Luminescent/Temperature-Sensing Properties of Multifunctional Rare-Earth Upconversion Kevlar Nanofiber Composite under 1550 nm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments and Characterization
2.3. CNPs Synthesi
2.3.1. Synthesis of NaYF4: Er UCNPs
2.3.2. Synthesis of NaYF4: Er@NaYF4 Core–Shell UCNPs
2.3.3. PEG Modification of NaYF4: Er@NaYF4 Core–Shell UCNPs
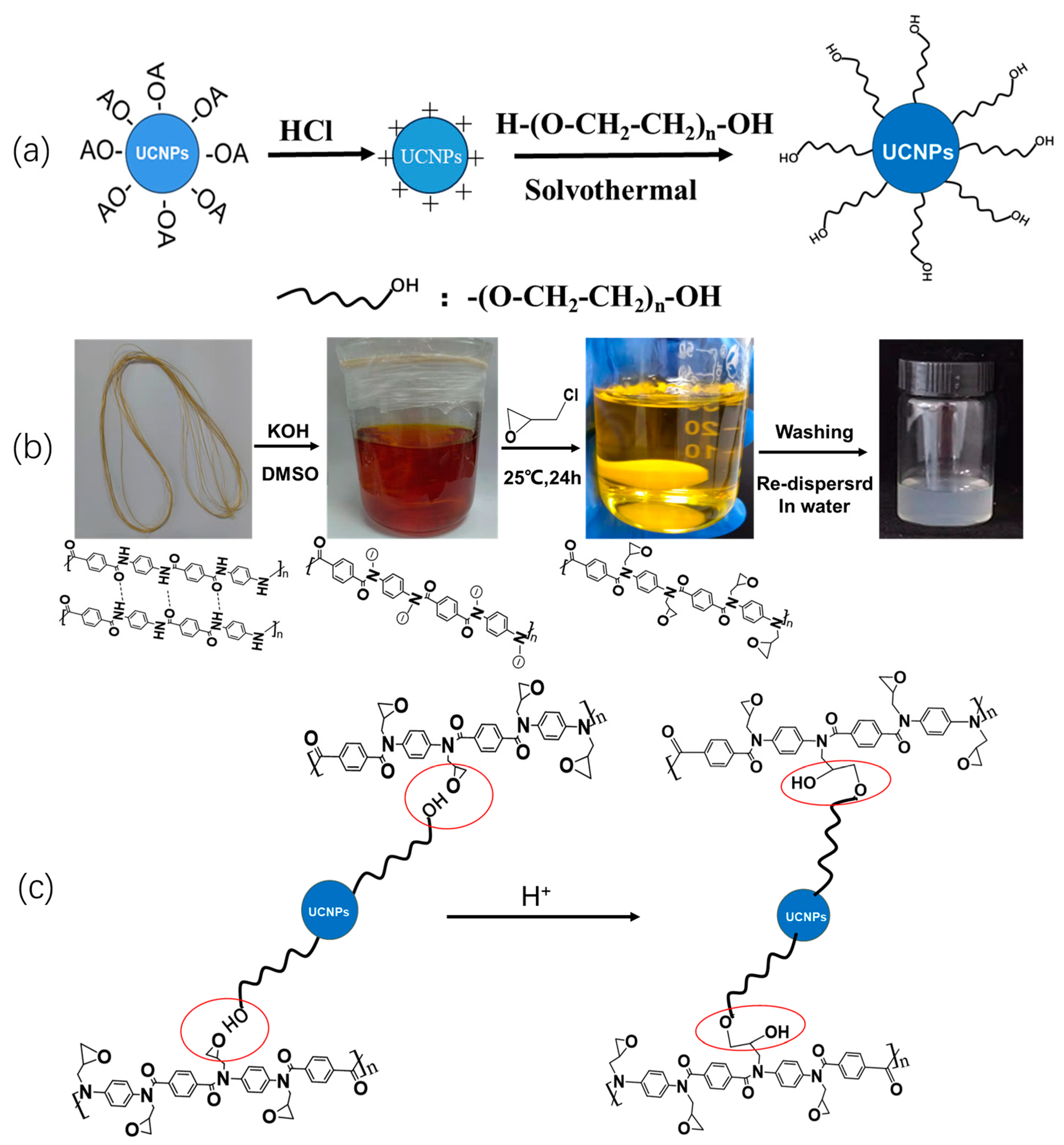
2.4. Synthesis and Water-Soluble Modification of KNFs
2.5. Synthesis and Film Preparation of UCNP/KNF Composites
3. Results and Discussion
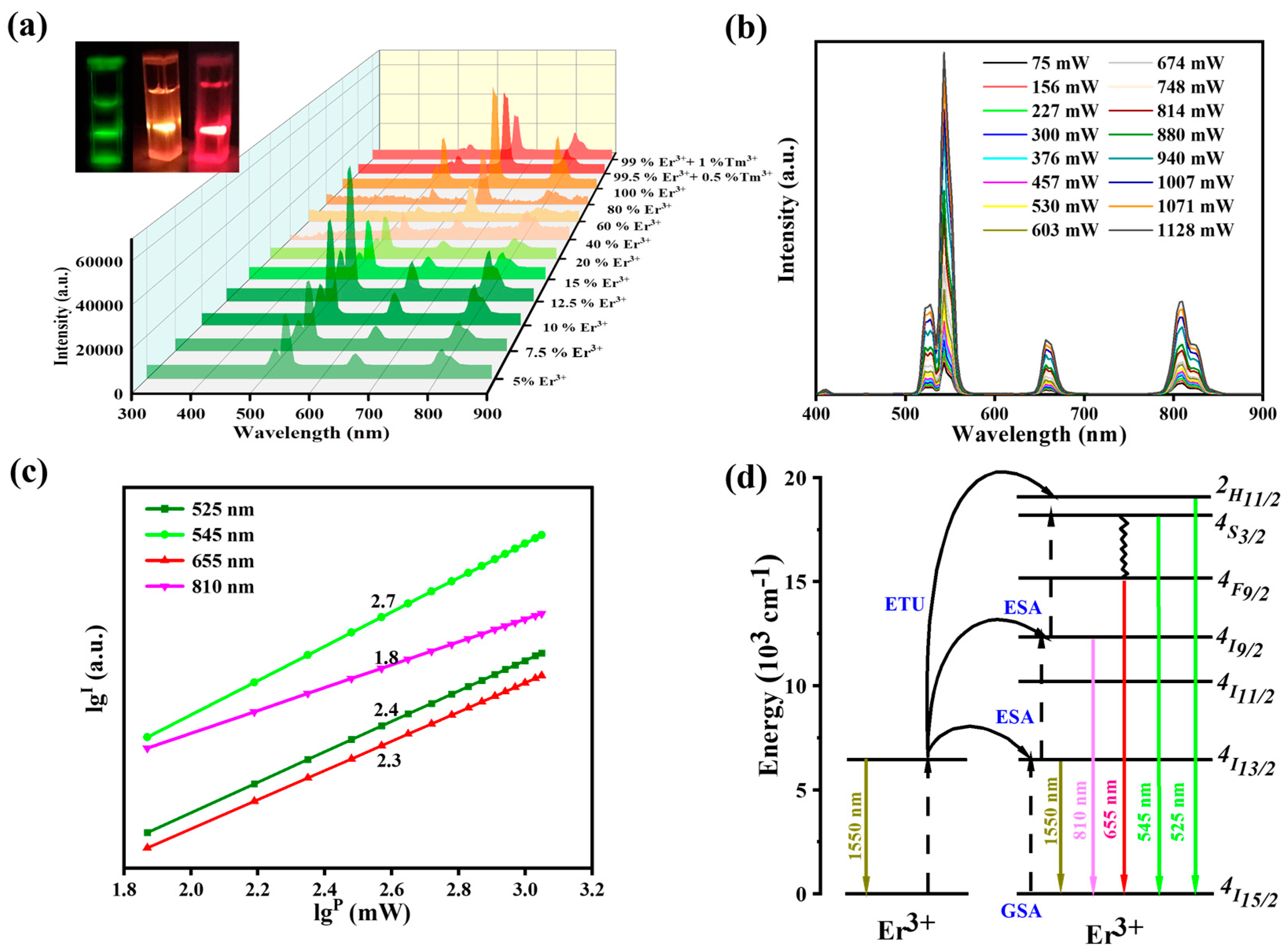
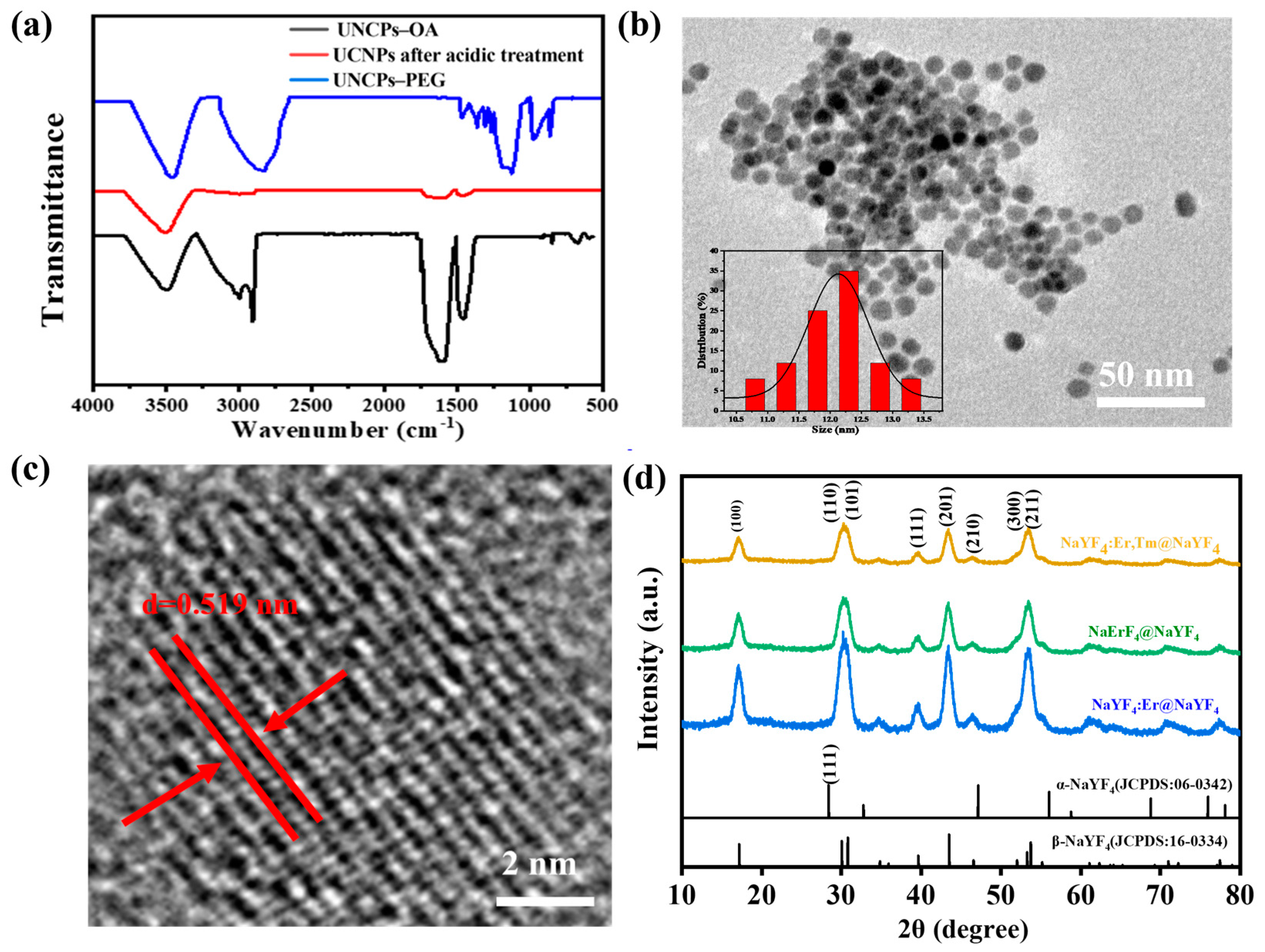
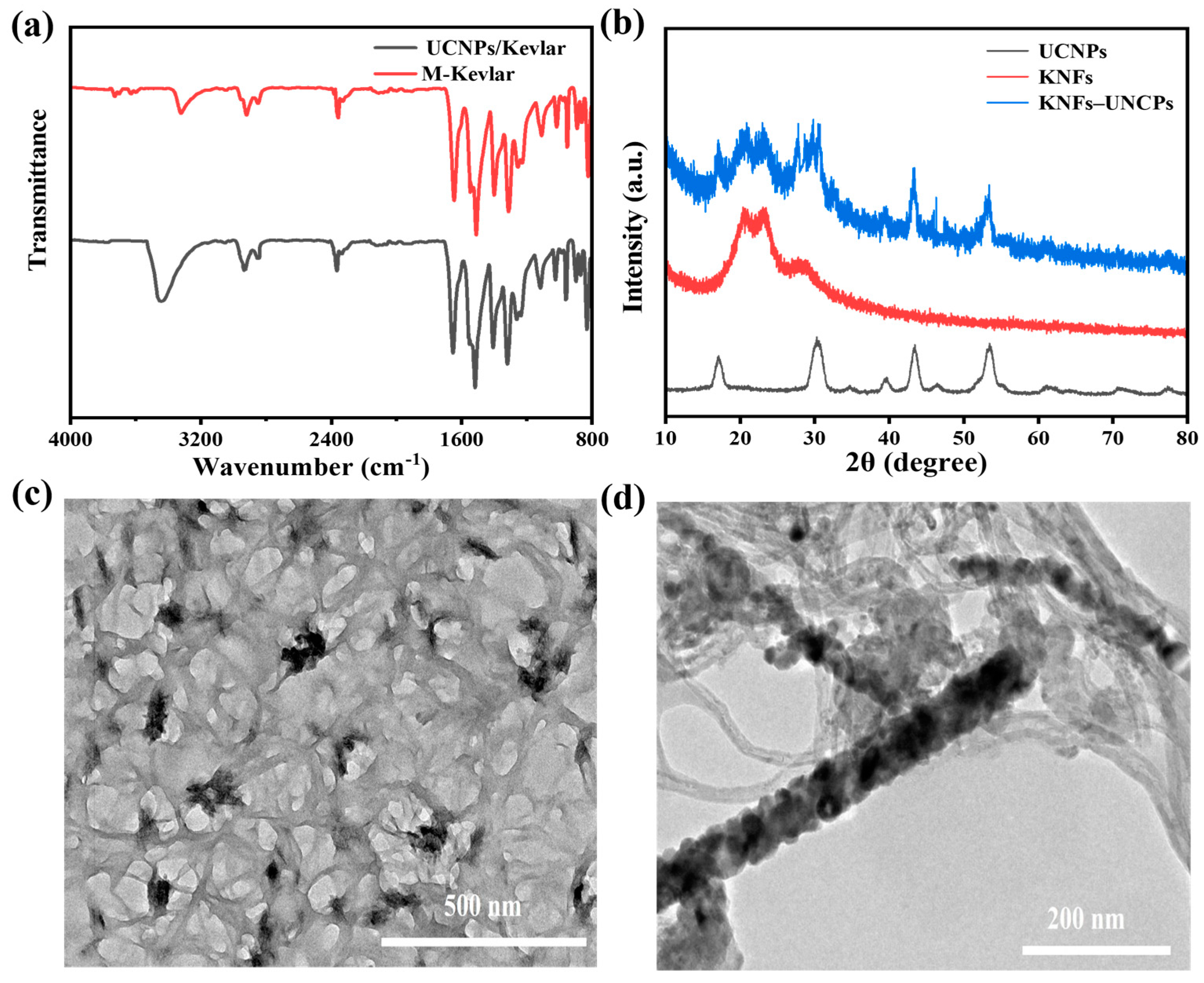
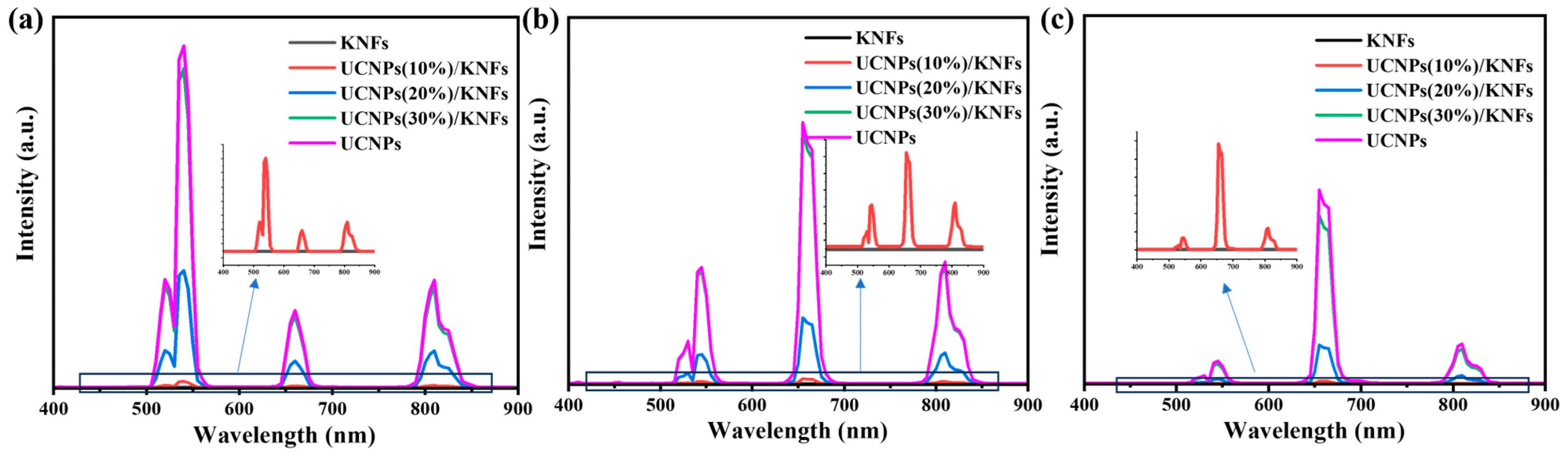
3.1. Morphology and Spectroscopic Properties of UCNPs Modified with PEG
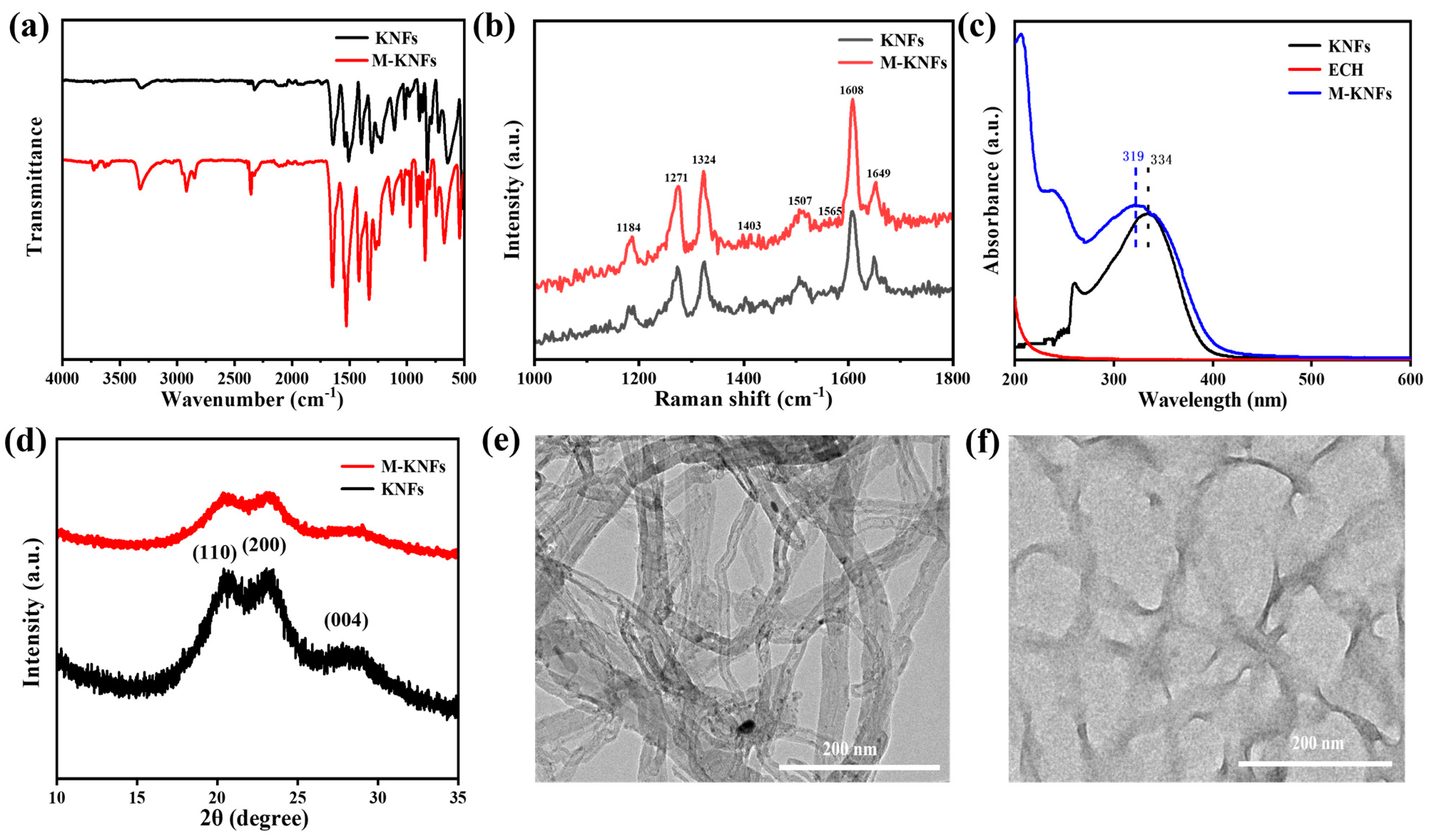
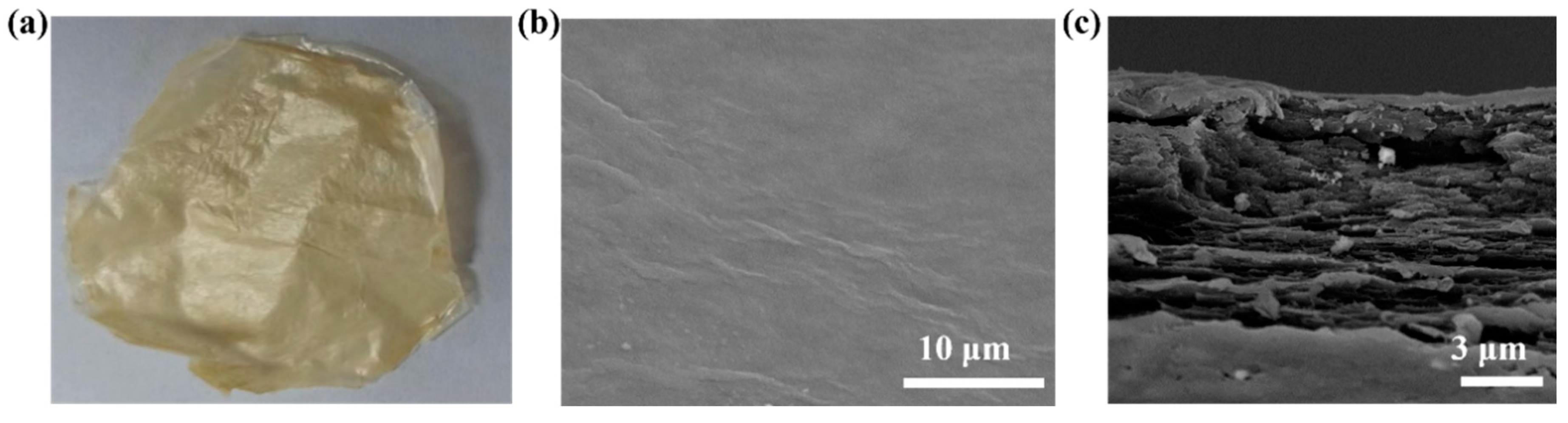
3.2. KNF Morphology
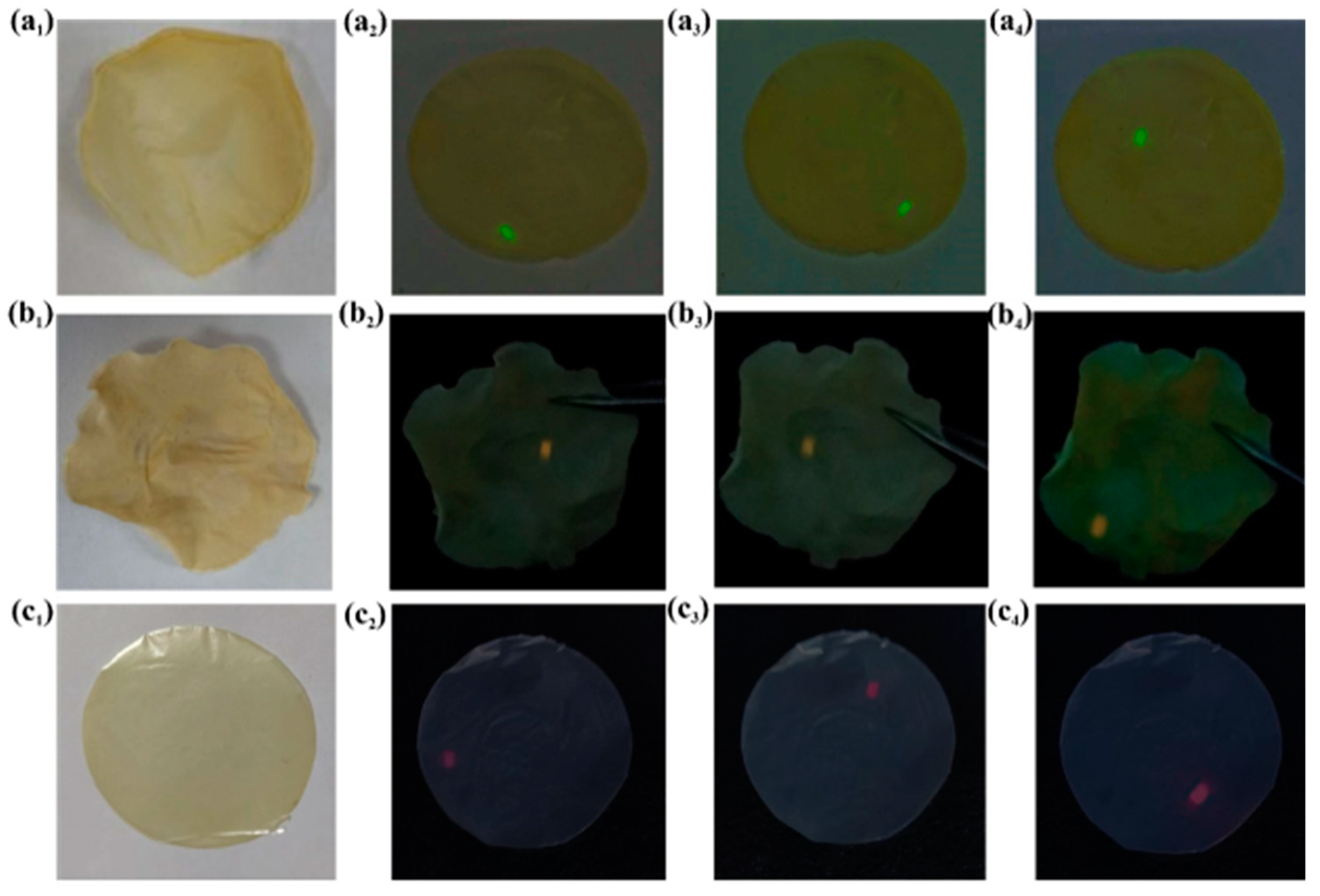
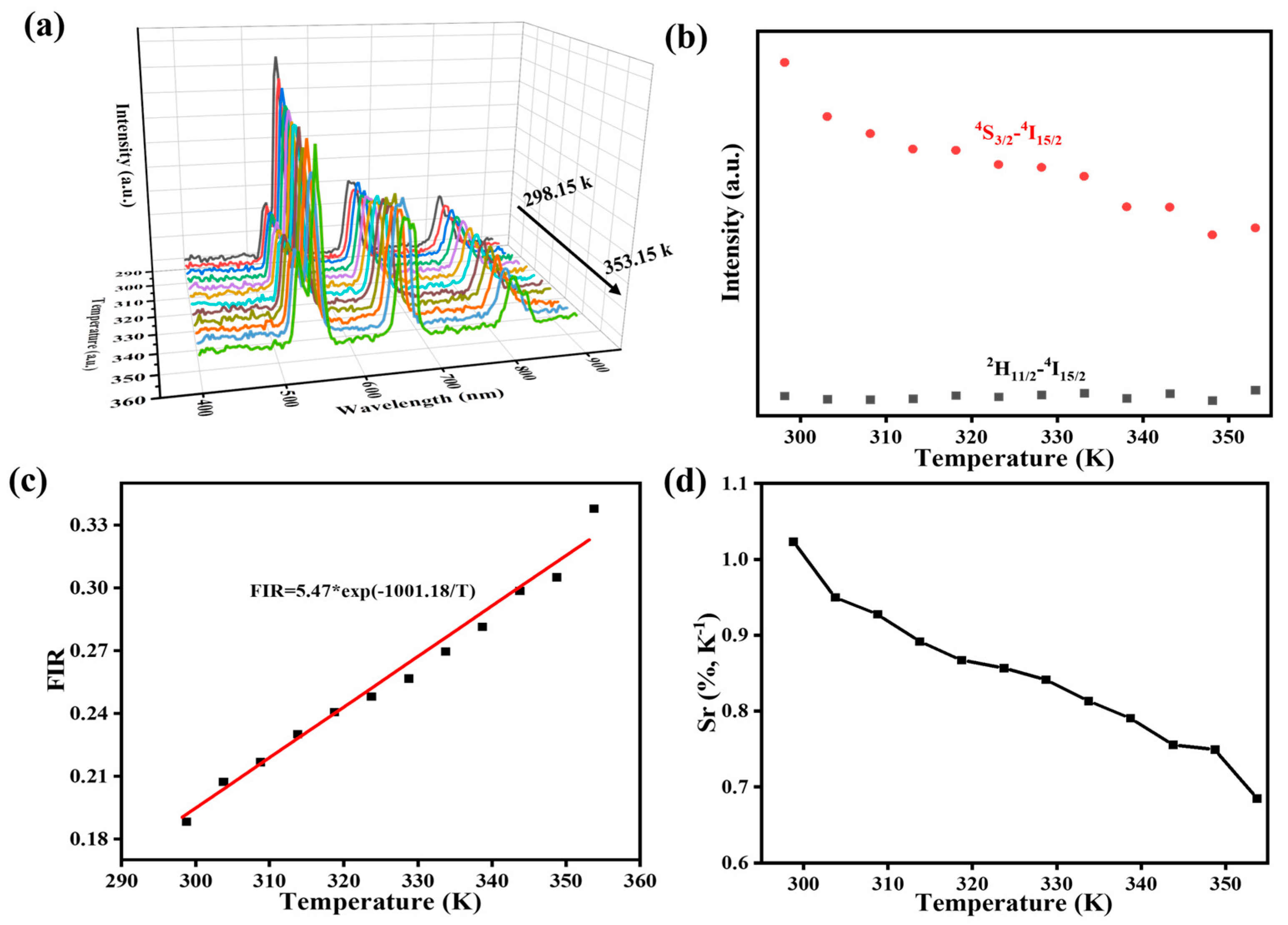
3.3. Morphology, Spectroscopic Properties, and Performance Analysis of UCNP/KNF Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Kankala, R.K.; Liu, C.G.; Wang, S.; Chen, A.; Zhang, Y. Lanthanides-doped near-infrared active upconversion nanocrystals: Upconversion mechanisms and synthesis. Coord. Chem. Rev. 2021, 438, 213870–213886. [Google Scholar] [CrossRef]
