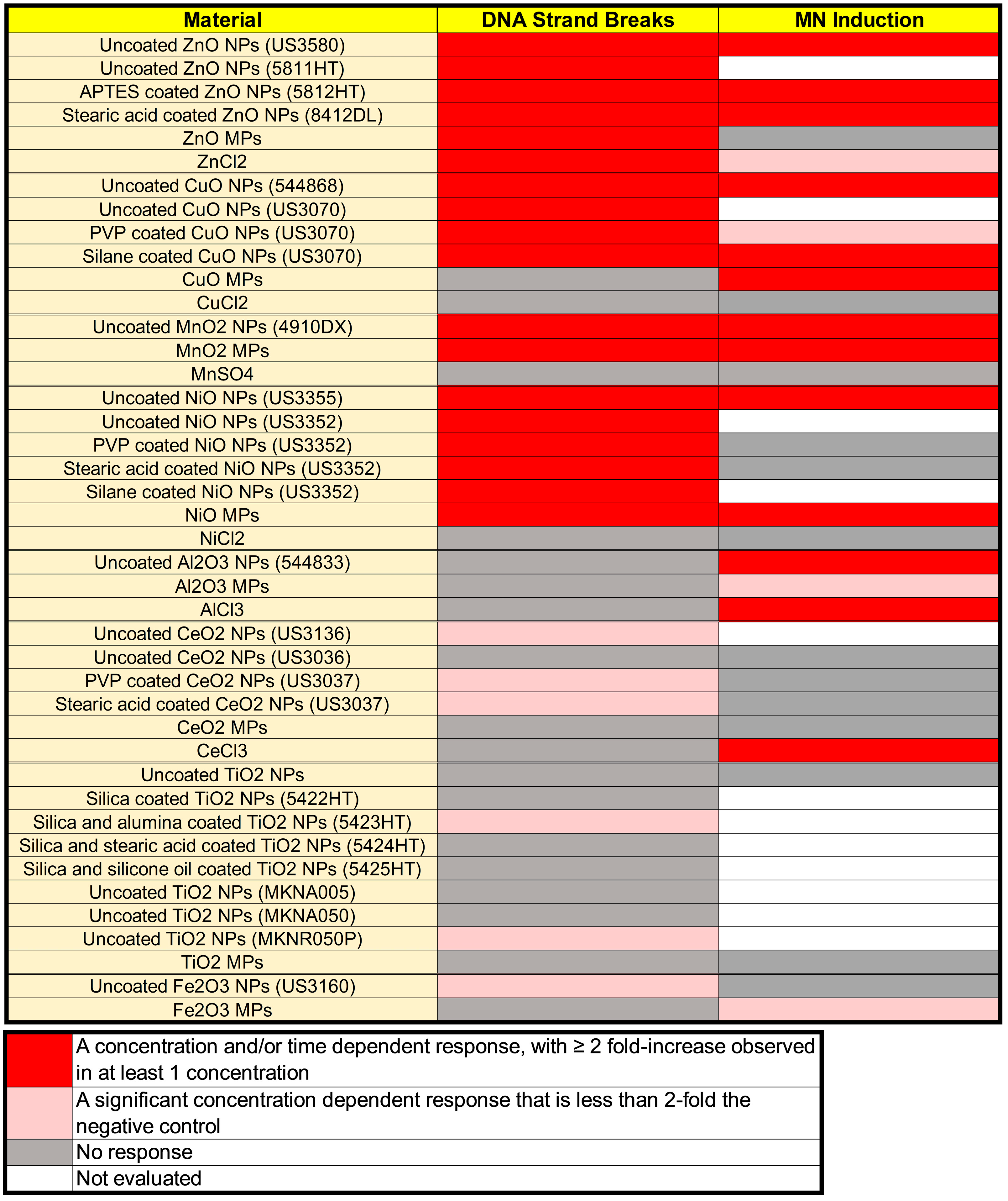
3.3. DNA Strand Break Induction
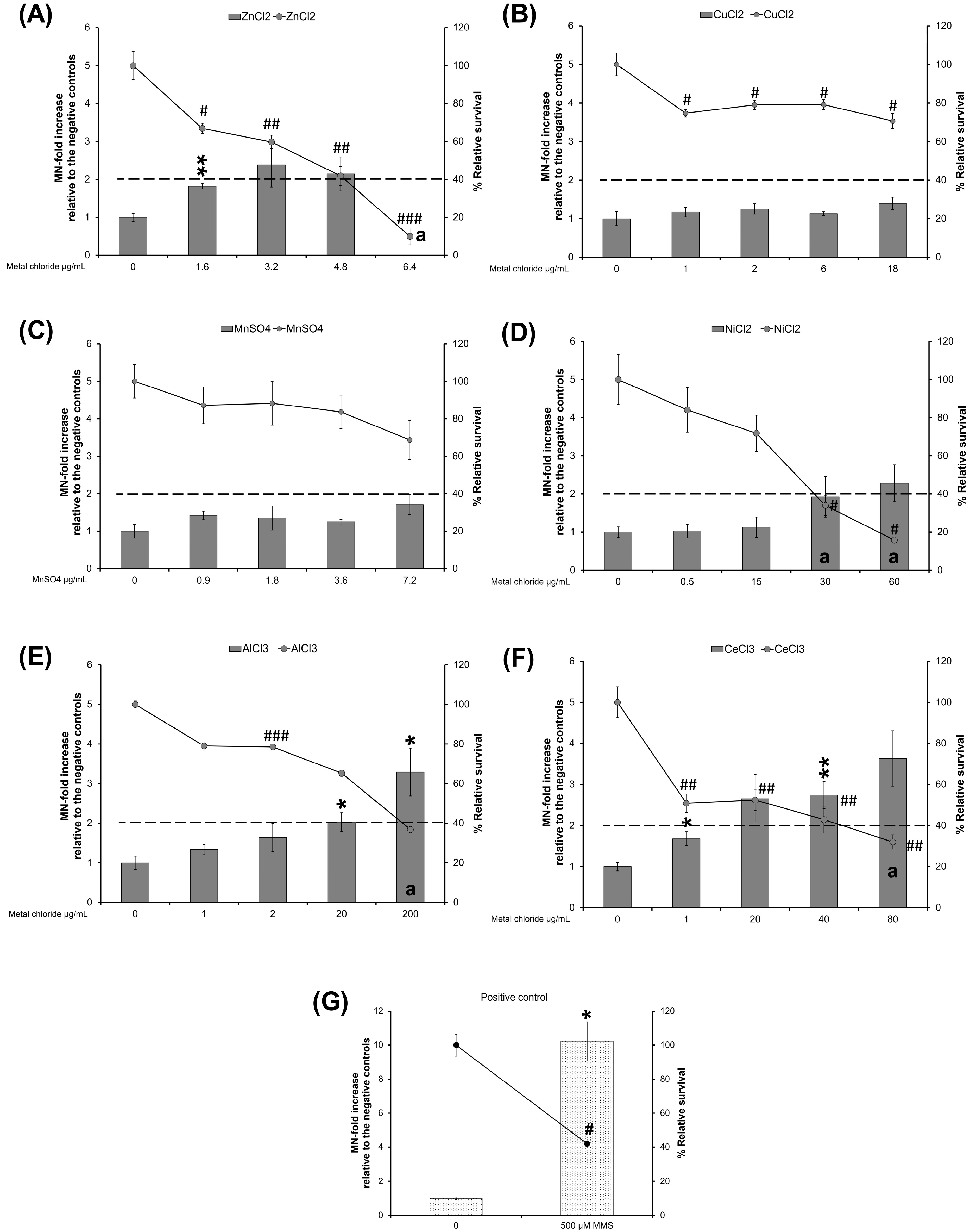
3.3.1. ZnO Variants and ZnCl2
3.3.2. CuO Variants and CuCl2
3.3.3. MnO2 Variants
3.3.4. NiO Variants and NiCl2
3.3.5. Al2O3 Variants and AlCl3
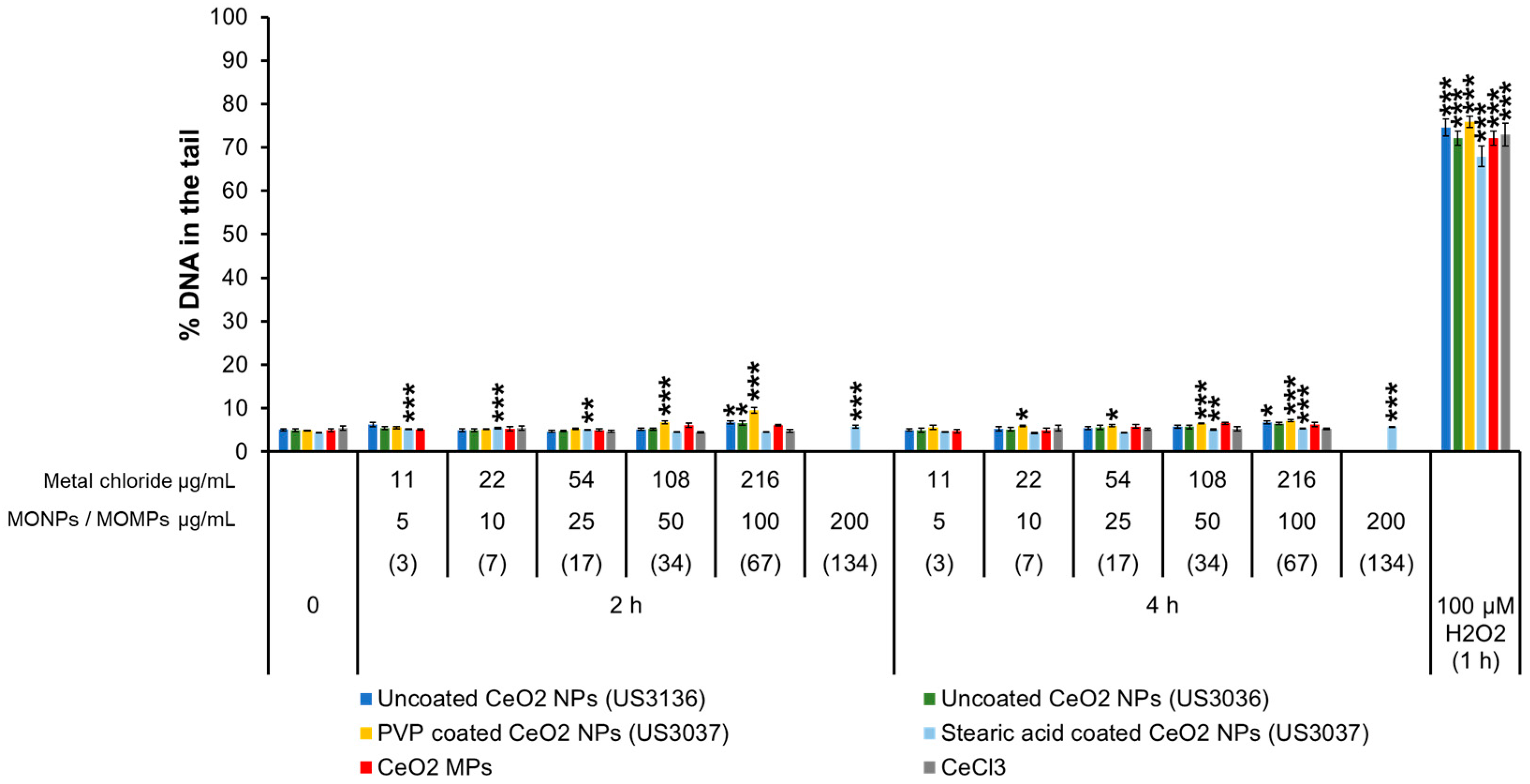
3.3.6. CeO2 Variants and CeCl3
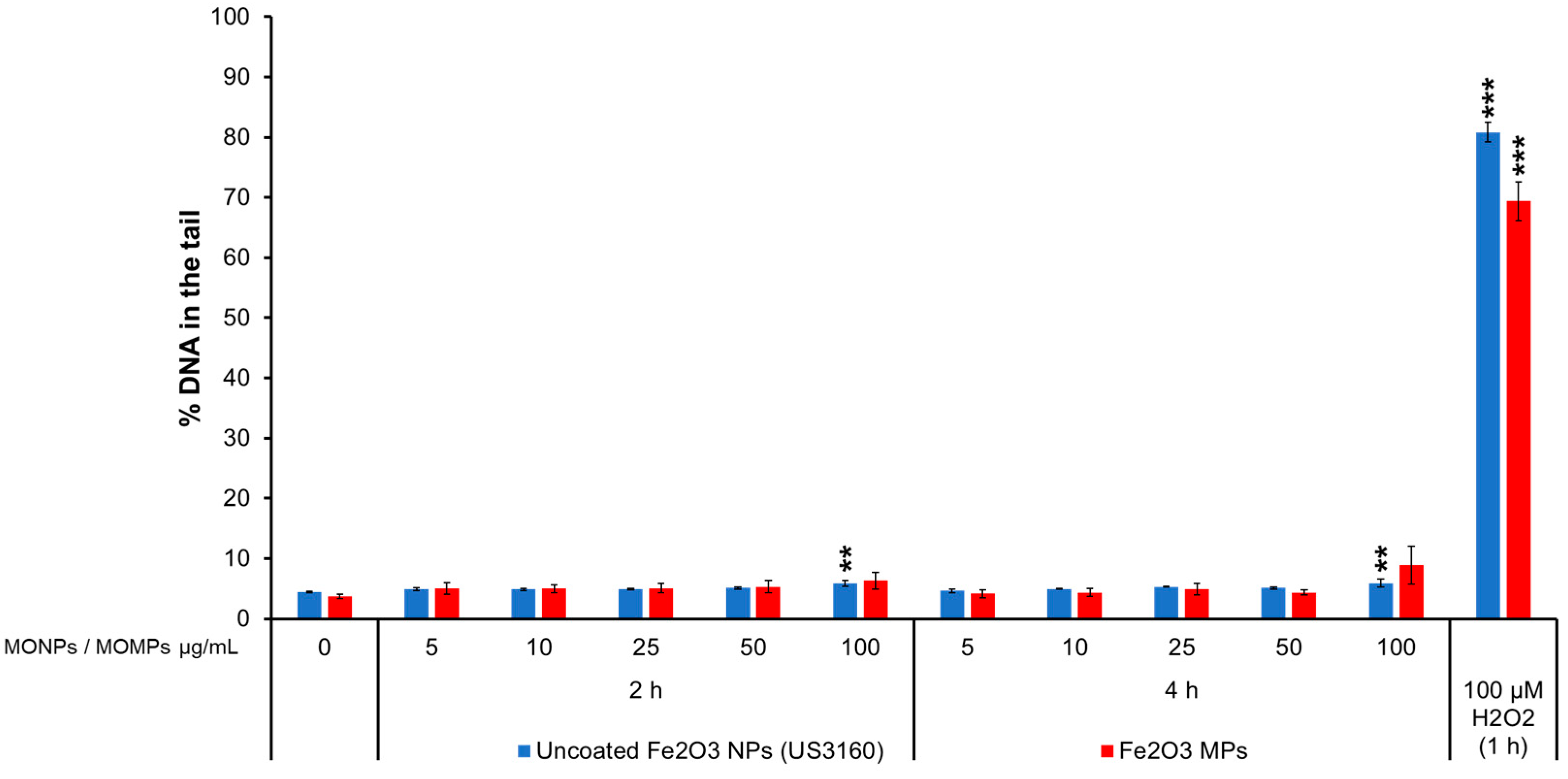
3.3.7. Fe2O3 Variants
3.4. MN Induction
3.4.1. ZnO Variants and ZnCl2
3.4.2. CuO Variants and CuCl2
3.4.3. MnO2 Variants
3.4.4. NiO Variants and NiCl2
