Nitrogen-Doped Porous Carbon Derived from Covalent Triazine Framework for Catalytic Oxidation of Benzyl Alcohol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
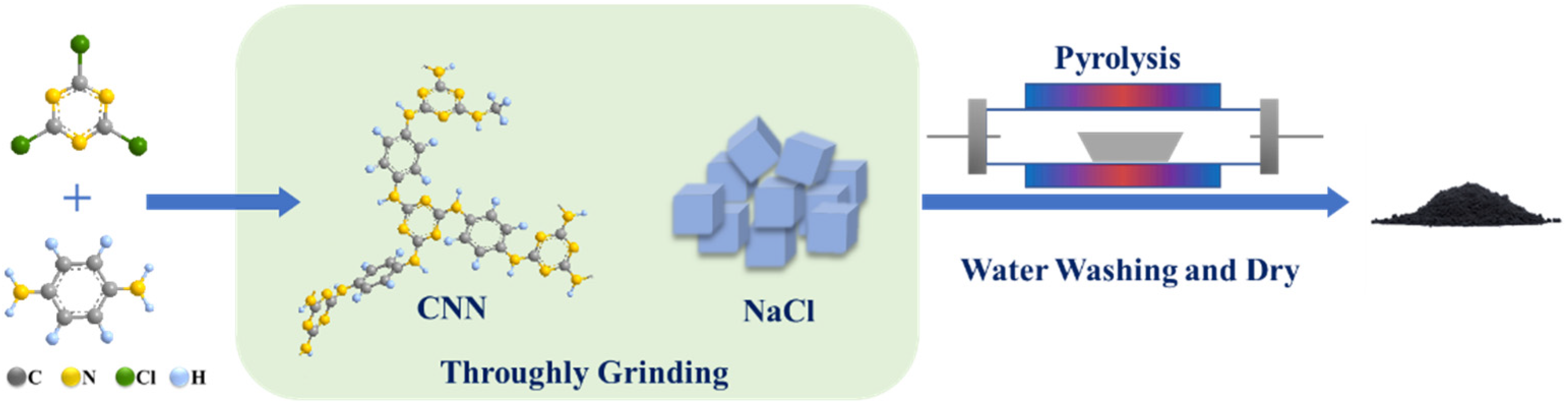
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Activity