- Lee, C.; Schuck, P.J. Photodarkening, photobrightening, and the role of color centers in emerging applications of lanthanide-based upconverting nanomaterials. Annu. Rev. Phys. Chem. 2023, 74, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, X.; Ma, X.; Liu, K.; Han, Y.; Wu, Y.; Li, X. Rare earth upconversion luminescent composite based on energy transfer for specific and sensitive detection of cysteine. Analyst 2023, 148, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Skwierczynska, M.; Stopikowska, N.; Kulpinski, P.; Kłonowska, M.; Lis, S.; Runowski, M. Ratiometric upconversion temperature sensor based on cellulose fibers modified with yttrium fluoride nanoparticles. Nanomaterials 2022, 12, 1926. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Zhu, Q.; Zhang, X.; Chen, B.; Chen, J.; Wang, F. High-security anti-counterfeiting through upconversion luminescence. Mater. Today Phys. 2021, 21, 100520–100529. [Google Scholar] [CrossRef]
- Li, B.; Tian, F.; Cui, X.; Xiang, B.; Zhao, H.; Zhang, H.; Wang, D.; Li, J.; Wang, X.; Fang, X.; et al. Review for rare-earth-modified perovskite materials and optoelectronic applications. Nanomaterials 2022, 12, 1773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, W.; Gao, S.; Lu, X.; Zhang, D.; Zhang, X.; Wang, M. Photocatalytic performance of metal-organic framework material MIL-100(Fe) enhanced by rare earth upconversion material β-NaYF4:90%Yb,1%Tm. Appl. Phys. A 2022, 128, 499–510. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Yang, Y.; Liao, Y.; Law, W.; Tang, C. Photodegradable and pH responsive nanocapsules encapsulated with upconversion nanoparticles for diagnosis and treatment. Eur. Polym. J. 2023, 182, 111715–111724. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, S.; Yuan, C.; Best, A.; Kappl, M.; Koynov, K.; Butt, H.; Wu, S. Fabrication of anticounterfeiting nanocomposites with multiple security features via integration of a photoresponsive polymer and upconverting nanoparticles. Adv. Funct. Mater. 2021, 31, 2103908–2103914. [Google Scholar] [CrossRef]
- Tian, B.; Chen, B.; Sun, J.; Li, X.; Zhang, J.; Hua, R. Improved upconversion luminescence and temperature sensing in Mo6+-doped LuNbO4: Er3+ phosphor under 1550 nm excitation. Mater. Res. Express 2016, 3, 116201. [Google Scholar] [CrossRef]
- Grzyb, T.; Przybylska, D.; Szczeszak, A.; Smiechowicz, E.; Kulpiński, P.; Martín, I. Multifunctional cellulose fibers: Intense red upconversion under 1532 nm excitation and temperature-sensing properties. Carbohydr. Polym. 2022, 294, 119782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tang, B.; Zhang, C.; Qin, C.; Gu, Z.; Ma, Y.; Zhai, T.; Yao, J. Enhancing multiphoton upconversion through interfacial energy transfer in multilayered nanoparticles. Nat. Commun. 2020, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Brik, M.; Liu, D.; Feng, B.; Tian, Y.; Suchocki, A. Energy level schemes of fN electronic configurations for the di-, tri-, and tet-ravalent lanthanides and actinides in a free state. J. Lumin. 2016, 170, 369–374. [Google Scholar] [CrossRef]