3.4.5. Al2O3 Variants and AlCl3
3.4.6. CeO2 Variants and CelCl3
3.4.7. TiO2 Variants
3.4.8. Fe2O3 Variants
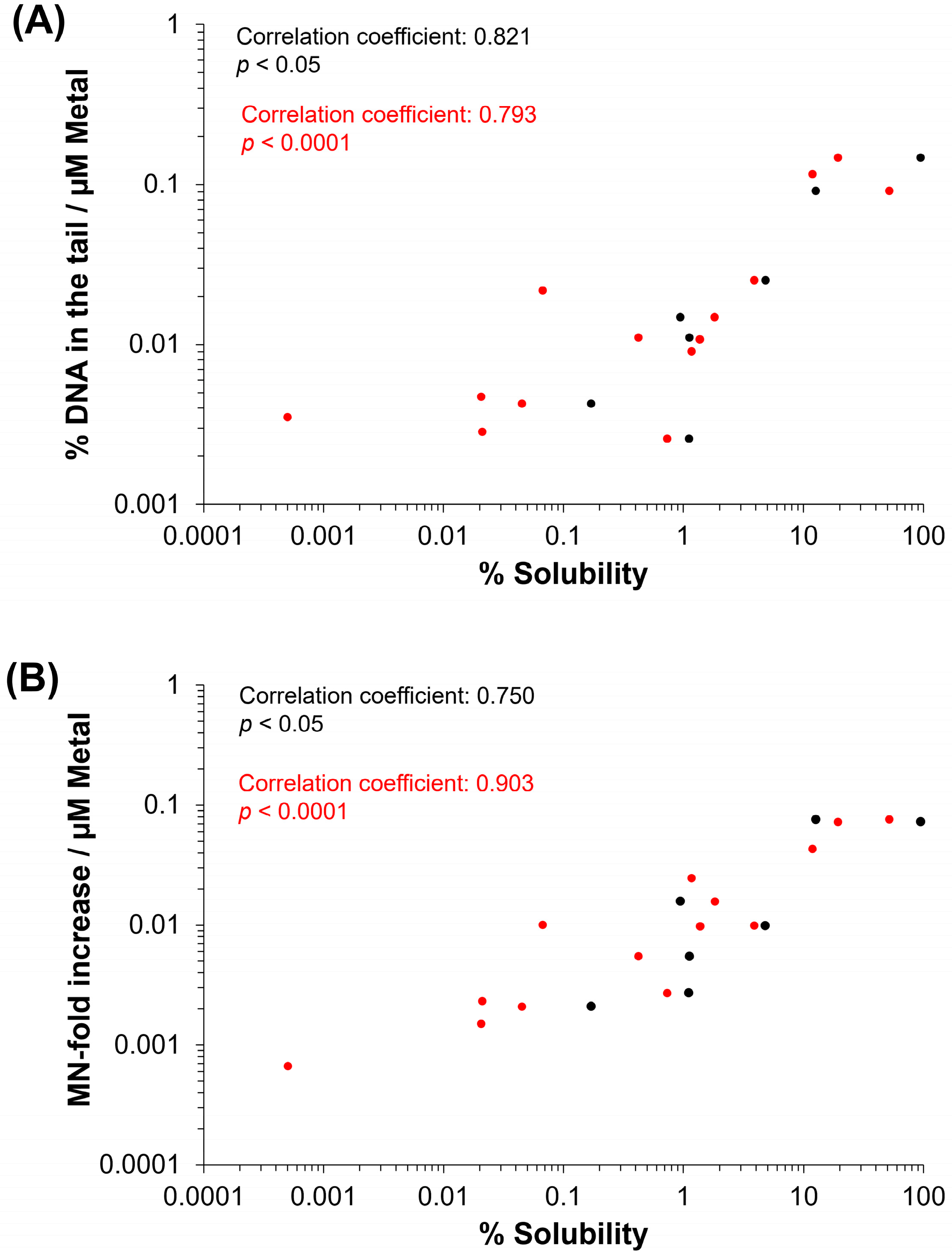
3.5. Spearman’s Correlation of Genotoxicity with Physicochemical Properties of MO
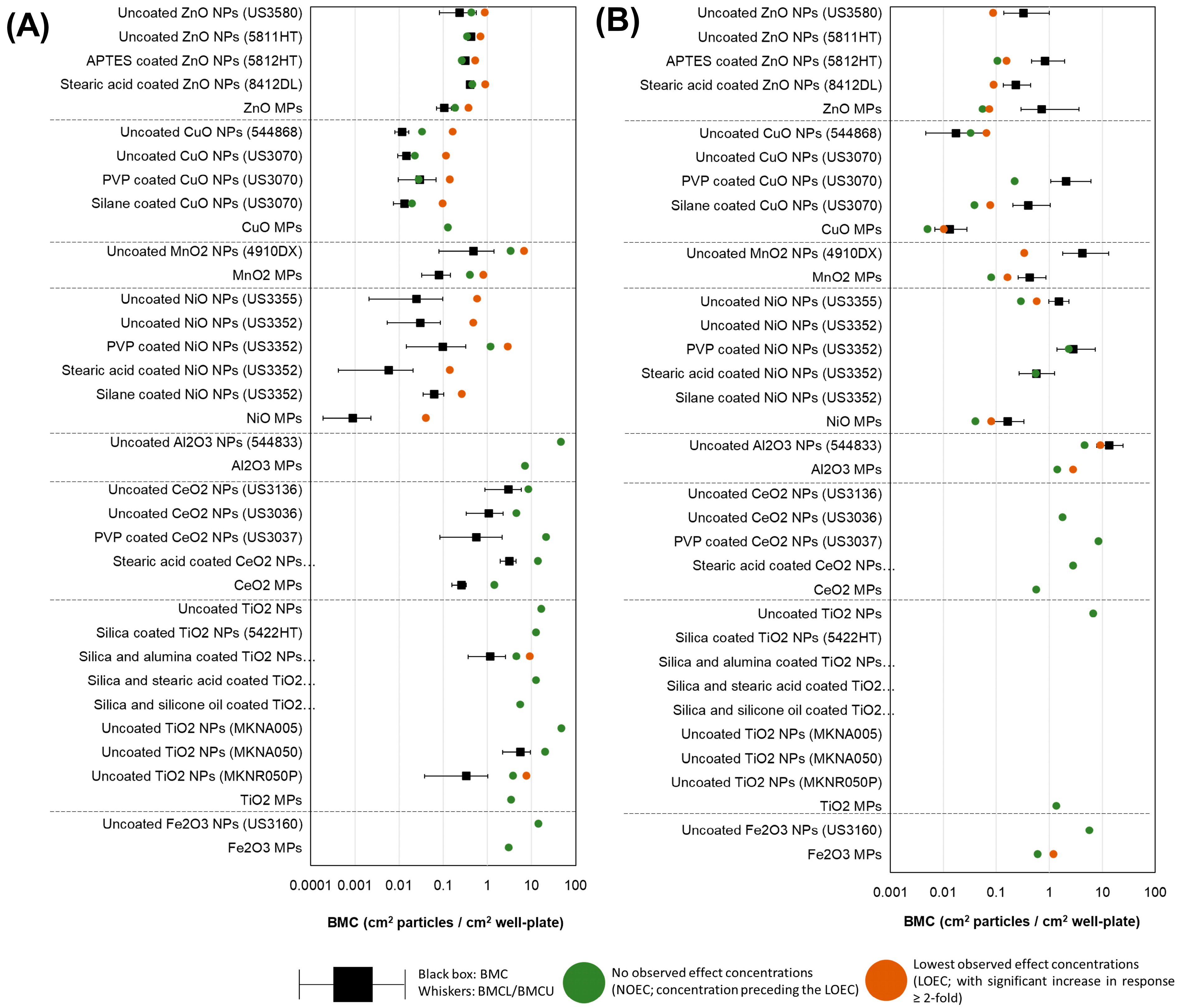
3.6. BMC Analysis and Potency Ranking of Metal Oxide Materials
4. Discussion
4.1. Impact of Particle Size, Solubility, and Surface Coating on MONP Genotoxicity
4.2. Relative Potency Ranking of MONP Genotoxic Potential
4.3. Mechanisms Affecting Genotoxic Potential of MONPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Health Canada. Nanomaterials. Available online: https://www.canada.ca/en/health-canada/services/chemical-substances/nanomaterials.html (accessed on 13 September 2023).
- Health Canada. Policy Statement on Health Canada’s Working Definition for Nanomaterial. Available online: https://www.canada.ca/en/health-canada/services/science-research/reports-publications/nanomaterial/policy-statement-health-canada-working-definition.html (accessed on 31 March 2024).
