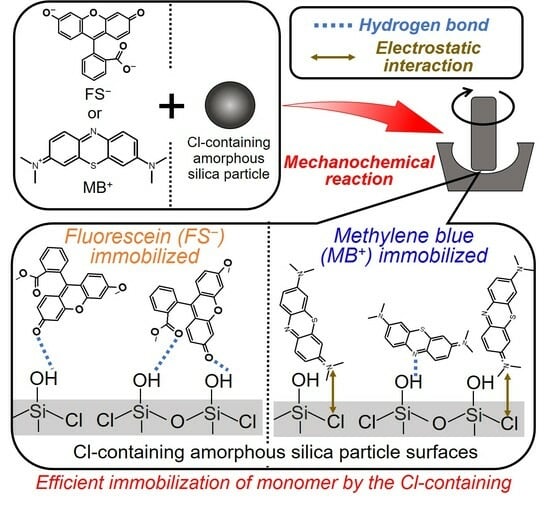
Mechanochemical Solid-State Immobilization of Photofunctional Dyes on Amorphous Silica Particles and Investigation of Their Interactive Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Cl-Containing ASPs
2.2. Mechanochemical Immobilization of Photofunctional Dyes
2.3. Characterization of the Mechanochemically Treated ASPs
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Sol-Gel Silica-Based Biomaterials and Bone Tissue Regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. New Trends in Bioactive Scaffolds: The Importance of Nanostructure. J. Eur. Ceram. Soc. 2009, 29, 1275–1281. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, H.; Bell, D.C.; Stein, A.; Francis, L.F. Effects of Materials Parameters on Mineralization and Degradation of Sol-Gel Bioactive Glasses with 3D-Ordered Macroporous Structures. J. Biomed. Mater. Res. A 2003, 66, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of Silica-Coated TiO2 and Its UV Absorption for Sunscreen Cosmetic Applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Zaccariello, G.; Back, M.; Zanello, M.; Canton, P.; Cattaruzza, E.; Riello, P.; Alimonti, A.; Benedetti, A. Formation and Controlled Growth of Bismuth Titanate Phases into Mesoporous Silica Nanoparticles: An Efficient Self-Sealing Nanosystem for UV Filtering in Cosmetic Formulation. ACS Appl. Mater. Interfaces 2017, 9, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Kramer, E.; Oomen, A.G.; Herrera Rivera, Z.E.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.P.; Weigel, S.; et al. Presence of Nano-Sized Silica during In Vitro Digestion of Foods Containing Silica as a Food Additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Larsericsdotter, H.; Oscarsson, S.; Buijs, J. Thermodynamic Analysis of Proteins Adsorbed on Silica Particles: Electrostatic Effects. J. Colloid Interface Sci. 2001, 237, 98–103. [Google Scholar] [CrossRef]
- Némethy, G.; Scheraga, H.A. Structure of Water and Hydrophobic Bonding in Proteins. IV. The Thermodynamic Properties of Liquid Deuterium Oxide. J. Chem. Phys. 2004, 41, 680–689. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Lee Fryar, K.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of Hydrogen Bonds to Protein Stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Chatani, S.; Inui, M.; Motozuka, S.; Liu, Z. Motohiro Tagaya Control of Biological Surface States on Chlorine-Doped Amorphous Silica Particles and Their Effective Absorptive Ability for Antibody Protein. Langmuir, 2024; in press. [Google Scholar]
- Heger, D.; Jirkovský, J.; Klán, P. Aggregation of Methylene Blue in Frozen Aqueous Solutions Studied by Absorption Spectroscopy. J. Phys. Chem. A 2005, 109, 6702–6709. [Google Scholar] [CrossRef] [PubMed]
- Bujdák, J.; Fujita, T.; Iyi, N. The Aggregation of Methylene Blue in Montmorillonite Dispersions. Clay Min. 2002, 37, 121–133. [Google Scholar] [CrossRef]
- Legentil, P.; Leroux, F.; Therias, S.; Mahiou, R.; Chadeyron, G. Revisiting Fluorescein and Layered Double Hydroxide Using a Synergistic Approach: A Complete Optical Study. J. Lumin. 2019, 215, 116634. [Google Scholar] [CrossRef]