- Wang, J.; Deng, R.; MacDonald, M.A.; Chen, B.; Yuan, J.; Wang, F. Enhancing multiphoton upconversion through energy clustering at sublattice level. Nat. Mater. 2014, 13, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Zheng, K.; Zhao, D.; Zhang, D.; Liu, N.; Qin, W. Ultraviolet upconversion fluorescence of Er3+ induced by 1560 nm laser excitation. Opt. Lett. 2010, 15, 2442–2444. [Google Scholar] [CrossRef] [PubMed]
- Mader, H.; Kele, P.; Saleh, S.; Wolfbeis, O. Upconverting luminescent nanoparticles for use in bioconjugation and bioimaging. Curr. Opin. Chem. Biol. 2010, 14, 582–596. [Google Scholar] [CrossRef]
- Boes, A.; Chang, L.; Langrock, C.; Yu, M.; Zhang, M.; Lin, Q.; Fejer, M.; Bowers, J.; Mitchell, A. Lithium niobate photonics: Unlocking the electromagnetic spectrum. Science 2023, 379, 6627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Shen, J.; Gao, C.; Bruggen, B. The potential of Kevlar aramid nanofiber composite membranes. J. Mater. Chem. A 2020, 8, 7548–7568. [Google Scholar] [CrossRef]
- Wang, B.; Mao, Z.; Li, D.; Zhang, K.; Zhou, G.; Ren, M.; Li, T. Multiscale insights into the stretching behavior of Kevlar fiber. Comput. Mater. Sci. 2020, 185, 109957–109966. [Google Scholar] [CrossRef]