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Environment. Support for 3rd Regulatory Review on Nanomaterials-Environmental Legislation; Broomfield, M., Foss Hansen, S., Pelsy, F., Eds.; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Naghdi, M.; Metahni, S.; Ouarda, Y.; Brar, S.K.; Das, R.K.; Cledon, M. Instrumental approach toward understanding nano-pollutants. Nanotechnol. Environ. Eng. 2017, 2, 3. [Google Scholar] [CrossRef]
- Kuhlbusch, T.A.; Asbach, C.; Fissan, H.; Göhler, D.; Stintz, M. Nanoparticle exposure at nanotechnology workplaces: A review. Part. Fibre Toxicol. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Bierkandt, F.S.; Leibrock, L.; Wagener, S.; Laux, P.; Luch, A. The impact of nanomaterial characteristics on inhalation toxicity. Toxicol. Res. 2018, 7, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.A.; Neu-Baker, N.M.; Eastlake, A.C.; Beaucham, C.C.; Geraci, C.L. NIOSH field studies team assessment: Worker exposure to aerosolized metal oxide nanoparticles in a semiconductor fabrication facility. J. Occup. Environ. Hyg. 2016, 13, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiang, J.; Liao, Y.; Wan, S.; Yao, Y.; Luo, Y.; Chen, X.; Qian, H.; Dai, X.; Yin, W.; et al. Risk for lung-related diseases associated with welding fumes in an occupational population: Evidence from a Cox model. Front. Public Health 2022, 10, 990547. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Leso, V.; Battistini, B.; Caimi, S.; Senofonte, M.; Fedele, M.; Cavallo, D.M.; Cattaneo, A.; Lovreglio, P.; Iavicoli, I. Human biomonitoring and personal air monitoring. An integrated approach to assess exposure of stainless-steel welders to metal-oxide nanoparticles. Environ. Res. 2023, 216, 114736. [Google Scholar] [CrossRef]
- Andujar, P.; Simon-Deckers, A.; Galateau-Sallé, F.; Fayard, B.; Beaune, G.; Clin, B.; Billon-Galland, M.-A.; Durupthy, O.; Pairon, J.-C.; Doucet, J.; et al. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part. Fibre Toxicol. 2014, 11, 23. [Google Scholar] [CrossRef]
- Schulte, P.A.; Leso, V.; Niang, M.; Iavicoli, I. Current state of knowledge on the health effects of engineered nanomaterials in workers: A systematic review of human studies and epidemiological investigations. Scand. J. Work. Environ. Health 2019, 45, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Schwarz, J.; Dvorackova, S.; Komarc, M.; Ondracek, J.; Kostejn, M.; Kacer, P.; Vlckova, S.; Fenclova, Z.; et al. Markers of Oxidative Stress in the Exhaled Breath Condensate of Workers Handling Nanocomposites. Nanomaterials 2018, 8, 611. [Google Scholar] [CrossRef]
- Monsé, C.; Hagemeyer, O.; Raulf, M.; Jettkant, B.; van Kampen, V.; Kendzia, B.; Gering, V.; Kappert, G.; Weiss, T.; Ulrich, N.; et al. Concentration-dependent systemic response after inhalation of nano-sized zinc oxide particles in human volunteers. Part. Fibre Toxicol. 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Labor. Occupational Safety and Health Administration. Zinc Oxide, Dust & Fume. Available online: https://www.osha.gov/chemicaldata/215 (accessed on 2 April 2024).
- Wang, D.; Li, H.; Liu, Z.; Zhou, J.; Zhang, T. Acute toxicological effects of zinc oxide nanoparticles in mice after intratracheal instillation. Int. J. Occup. Environ. Health 2017, 23, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.T.; Kim, Y.; Choi, S.; Yoon, B.L.; Kim, H.S.; Shim, I.; Sul, D. Pulmonary Toxicity and Proteomic Analysis in Bronchoalveolar Lavage Fluids and Lungs of Rats Exposed to Copper Oxide Nanoparticles. Int. J. Mol. Sci. 2022, 23, 3265. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Jeon, S.; Yu, H.S.; Cho, W.S.; Lee, S.; Kang, D.; Kim, Y.; Kim, Y.J.; Kim, S.Y. Exposure to Nickel Oxide Nanoparticles Induces Acute and Chronic Inflammatory Responses in Rat Lungs and Perturbs the Lung Microbiome. Int. J. Environ. Res. Public Health 2022, 19, 522. [Google Scholar] [CrossRef]
- Sárközi, L.; Horváth, E.; Kónya, Z.; Kiricsi, I.; Szalay, B.; Vezér, T.; Papp, A. Subacute intratracheal exposure of rats to manganese nanoparticles: Behavioral, electrophysiological, and general toxicological effects. Inhal. Toxicol. 2009, 21 (Suppl. S1), 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chung, Y.H.; Seo, D.S.; Choi, H.S.; Lim, C.H. Twenty-Eight-Day Repeated Inhalation Toxicity Study of Aluminum Oxide Nanoparticles in Male Sprague-Dawley Rats. Toxicol. Res. 2018, 34, 343–354. [Google Scholar] [CrossRef]
- Guo, C.; Robertson, S.; Weber, R.J.M.; Buckley, A.; Warren, J.; Hodgson, A.; Rappoport, J.Z.; Ignatyev, K.; Meldrum, K.; Römer, I.; et al. Pulmonary toxicity of inhaled nano-sized cerium oxide aerosols in Sprague-Dawley rats. Nanotoxicology 2019, 13, 733–750. [Google Scholar] [CrossRef]