- Chappey, B.; Myara, I.; Benoit, M.-O.; Mazière, C.; Mazière, J.-C.; Moatti, N. Characteristics of Ten Charge-Differing Subfractions Isolated from Human Native Low-Density Lipoproteins (LDL). No Evidence of Peroxidative Modifications. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1995, 1259, 261–270. [Google Scholar] [CrossRef]
- Lykke-Andersen, J.; Christiansen, J. The C-Terminal Carboxy Group of T7 RNA Polymerase Ensures Efficient Magnesium Ion-Dependent Catalysis. Nucleic Acids Res. 1998, 26, 5630–5635. [Google Scholar] [CrossRef] [PubMed]
- Salmio, H.; Brühwiler, D. Distribution of Amino Groups on a Mesoporous Silica Surface after Submonolayer Deposition of Aminopropylsilanes from an Anhydrous Liquid Phase. J. Phys. Chem. C 2007, 111, 923–929. [Google Scholar] [CrossRef]
- Ritter, H.; Ramm, J.H.; Brühwiler, D. Influence of the Structural Properties of Mesoporous Silica on the Adsorption of Guest Molecules. Materials 2010, 3, 4500–4509. [Google Scholar] [CrossRef]
- Melnyk, I.V.; Tomina, V.V.; Stolyarchuk, N.V.; Seisenbaeva, G.A.; Kessler, V.G. Organic Dyes (Acid Red, Fluorescein, Methylene Blue) and Copper(II) Adsorption on Amino Silica Spherical Particles with Tailored Surface Hydrophobicity and Porosity. J. Mol. Liq. 2021, 336, 116301. [Google Scholar] [CrossRef]
- Motozuka, S.; Tagaya, M.; Hayashi, K.; Kameyama, T.; Oguri, H.; Xu, Z. Mechanochemical Surface Modification of Carbon Fibers Using a Simple Rubbing Method. J. Compos. Mater. 2017, 51, 3577–3584. [Google Scholar] [CrossRef]
- Motozuka, S.; Tagaya, M.; Nishiyama, H.; Nishikawa, M.; Ikoma, T.; Yoshioka, T.; Samitsu, S.; Tanaka, J. Effective Functionalization of Disordered Oxide Lattices on Iron Particle Surfaces Using Mechanochemical Reactions. J. Phys. Chem. C 2013, 117, 9908–9919. [Google Scholar] [CrossRef]
- Watanabe, T.; Liao, J.; Senna, M. Changes in the Basicity and Species on the Surface of Me(OH)2-SiO2 (Me = Ca, Mg, Sr) Mixtures Due to Mechanical Activation. J. Solid State Chem. 1995, 115, 390–394. [Google Scholar] [CrossRef]
- Tagaya, M.; Motozuka, S.; Kobayashi, T.; Ikoma, T.; Tanaka, J. Mechanochemical Preparation of 8-Hydroxyquinoline/Hydroxyapatite Hybrid Nanocrystals and Their Photofunctional Interfaces. Ind. Eng. Chem. Res. 2012, 51, 11294–11300. [Google Scholar] [CrossRef]
- Thongsamakphan, S.P.; Ogawa, M. Hybridization of Quinacridone and Synthetic Hectorite and the Photoluminescence Quenching by Metal Ions. Appl. Clay Sci. 2023, 245, 107148. [Google Scholar] [CrossRef]
- Tagaya, M.; Motozuka, S.; Kobayashi, T.; Ikoma, T.; Tanaka, J. Efficient Incorporation of Monomeric Anthracene into Nanoporous Silica/Surfactant Nanocomposite Spheres Using a Mechanochemical Solid State Reaction. J. Mater. Chem. 2012, 22, 18741–18743. [Google Scholar] [CrossRef]
- De, S.; Kundu, R. Spectroscopic Studies with Fluorescein Dye—Protonation, Aggregation and Interaction with Nanoparticles. J. Photochem. Photobiol. A Chem. 2011, 223, 71–81. [Google Scholar] [CrossRef]
- Das, S.; Chattopadhyay, A.P.; De, S. Controlling J Aggregation in Fluorescein by Bile Salt Hydrogels. J. Photochem. Photobiol. A Chem. 2008, 197, 402–414. [Google Scholar] [CrossRef]