- Yang, M.; Cao, K.; Sui, L.; Qi, Y.; Zhu, J.; Waas, A.; Arruda, E.; Kieffer, J.; Thouless, M.D.; Kotov, N.A. Dispersions of Aramid Nanofibers: A New Nanoscale Building Block. ACS Nano 2011, 5, 6945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Yang, B.; Tan, J.; Ding, X. Highly compressible, thermally stable, light- weight, and robust aramid nanofibers/Ti3AlC2 MXene composite aerogel for sensitive pressure sensor. ACS Nano 2020, 14, 10633–10647. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, P.; Dixit, A.; Mali, H.S. High strength Kevlar fiber reinforced advanced textile composites. Iran. Polym. J. 2019, 28, 621–638. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Sang, M.; Liu, S.; Yuan, F.; Wang, S.; Sun, S.; Gong, X. Advanced functional Kevlar composite with excellent mechanical properties for thermal management and intelligent safeguarding. Chem. Eng. J. 2022, 428, 131878–131889. [Google Scholar] [CrossRef]
- Li, D.; Guo, Z. Metal-organic framework superhydrophobic coating on Kevlar fabric with efficient drag reduction and wear resistance. Appl. Surf. Sci. 2018, 443, 548–557. [Google Scholar] [CrossRef]
- Lee, D.; Cho, J.; Son, J.G.; Yeom, B. Highly aligned aramid nanofibrillar nanocomposites for enhanced dynamic mechanical properties. Compos. Part B Eng. 2022, 229, 109467–109478. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.G. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4: Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 2008, 19, 345606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Yin, Q.; Wu, J.; Song, W.; Mohamed, A.; Jia, H.; Yang, F.; Rui, X. Highly improved compatibility and mechanical properties of carboxylated nitrile rubber/styrene butadiene rubber by incorporating modified Kevlar nanofibers. Mater. Chem. Phys. 2019, 238, 121926–121931. [Google Scholar] [CrossRef]
- Ermakova, J.; Madirov, E.; Fedorov, P.P.; Alexandrov, A.A.; Kuznetsov, S. Effect of the fluorinating agent type (NH4F, NaF, KF) on the particle size and emission properties of SrF2: Yb: Er luminophores. J. Mater. Chem. C 2024, 12, 1406–1411. [Google Scholar] [CrossRef]
- Shao, W.; Chen, G.; Damasco, J.; Wang, X.; Kachynski, A.; Ohulchanskyy, T.; Yang, H.; Ågren, H.; Prasad, P. Enhanced upconversion emission in colloidal (NaYF4:Er3+)/NaYF4 core/shell nanoparticles excited at 1523 nm. Opt. Lett. 2014, 39, 1386–1389. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Butt, H.J. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, X.; Huang, B.; Liang, L.; Han, S.; Yi, Z.; Wang, Y.; Li, Y.; Fan, D.; Huang, L.; et al. Confining Excitation Energy in Er3+ Nanocrystals through Tm3+ Mediated Transient Energy Trapping. Angew. Chem. Int. Ed. 2017, 56, 7605–7609. [Google Scholar] [CrossRef] [PubMed]
- Songfeng, E.; Ma, Q.; Huang, J.Z.; Ning, D.; Lu, Z. Polyvinyl alcohol-mediated splitting of Kevlar fibers and superior mechanical performances of the subsequently assembled nanopapers. Nanoscale 2021, 13, 18201–18209. [Google Scholar]