- Rahman, L.; Wu, D.; Johnston, M.; William, A.; Halappanavar, S. Toxicogenomics analysis of mouse lung responses following exposure to titanium dioxide nanomaterials reveal their disease potential at high doses. Mutagenesis 2017, 32, 59–76. [Google Scholar] [CrossRef]
- Hadrup, N.; Saber, A.T.; Kyjovska, Z.O.; Jacobsen, N.R.; Vippola, M.; Sarlin, E.; Ding, Y.; Schmid, O.; Wallin, H.; Jensen, K.A.; et al. Pulmonary toxicity of Fe2O3, ZnFe2O4, NiFe2O4 and NiZnFe4O8 nanomaterials: Inflammation and DNA strand breaks. Environ. Toxicol. Pharmacol. 2020, 74, 103303. [Google Scholar] [CrossRef]
- Hadrup, N.; Sørli, J.B.; Sharma, A.K. Pulmonary toxicity, genotoxicity, and carcinogenicity evaluation of molybdenum, lithium, and tungsten: A review. Toxicology 2022, 467, 153098. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.; Bian, Q.; Ning, J.; Yong, L.; Ou, T.; Song, Y.; Wei, S. Genotoxicity Evaluation of Titanium Dioxide Nanoparticles In Vivo and In Vitro: A Meta-Analysis. Toxics 2023, 11, 882. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; de Melo, T.C.; Mendes, T.B.; de Sá Júnior, P.L.; Nozima, B.H.; Ito, E.T.; de Carvalho, R.F.; de Souza, E.B.; de Cassia Stocco, R. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed. Pharmacother. 2015, 72, 74–82. [Google Scholar] [CrossRef]
- OECD. Test No. 489: In Vivo Mammalian Alkaline Comet Assay; OECD: Paris, France, 2014. [Google Scholar]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test; OECD: Paris, France, 2023. [Google Scholar]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Collins, A.R.; Annangi, B.; Rubio, L.; Marcos, R.; Dorn, M.; Merker, C.; Estrela-Lopis, I.; Cimpan, M.R.; Ibrahim, M.; Cimpan, E.; et al. High throughput toxicity screening and intracellular detection of nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1413. [Google Scholar] [CrossRef]
- Boyadzhiev, A.; Solorio-Rodriguez, S.A.; Wu, D.; Avramescu, M.L.; Rasmussen, P.; Halappanavar, S. The High-Throughput In Vitro CometChip Assay for the Analysis of Metal Oxide Nanomaterial Induced DNA Damage. Nanomaterials 2022, 12, 1844. [Google Scholar] [CrossRef]
- Watson, C.; Ge, J.; Cohen, J.; Pyrgiotakis, G.; Engelward, B.P.; Demokritou, P. High-throughput screening platform for engineered nanoparticle-mediated genotoxicity using CometChip technology. ACS Nano 2014, 8, 2118–2133. [Google Scholar] [CrossRef]
- Vecchio, G.; Fenech, M.; Pompa, P.P.; Voelcker, N.H. Lab-on-a-chip-based high-throughput screening of the genotoxicity of engineered nanomaterials. Small 2014, 10, 2721–2734. [Google Scholar] [CrossRef]
- Lebedová, J.; Hedberg, Y.S.; Odnevall Wallinder, I.; Karlsson, H.L. Size-dependent genotoxicity of silver, gold and platinum nanoparticles studied using the mini-gel comet assay and micronucleus scoring with flow cytometry. Mutagenesis 2018, 33, 77–85. [Google Scholar] [CrossRef]
- Avramescu, M.L.; Chénier, M.; Beauchemin, S.; Rasmussen, P. Dissolution Behaviour of Metal-Oxide Nanomaterials in Various Biological Media. Nanomaterials 2022, 13, 26. [Google Scholar] [CrossRef]
- Avramescu, M.L.; Chénier, M.; Gardner, H.D.; Rasmussen, P.E. Solubility of metal oxide nanomaterials: Cautionary notes on sample preparation. J. Phys. Conf. Ser. 2019, 1323, 012001. [Google Scholar] [CrossRef]
- Bushell, M.; Beauchemin, S.; Kunc, F.; Gardner, D.; Ovens, J.; Toll, F.; Kennedy, D.; Nguyen, K.; Vladisavljevic, D.; Rasmussen, P.E.; et al. Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area. Nanomaterials 2020, 10, 1812. [Google Scholar] [CrossRef]
- Avramescu, M.-L.; Chénier, M.; Palaniyandi, S.; Rasmussen, P.E. Dissolution behavior of metal oxide nanomaterials in cell culture medium versus distilled water. J. Nanopart. Res. 2020, 22, 222. [Google Scholar] [CrossRef]
- Rahman, L.; Jacobsen, N.R.; Aziz, S.A.; Wu, D.; Williams, A.; Yauk, C.L.; White, P.; Wallin, H.; Vogel, U.; Halappanavar, S. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: Investigating the mechanisms of pulmonary carcinogenesis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 823, 28–44. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- White, P.A.; Douglas, G.R.; Gingerich, J.; Parfett, C.; Shwed, P.; Seligy, V.; Soper, L.; Berndt, L.; Bayley, J.; Wagner, S.; et al. Development and characterization of a stable epithelial cell line from Muta Mouse lung. Environ. Mol. Mutagen. 2003, 42, 166–184. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; Pojana, G.; White, P.; Møller, P.; Cohn, C.A.; Korsholm, K.S.; Vogel, U.; Marcomini, A.; Loft, S.; Wallin, H. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C(60) fullerenes in the FE1-Mutatrade markMouse lung epithelial cells. Environ. Mol. Mutagen. 2008, 49, 476–487. [Google Scholar] [CrossRef]