Test
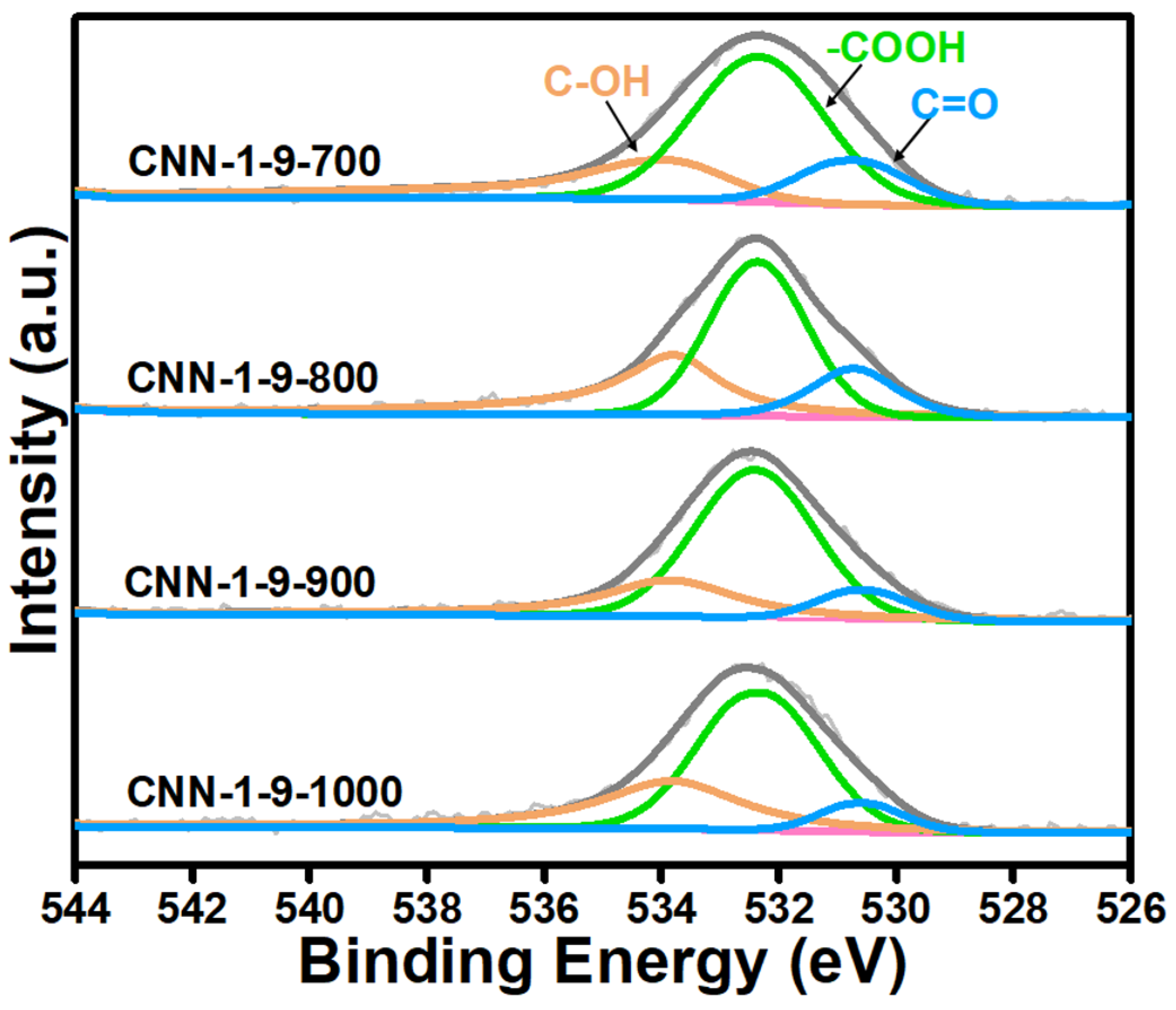
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