- Zhou, Q.; Lyu, J.; Wang, G.; Robertson, M.; Qiang, Z.; Sun, B.; Ye, C.; Zhu, M. Mechanically strong and multifunctional hybrid hydrogels with ultrahigh electrical conductivity. Adv. Funct. Mater. 2021, 31, 2104536. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Q.; Zhang, X.; Zhang, W.; Jia, H.; Ji, Q.; Yang, F.; Rui, X. Rational design of multifunctional properties for styrene-butadiene rubber reinforced by modified Kevlar nanofibers. Compos. Part B 2019, 166, 196–203. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, Z.; Liu, E.; Liu, Q. Synthesis, up-conversion luminescence and thermometry of Yb3+/Er3+ co-doped La2.4Mo1.6O8 phosphors. Dalton Trans. 2016, 45, 16240–16245. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; He, Y. Fabrication, microstructure, and temperature sensing behavior based on upconversion luminescence of novel Er3+, Yb3+ co-doped YOF ceramic. J. Lumin. 2018, 201, 18–23. [Google Scholar] [CrossRef]
- Geitenbeek, R.G.; Prins, P.T.; Albrecht, W.; Blaaderen, A.; Weckhuysen, B.M.; Meijerink, A. NaYF4:Er3+, Yb3+/SiO2 core/shell upconverting nanocrystals for luminescence thermometry up to 900 K. J. Phys. Chem. C 2017, 121, 3503–3510. [Google Scholar] [CrossRef]
- Qiang, Q.; Du, S.; Ma, X.; Chen, W.; Zhang, G.; Wang, Y. A temperature sensor based on the enhanced upconversion luminescence of Li+ doped NaLu4:Yb3+, Tm3+/Er3+ nano/microcrystals. Dalton Trans. 2018, 47, 8656–8662. [Google Scholar] [CrossRef]