- Bengtson, S.; Kling, K.; Madsen, A.M.; Noergaard, A.W.; Jacobsen, N.R.; Clausen, P.A.; Alonso, B.; Pesquera, A.; Zurutuza, A.; Ramos, R.; et al. No cytotoxicity or genotoxicity of graphene and graphene oxide in murine lung epithelial FE1 cells in vitro. Environ. Mol. Mutagen. 2016, 57, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Decan, N.; Wu, D.; Williams, A.; Bernatchez, S.; Johnston, M.; Hill, M.; Halappanavar, S. Characterization of in vitro genotoxic, cytotoxic and transcriptomic responses following exposures to amorphous silica of different sizes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 796, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Maertens, R.M.; Long, A.S.; White, P.A. Performance of the in vitro transgene mutation assay in MutaMouse FE1 cells: Evaluation of nine misleading (“False”) positive chemicals. Environ. Mol. Mutagen. 2017, 58, 582–591. [Google Scholar] [CrossRef]
- Boyadzhiev, A.; Avramescu, M.L.; Wu, D.; Williams, A.; Rasmussen, P.; Halappanavar, S. Impact of copper oxide particle dissolution on lung epithelial cell toxicity: Response characterization using global transcriptional analysis. Nanotoxicology 2021, 15, 380–399. [Google Scholar] [CrossRef] [PubMed]
- White, P.A.; Luijten, M.; Mishima, M.; Cox, J.A.; Hanna, J.N.; Maertens, R.M.; Zwart, E.P. In vitro mammalian cell mutation assays based on transgenic reporters: A report of the International Workshop on Genotoxicity Testing (IWGT). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 847, 403039. [Google Scholar] [CrossRef] [PubMed]
- Hölzl-Armstrong, L.; Nævisdal, A.; Cox, J.A.; Long, A.S.; Chepelev, N.L.; Phillips, D.H.; White, P.A.; Arlt, V.M. In vitro mutagenicity of selected environmental carcinogens and their metabolites in MutaMouse FE1 lung epithelial cells. Mutagenesis 2020, 35, 453–463. [Google Scholar] [CrossRef]
- Fortin, A.V.; Long, A.S.; Williams, A.; Meier, M.J.; Cox, J.; Pinsonnault, C.; Yauk, C.L.; White, P.A. Application of a new approach methodology (NAM)-based strategy for genotoxicity assessment of data-poor compounds. Front. Toxicol. 2023, 5, 1098432. [Google Scholar] [CrossRef]
- Avlasevich, S.; Pellegrin, T.; Godse, M.; Bryce, S.; Bemis, J.; Bajorski, P.; Dertinger, S. Biomarkers of DNA damage response improve in vitro micronucleus assays by revealing genotoxic mode of action and reducing the occurrence of irrelevant positive results. Mutagenesis 2021, 36, 407–418. [Google Scholar] [CrossRef]
- Boyadzhiev, A.; Wu, D.; Avramescu, M.-L.; Williams, A.; Rasmussen, P.; Halappanavar, S. Toxicity of Metal Oxide Nanoparticles: Looking through the Lens of Toxicogenomics. Int. J. Mol. Sci. 2024, 25, 529. [Google Scholar] [CrossRef]
- Chakraborty, S.; Misra, S.K. A comparative analysis of dialysis based separation methods for assessing copper oxide nanoparticle solubility. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100258. [Google Scholar] [CrossRef]
- Shinohara, N.; Zhang, G.; Oshima, Y.; Kobayashi, T.; Imatanaka, N.; Nakai, M.; Sasaki, T.; Kawaguchi, K.; Gamo, M. Kinetics and dissolution of intratracheally administered nickel oxide nanomaterials in rats. Part. Fibre Toxicol. 2017, 14, 48. [Google Scholar] [CrossRef]
- Holmfred, E.; Loeschner, K.; Sloth, J.J.; Jensen, K.A. Validation and Demonstration of an Atmosphere-Temperature-pH-Controlled Stirred Batch Reactor System for Determination of (Nano)Material Solubility and Dissolution Kinetics in Physiological Simulant Lung Fluids. Nanomaterials 2022, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yu, Y.; Lian, H.-Z.; Hu, X.; Fu, H.; Chen, Y.-J. Solubility of nano-sized metal oxides evaluated by using in vitro simulated lung and gastrointestinal fluids: Implication for health risks. J. Nanopart. Res. 2017, 19, 375. [Google Scholar] [CrossRef]
- Helmig, S.; Haibel, N.; Walter, D. In vitro toxicity studies of aluminum compounds. J. Therm. Anal. Calorim. 2018, 134, 643–651. [Google Scholar] [CrossRef]
- Berry, J.P.; Masse, R.; Escaig, F.; Galle, P. Intracellular localization of cerium. A microanalytical study using an electron microprobe and ionic microanalysis. Hum. Toxicol. 1989, 8, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, S.; Saber, A.T.; Decan, N.; Jensen, K.A.; Wu, D.; Jacobsen, N.R.; Guo, C.; Rogowski, J.; Koponen, I.K.; Levin, M.; et al. Transcriptional profiling identifies physicochemical properties of nanomaterials that are determinants of the in vivo pulmonary response. Environ. Mol. Mutagen. 2015, 56, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lee, A.; Ji, Z.; Huang, C.; Chang, C.H.; Wang, X.; Liao, Y.P.; Xia, T.; Li, R. Reduction of pulmonary toxicity of metal oxide nanoparticles by phosphonate-based surface passivation. Part. Fibre Toxicol. 2017, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- García-Torra, V.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; Barroso, E.; Vazquez-Carrera, M.; García, M.L.; Sánchez-López, E.; Souto, E.B. State of the Art on Toxicological Mechanisms of Metal and Metal Oxide Nanoparticles and Strategies to Reduce Toxicological Risks. Toxics 2021, 9, 195. [Google Scholar] [CrossRef]