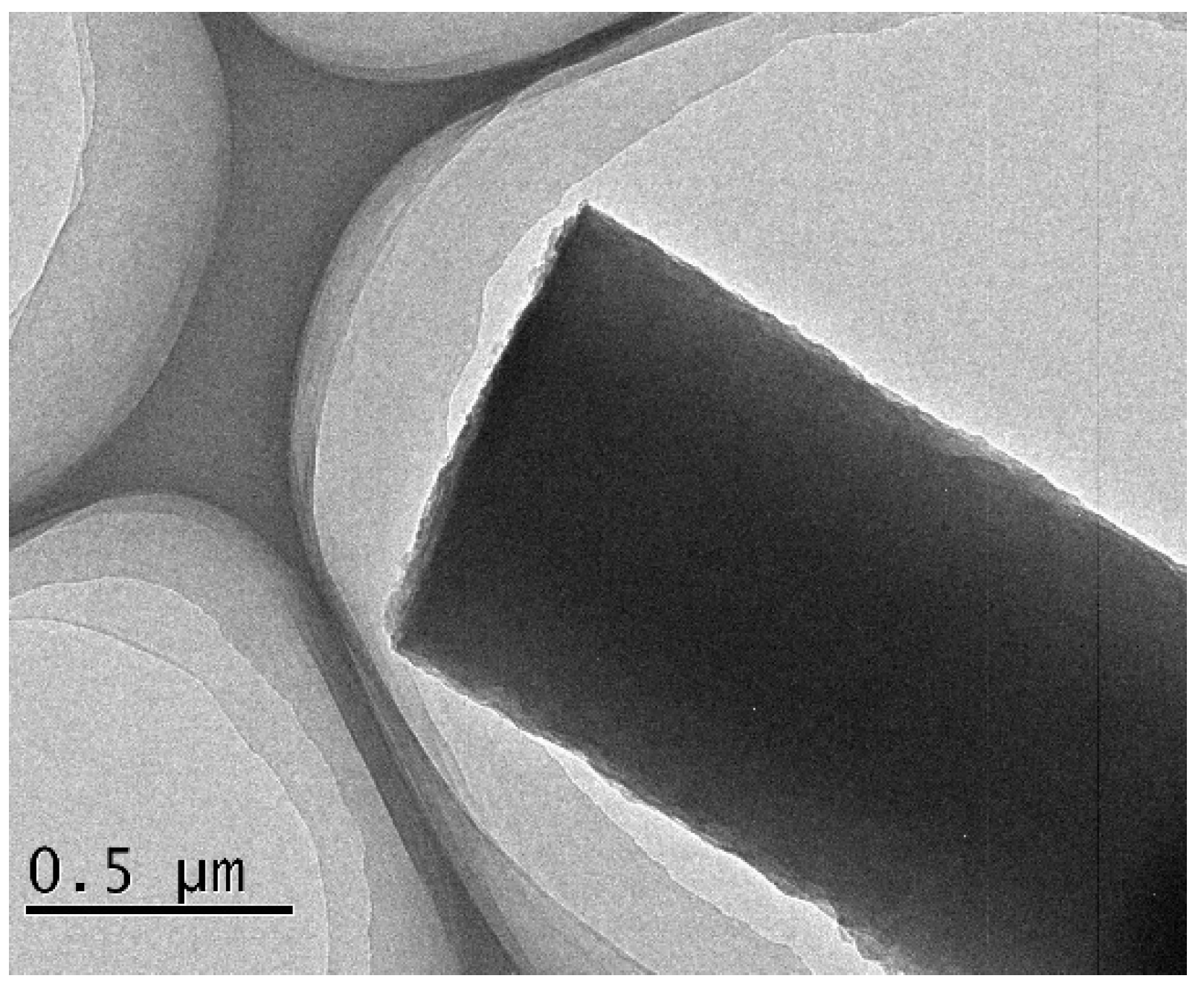
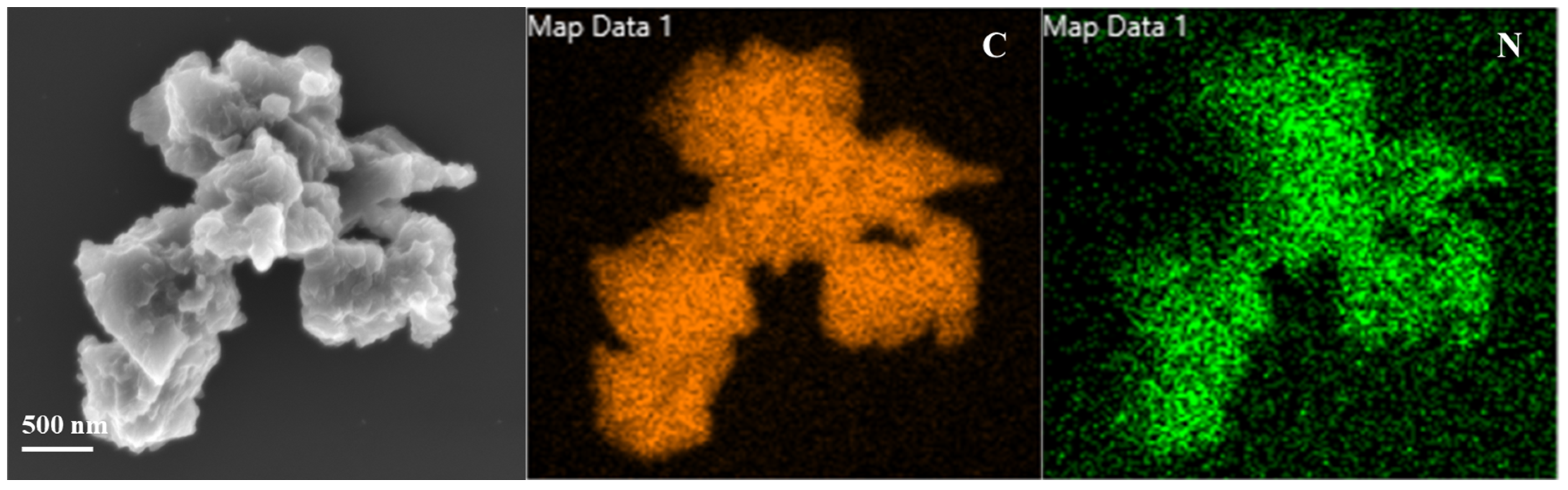
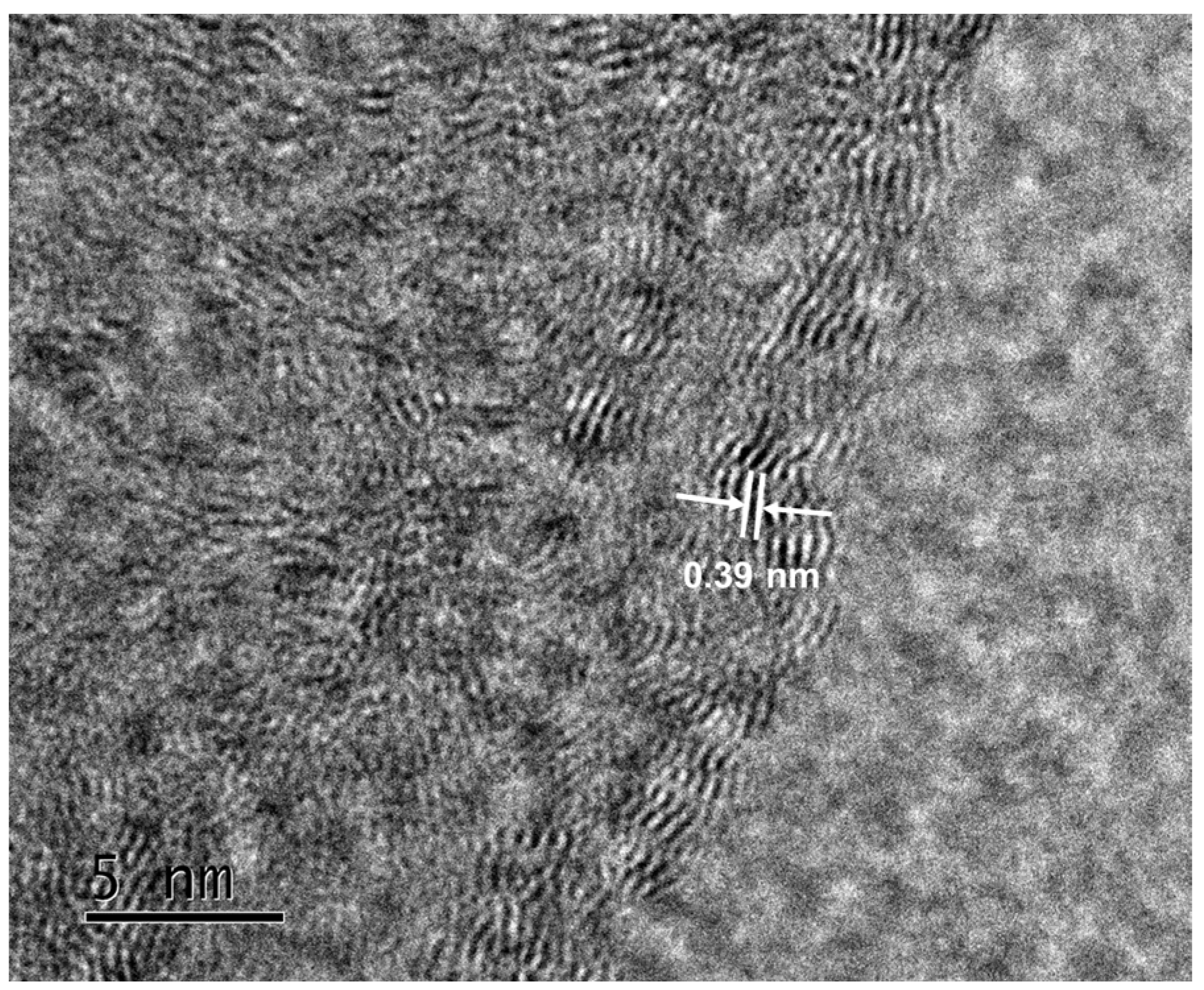