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and Fluorescence Properties of Fluorescein. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Grumelli, D.; Méndez De Leo, L.P.; Bonazzola, C.; Zamlynny, V.; Calvo, E.J.; Salvarezza, R.C. Methylene Blue Incorporation into Alkanethiol SAMs on Au(111): Effect of Hydrocarbon Chain Ordering. Langmuir 2010, 26, 8226–8232. [Google Scholar] [CrossRef]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of Intermolecular Interactions in FTIR Spectra of Methylene Blue Molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Seo, S.-H.; Kim, B.-M.; Joe, A.; Han, H.-W.; Chen, X.; Cheng, Z.; Jang, E.-S. NIR-Light-Induced Surface-Enhanced Raman Scattering for Detection and Photothermal/Photodynamic Therapy of Cancer Cells Using Methylene Blue-Embedded Gold Nanorod@SiO2 Nanocomposites. Biomaterials 2014, 35, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, V.D.; Raman, B.; Rao, C.M.; Ramakrishna, T. Co-Refolding Denatured-Reduced Hen Egg White Lysozyme with Acidic and Basic Proteins. FEBS Lett. 1997, 418, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Aslan, F.M.; Yu, Y.; Vajda, S.; Mohr, S.C.; Cantor, C.R. Engineering a Novel, Stable Dimeric Streptavidin with Lower Isoelectric Point. J. Biotechnol. 2007, 128, 213–225. [Google Scholar] [CrossRef]
- Tang, Y.; Cain, P.; Anguiano, V.; Shih, J.J.; Chai, Q.; Feng, Y. Impact of IgG Subclass on Molecular Properties of Monoclonal Antibodies. mAbs 2021, 13, 1993768. [Google Scholar] [CrossRef]






| Assignments | Peak Position [nm] | Peak Position [eV] | |
|---|---|---|---|
| FS−-immobilized ASPs | M-mono | 590 | 2.1 |
| J-aggr | 561 | 2.21 | |
| Di-mono | 544 | 2.28 | |
| H-aggr | 515 | 2.41 | |
| FS− solution | M-mono | 564 | 2.2 |
| Di-mono | 535 | 2.32 | |
| H-aggr | 514 | 2.41 | |
| MB+-immobilized ASPs | J-aggr | 708–664 | 1.75–1.87 |
| Di-mono | 644–607 | 1.93–2.04 | |
| H-Dimer | 592–548 | 2.09–2.26 | |
| H-aggr | 539–518 | 2.30–2.39 | |
| MB+ solution | J-aggr | 761 | 1.63 |
| Di-mono | 663 | 1.87 | |
| H-Dimer | 608 | 2.04 | |
| H-aggr | 577 | 2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, R.; Chatani, S.; Inui, M.; Motozuka, S.; Yamada, I.; Tagaya, M. Mechanochemical Solid-State Immobilization of Photofunctional Dyes on Amorphous Silica Particles and Investigation of Their Interactive Mechanisms. Nanomaterials 2024, 14, 741. https://doi.org/10.3390/nano14090741
Kimura R, Chatani S, Inui M, Motozuka S, Yamada I, Tagaya M. Mechanochemical Solid-State Immobilization of Photofunctional Dyes on Amorphous Silica Particles and Investigation of Their Interactive Mechanisms. Nanomaterials. 2024; 14(9):741. https://doi.org/10.3390/nano14090741
Chicago/Turabian StyleKimura, Reo, Sunao Chatani, Masahiko Inui, Satoshi Motozuka, Iori Yamada, and Motohiro Tagaya. 2024. "Mechanochemical Solid-State Immobilization of Photofunctional Dyes on Amorphous Silica Particles and Investigation of Their Interactive Mechanisms" Nanomaterials 14, no. 9: 741. https://doi.org/10.3390/nano14090741