- Zhou, F.; Liao, F.; Chen, L.; Liu, Y.; Wang, W.; Feng, S. The size-dependent genotoxicity and oxidative stress of silica nanoparticles on endothelial cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 1911–1920. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef]
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 2016, 99, 133–143. [Google Scholar] [CrossRef]
- Hadrup, N.; Sahlgren, N.; Jacobsen, N.R.; Saber, A.T.; Hougaard, K.S.; Vogel, U.; Jensen, K.A. Toxicity dose descriptors from animal inhalation studies of 13 nanomaterials and their bulk and ionic counterparts and variation with primary particle characteristics. Nanotoxicology 2023, 17, 338–371. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H.; Maryon, E.B. How Mammalian Cells Acquire Copper: An Essential but Potentially Toxic Metal. Biophys. J. 2016, 110, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Maryon, E.B.; Molloy, S.A.; Ivy, K.; Yu, H.; Kaplan, J.H. Rate and regulation of copper transport by human copper transporter 1 (hCTR1). J. Biol. Chem. 2013, 288, 18035–18046. [Google Scholar] [CrossRef] [PubMed]
- Nies, A.T. Membrane Transporters. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1826–1829. [Google Scholar]
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. Met. Ions Life Sci. 2013, 13, 199–227. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise Review of Nickel Human Health Toxicology and Ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10 (Suppl. S1), 1–269. [Google Scholar] [CrossRef]
- Husain, M.; Saber, A.T.; Guo, C.; Jacobsen, N.R.; Jensen, K.A.; Yauk, C.L.; Williams, A.; Vogel, U.; Wallin, H.; Halappanavar, S. Pulmonary instillation of low doses of titanium dioxide nanoparticles in mice leads to particle retention and gene expression changes in the absence of inflammation. Toxicol. Appl. Pharmacol. 2013, 269, 250–262. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Kiliç, G.; Costa, C.; Fernández-Bertólez, N.; Pásaro, E.; Teixeira, J.P.; Laffon, B. Effects of iron oxide nanoparticles: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ. Mol. Mutagen. 2015, 56, 125–148. [Google Scholar] [CrossRef]
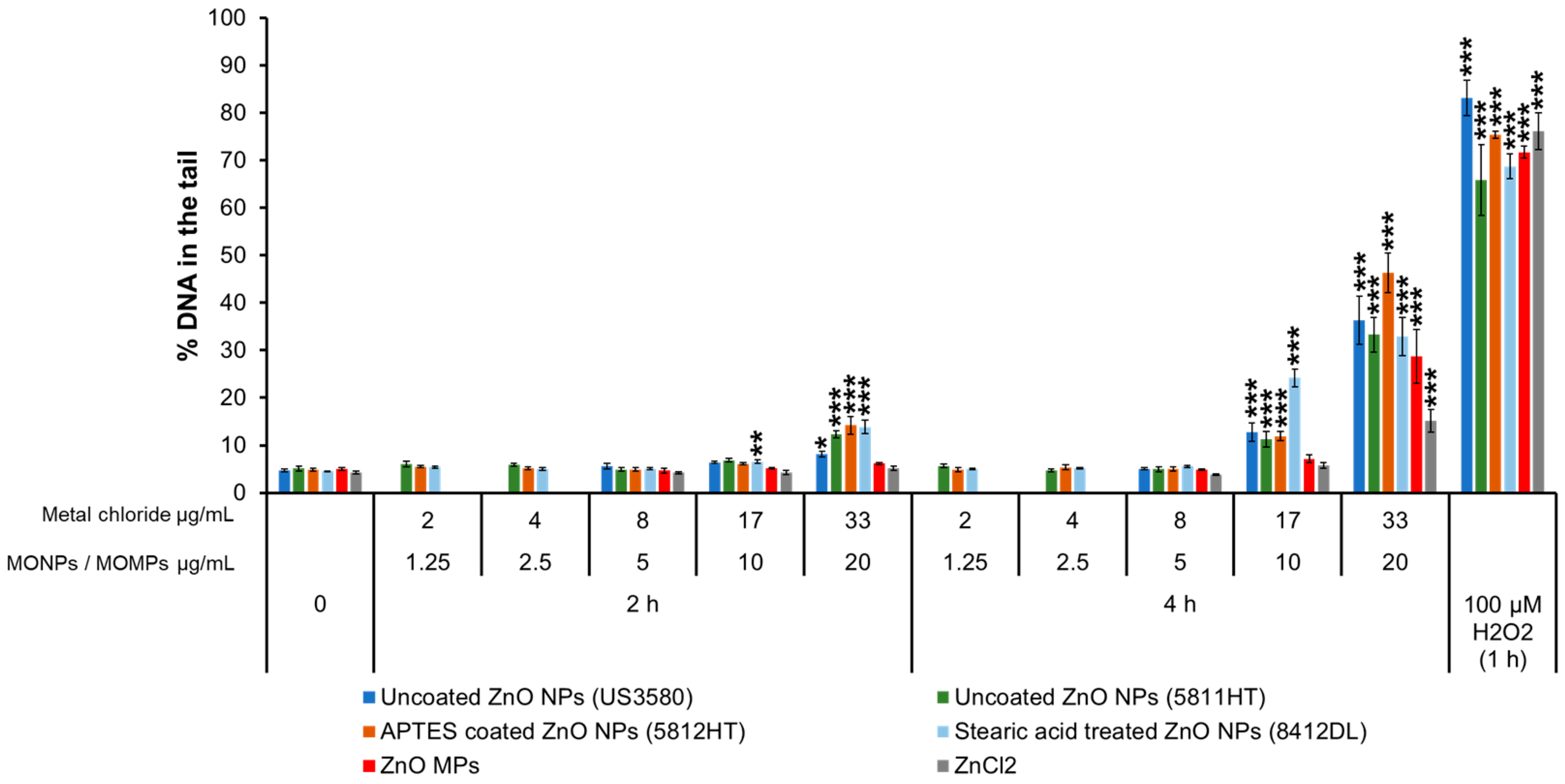
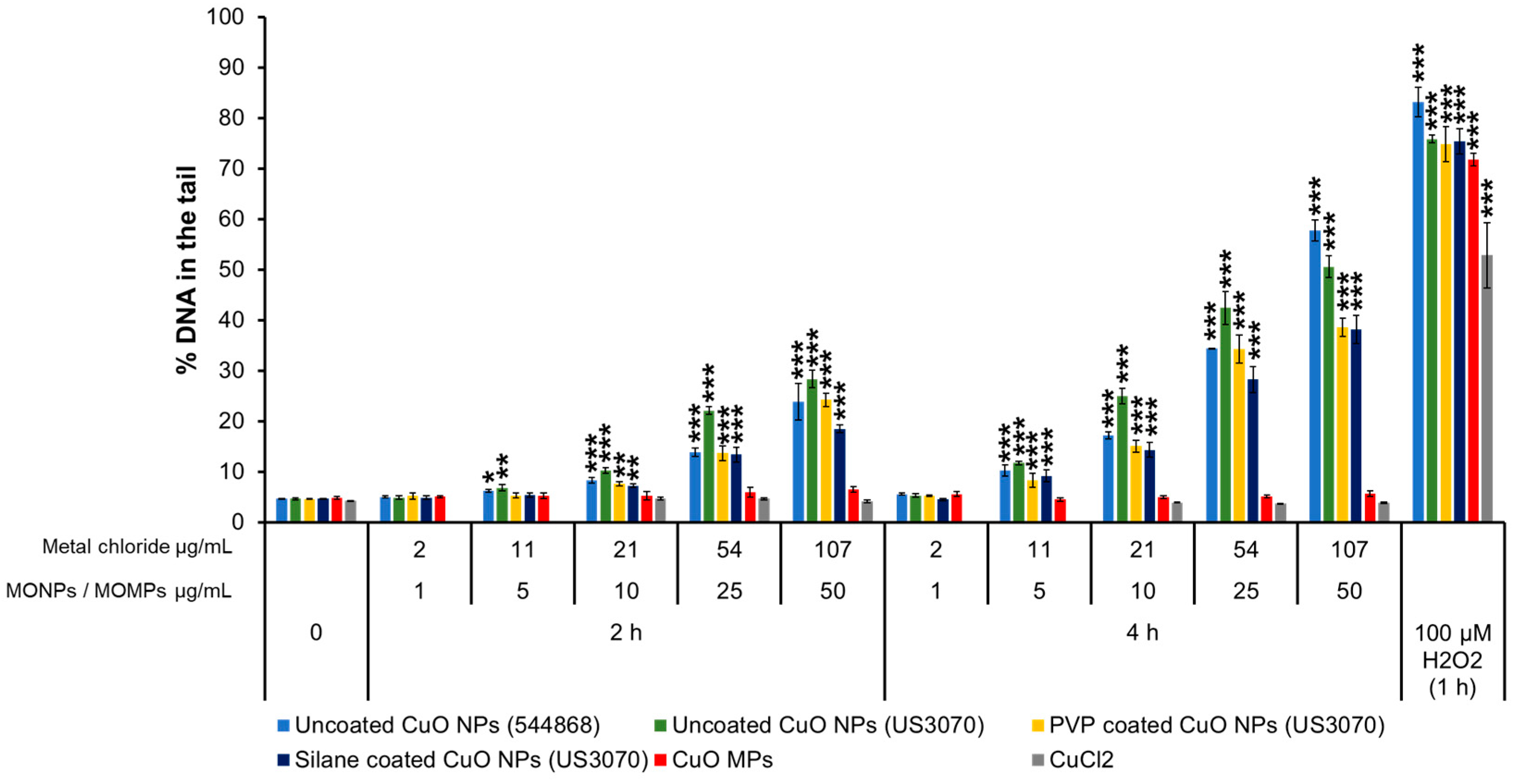
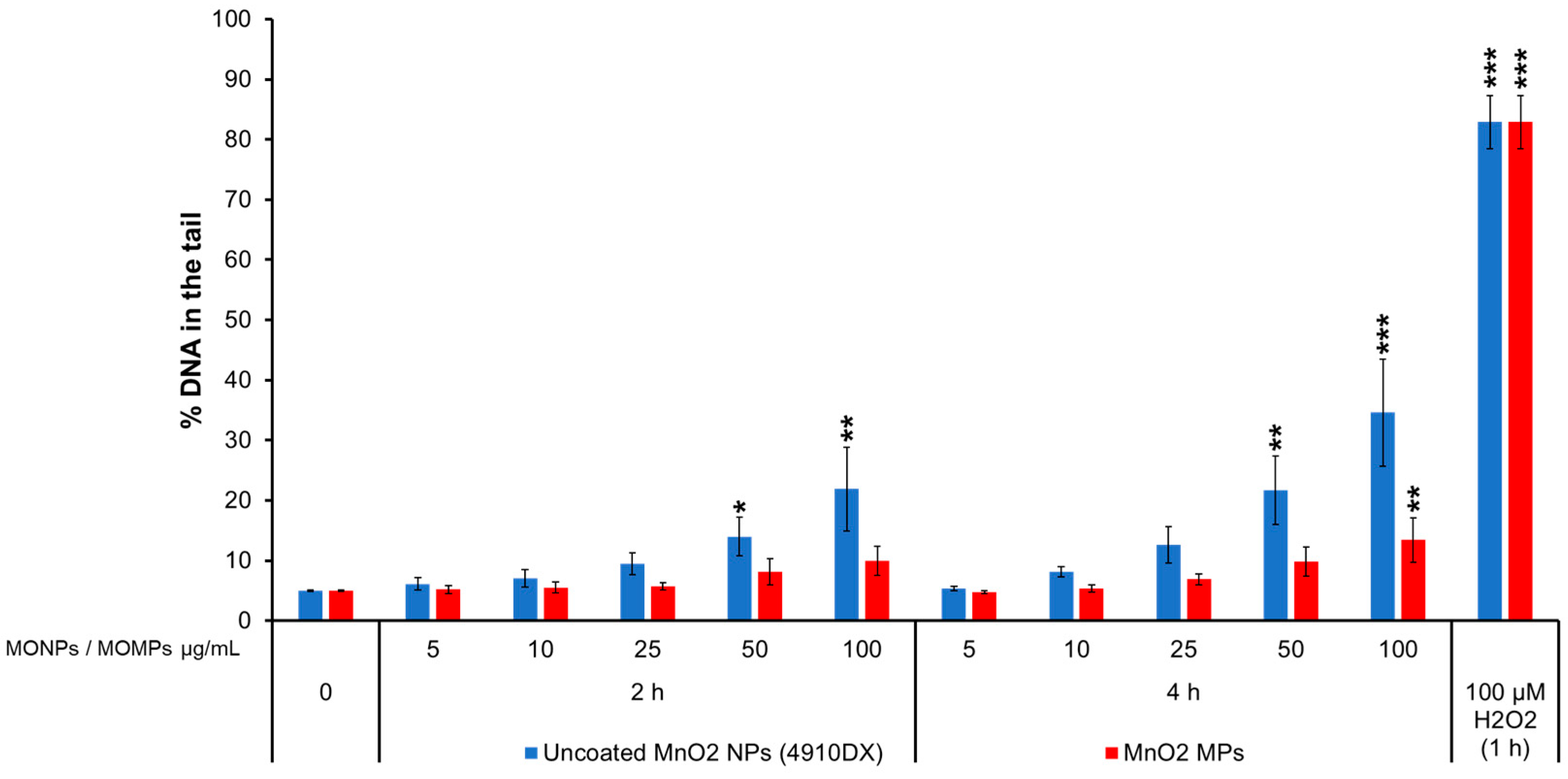
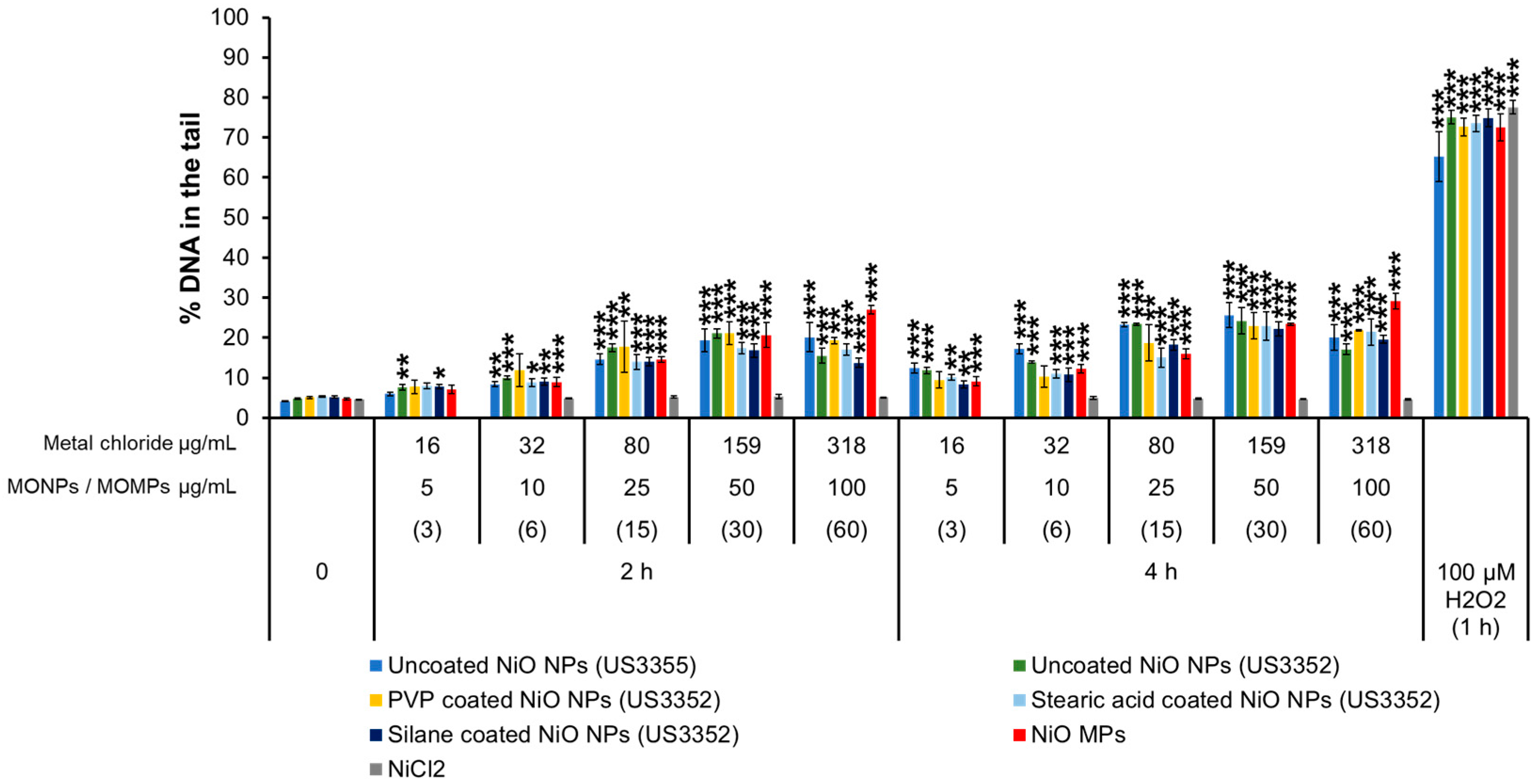



| MONPs | Manufacturer | Coating | Purity (%) | Primary Size (nm) | SSA (m2/g) |
|---|---|---|---|---|---|
| ZnO US3580 | US Research Nanomaterials, Inc., Houston, TX, USA | Uncoated | 99+ | 35–45 | 65 |
| ZnO 5811HT | Nanostructured and Amorphous Materials, Inc., Katy, TX, USA | Uncoated | 99+ | 30 | >15 |
| ZnO 5812HT | Nanostructured and Amorphous Materials, Inc., Katy, TX, USA | APTES-coated | 98+ | 30 | >8 |
| ZnO 8412DL | SkySpring Nanomaterials, Inc., Houston, TX, USA | Stearic acid-treated | 99 | 10–30 | >60 |
| CuO 544868 | Sigma–Aldrich, Oakville, ON, Canada | Uncoated | 97.8 | <50 | 29 |
| CuO US3070 | US Research Nanomaterials, Inc., Houston, TX, USA | Uncoated | 99 | 40 | ~45 |
| CuO US3070 | US Research Nanomaterials, Inc., Houston, TX, USA | PVP-coated | 99 | 40 | ~45 |
| CuO US3070 | US Research Nanomaterials, Inc., Houston, TX, USA | Silane-coated | 99 | 40 | ~45 |
| MnO2 4910DX * | SkySpring Nanomaterials, Inc., Houston, TX, USA | Uncoated | 98+ | 40–60 | ~13.5 |
| NiO US3355 | US Research Nanomaterials, Inc., Houston, TX, USA | Uncoated | 99.5+ | 15–35 | 50–100 |
| NiO US3352 | US Research Nanomaterials, Inc., Houston, TX, USA | Uncoated | 99.98 | 18 | 50–100 |
| NiO US3352 | US Research Nanomaterials, Inc., Houston, TX, USA | PVP-coated | 99.98 | 18 | 50–100 |
| NiO US3352 | US Research Nanomaterials, Inc., Houston, TX, USA | Stearic acid-coated | 99.98 | 18 | 50–100 |
| NiO US3352 | US Research Nanomaterials, Inc., Houston, TX, USA | Silane-coated | 99.98 | 18 | 50–100 |
| Al2O3 544833 | Sigma–Aldrich, Oakville, ON, Canada | Uncoated | - | <50 | >40 |
| CeO2 US3136 | US Research Nanomaterials, Inc., Houston, TX, USA | Uncoated | 99.97 | 50 | 30–35 |
| CeO2 US3036 | US Research Nanomaterials, Inc., Houston, TX, USA | Uncoated | 99.97 | 10–30 | 30–50 |
| CeO2 US3037 | US Research Nanomaterials, Inc., Houston, TX, USA | PVP-coated | 99.99 | 10 | 35–70 |
| CeO2 US3037 | US Research Nanomaterials, Inc., Houston, TX, USA | Stearic acid-coated | 99.99 | 10 | 35–70 |