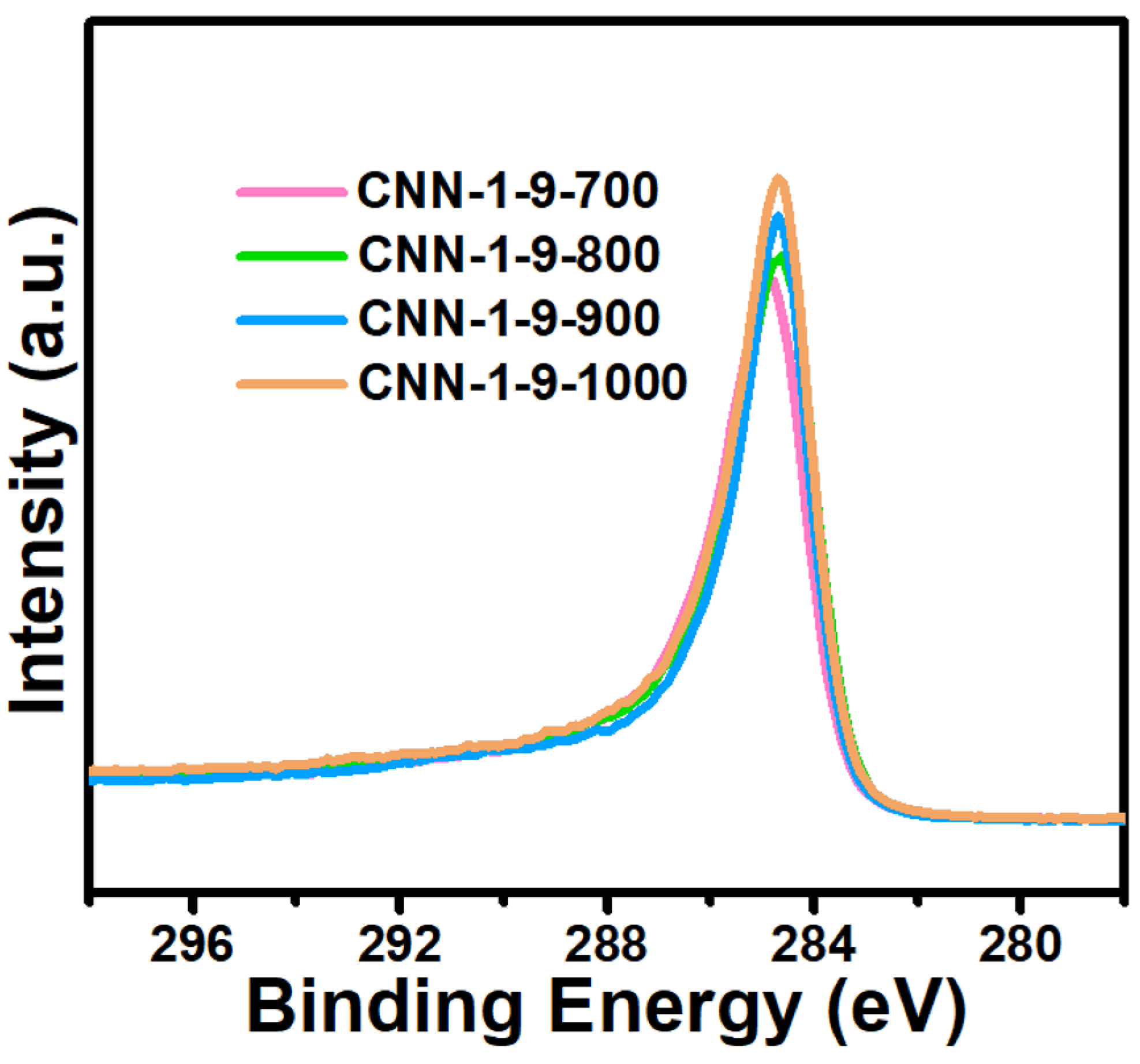
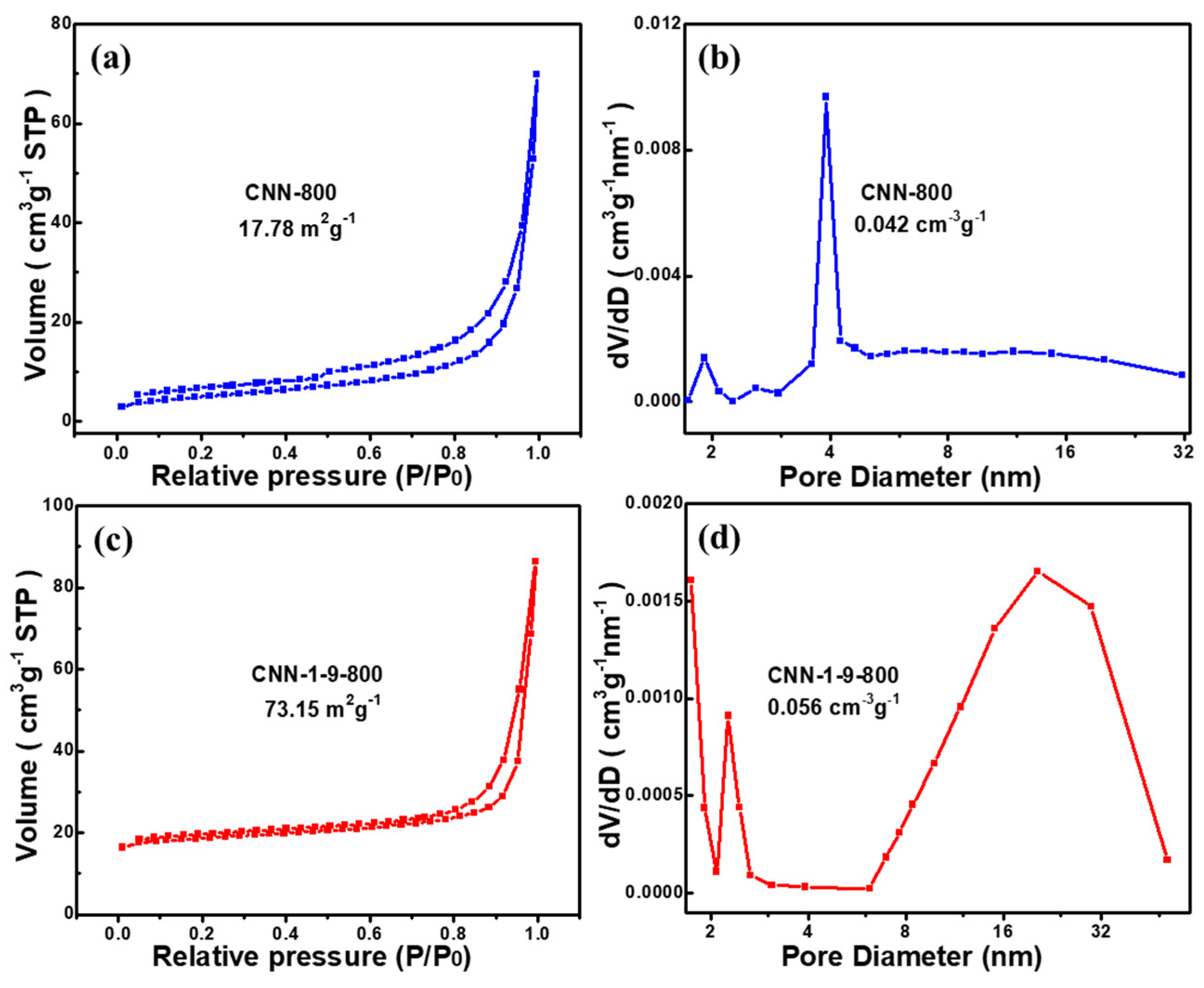
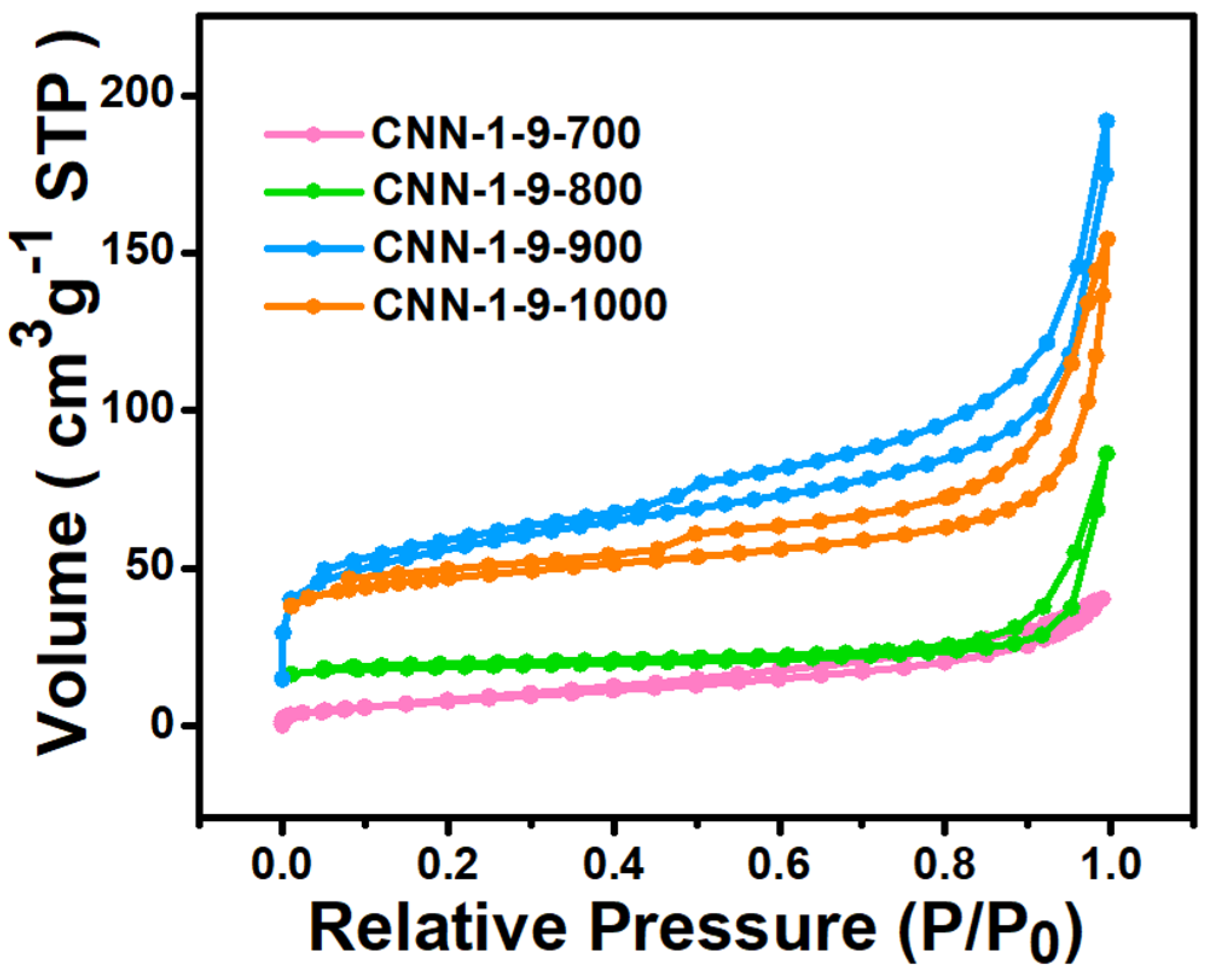