- Li, D.D.; Shao, Q.Y.; Dong, Y.; Jiang, J. Thermal sensitivity and stability of NaYF4:Yb3+, Er3+ upconversion nanowires, nanorods and nanoplates. Mater. Lett. 2013, 110, 233–236. [Google Scholar] [CrossRef]
- Chen, D.; Xu, M.; Huang, P. Core@shell upconverting nanoarchitectures for luminescent sensing of temperature. Sens. Actuators B Chem. 2016, 231, 576–583. [Google Scholar] [CrossRef]
- Xu, M.; Ge, W.; Tian, Y.; Wu, Y.; Li, Y. Tunable upconversion luminescence and enhanced temperature sensitive properties from Bi2Ti2O7:Yb3+/ Er3+ nanofibers. J. Mater. Sci.-Mater. Electron. 2021, 56, 9302–9314. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, S.; Liu, Y.; Cao, S. Luminescent/Temperature-Sensing Properties of Multifunctional Rare-Earth Upconversion Kevlar Nanofiber Composite under 1550 nm. Nanomaterials 2024, 14, 740. https://doi.org/10.3390/nano14090740
Li J, Xu S, Liu Y, Cao S. Luminescent/Temperature-Sensing Properties of Multifunctional Rare-Earth Upconversion Kevlar Nanofiber Composite under 1550 nm. Nanomaterials. 2024; 14(9):740. https://doi.org/10.3390/nano14090740
Chicago/Turabian StyleLi, Juan, Shengang Xu, Yingliang Liu, and Shaokui Cao. 2024. "Luminescent/Temperature-Sensing Properties of Multifunctional Rare-Earth Upconversion Kevlar Nanofiber Composite under 1550 nm" Nanomaterials 14, no. 9: 740. https://doi.org/10.3390/nano14090740