| TiO2 NIST # | National Institute of Standards and Technology (NIST,1989), Gaithersburg, MD, USA | Uncoated | 99.4 a | 19-Anatase (76%) 37-Rutile (24%) | 55.55 |
| Fe2O3 US3160 | US Research Nanomaterials, Inc., Houston, TX, USA | Uncoated | 99.5+ | 30 | 20–60 |
| MOMPs | Manufacturer | Purity (%) | Primary Size (µm) | SSA (m2/g) |
|---|---|---|---|---|
| ZnO US1003M | US Research Nanomaterials, Inc., Houston, TX, USA | 99.9+ | 1 | 2–15.8 |
| CuO US1140M | US Research Nanomaterials, Inc., Houston, TX, USA | 99.5 | 5 | 4–6 |
| MnO2 4930DX | SkySpring Nanomaterials, Inc., Houston, TX, USA | 99+ | ~5 (50%), <10 (90%) | - |
| NiO US1014M | US Research Nanomaterials, Inc., Houston, TX, USA | 99+ a | 5 | 5–20 |
| Al2O3 1331DL | SkySpring Nanomaterials, Inc., Houston, TX, USA | 99.9 | 0.4–1.5 | ~110 |
| CeO2 2118CG | SkySpring Nanomaterials, Inc., Houston, TX, USA | 99.9 | 5–20 | 5–8 |
| TiO2 US1017M | US Research Nanomaterials, Inc., Houston, Tx, USA | 99.9+ | 1.5 (Anatase) 1.5 (Rutile) | 5–8 |
| Fe2O3 US1139M | US Research Nanomaterials, Inc., Houston, TX, USA | 99 | 5 | - |
| MONPs | Primary Size d (nm) | Aspect Ratio e | Cell Culture Medium f | ||
|---|---|---|---|---|---|
| Length Width | |||||
| HD (nm) | PDI | ZP (mv) | |||
| Uncoated ZnO (US3580) | (23.9 ± 7.2) a (19.4 ± 5.5) a | 1.23 ± 0.17 a | 323 ± 125 a | 0.56 ± 0.12 a | −9.21 ± 1.15 |
| Uncoated ZnO (5811HT) | (44.06 ± 19.4) (33.88 ± 13.2) | 1.31 ± 0.32 | 290 ± 13.5 | 0.27 ± 0.05 | −9.05 ± 0.65 |
| APTES-coated ZnO (5812HT) | (53.51 ± 38.5) (40.4 ± 25.6) | 1.31 ± 0.30 | 322 ± 24.8 | 0.29 ± 0.05 | −10.8 ± 0.60 |
| Stearic acid-treated ZnO (8412DL) | (33.69 ± 16.57) (25.06 ± 9.56) | 1.35 ± 0.36 | 361 ± 65.9 | 0.42 ± 0.04 | −9.00 ± 0.96 |
| Uncoated CuO (544868) | (64.8 ± 47.0) b,c (45.9 ± 28.0) b,c | 1.39 ± 0.39 b,c | 396 ± 34.9 | 0.35 ± 0.05 | −11.7 ± 0.73 |
| Uncoated CuO (US3070) | (75.97 ± 42.87) (58.06 ± 34.36) | 1.35 ± 0.32 | 265 ± 8.79 | 0.26 ± 0.03 | −11.7 ± 0.73 |
| PVP-coated CuO (US3070) | (63.07 ± 34.05) (48.07 ± 24.7) | 1.32 ± 0.28 | 272 ± 12.6 | 0.29 ± 0.04 | −11.3 ± 0.57 |
| Silane-coated CuO (US3070) | (84.21 ± 55.46) (62.35 ± 36.28) | 1.35 ± 0.41 | 494 ± 49.1 | 0.34 ± 0.11 | −14.9 ± 0.75 |
| Uncoated MnO2 (4910DX) | (36.06 ± 34.88) (13.12 ± 6.33) | 2.80 ± 1.97 | 151 ± 2.42 | 0.19 ± 0.03 | −12.3 ± 0.87 |
| Uncoated NiO (US3355) | (27.29 ± 10.26) c (21.84 ± 7.90) c | 1.25 ± 0.20 c | 229 ± 18.5 | 0.30 ± 0.05 | −12.9 ± 1.03 |
| Uncoated NiO (US3352) | (25.56 ± 13.16) (20.71 ± 10.08) | 1.23 ± 0.24 | 231 ± 4.11 | 0.21 ± 0.02 | −14.4 ± 1.06 |
| PVP-coated NiO (US3352) | (31.99 ± 14.7) (24.66 ± 11.28) | 1.31 ± 0.29 | 213 ± 4.37 | 0.20 ± 0.02 | −13.6 ± 0.52 |
| Stearic acid-coated NiO (US3352) | (29.59 ± 14.42) (22.81 ± 10.38) | 1.30 ± 0.25 | 285 ± 9.62 | 0.28 ± 0.05 | −16.7 ± 0.82 |
| Silane-coated NiO (US3352) | (29.51 ± 12.46) (23.90 ± 9.98) | 1.24 ± 0.19 | 222 ± 8.49 | 0.29 ± 0.04 | −13.6 ± 0.83 |
| Uncoated Al2O3 (544833) | (23.92 ± 11.84) c (10.68 ± 6.85) c | 2.63 ± 1.40 c | 385 ± 48.6 | 0.37 ± 0.05 | −12.4 ± 0.81 |
| Uncoated CeO2 (US3136) | (39.78 ± 14.55) (31.25 ± 9.66) | 1.27 ± 0.22 | 345 ± 6.72 | 0.27 ± 0.03 | −14.0 ± 1.12 |
| Uncoated CeO2 (US3036) | (12.05 ± 3.42) (9.90 ± 2.73) | 1.22 ± 0.20 | 357 ± 15.2 | 0.32 ± 0.05 | −13.9 ± 1.91 |
| PVP-coated CeO2 (US3037) | (14.12 ± 4.54) (10.92 ± 3.42) | 1.29 ± 0.22 | 383 ± 20.4 | 0.32 ± 0.05 | −11.9 ±0.99 |
| Stearic acid-coated CeO2 (US3037) | (10.61 ± 2.71) (8.23 ± 2.05) | 1.30 ± 0.20 | 405 ± 23.4 | 0.40 ± 0.05 | −12.2 ± 0.68 |
| Uncoated TiO2 NPs | (26.8 ± 8.9) a,c (20.8 ± 6.8) a,c | 1.30 ± 0.26 c | 421 ± 31 c | 0.23 ± 0.06 c | −11.5 ± 0.2 c |
| Uncoated Fe2O3 (US3160) | (30.95 ± 13.12) (24.99 ± 11.24) | 1.26 ± 0.24 | 193 ± 12.9 | 0.28 ± 0.03 | −11.4 ± 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© Health Canada, 2024. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solorio-Rodriguez, S.A.; Wu, D.; Boyadzhiev, A.; Christ, C.; Williams, A.; Halappanavar, S. A Systematic Genotoxicity Assessment of a Suite of Metal Oxide Nanoparticles Reveals Their DNA Damaging and Clastogenic Potential. Nanomaterials 2024, 14, 743. https://doi.org/10.3390/nano14090743
Solorio-Rodriguez SA, Wu D, Boyadzhiev A, Christ C, Williams A, Halappanavar S. A Systematic Genotoxicity Assessment of a Suite of Metal Oxide Nanoparticles Reveals Their DNA Damaging and Clastogenic Potential. Nanomaterials. 2024; 14(9):743. https://doi.org/10.3390/nano14090743
Chicago/Turabian StyleSolorio-Rodriguez, Silvia Aidee, Dongmei Wu, Andrey Boyadzhiev, Callum Christ, Andrew Williams, and Sabina Halappanavar. 2024. "A Systematic Genotoxicity Assessment of a Suite of Metal Oxide Nanoparticles Reveals Their DNA Damaging and Clastogenic Potential" Nanomaterials 14, no. 9: 743. https://doi.org/10.3390/nano14090743