| Sample | SBET (m2g−1) | Vt (cm3g−1) |
|---|---|---|
| CNN-1-9-700 | 30.34 | 0.045 |
| CNN-1-9-800 | 73.15 | 0.056 |
| CNN-1-9-900 | 199.03 | 0.29 |
| CNN-1-9-1000 | 148.33 | 0.24 |
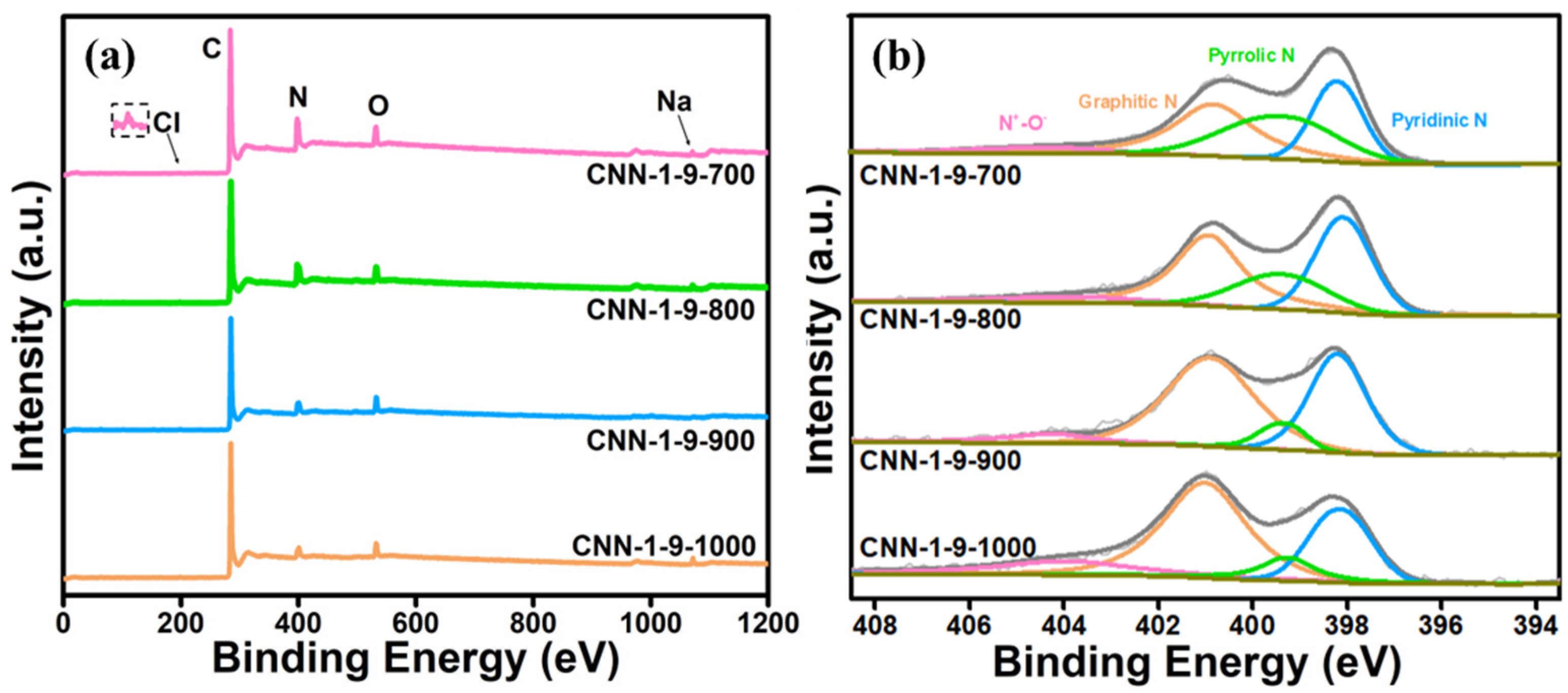
| Sample | N (Total, at%) | Relative Proportion of Different N Species in the Total N | ||||
|---|---|---|---|---|---|---|
| Pyridinic N + Graphitic N (%) | Pyridinic N (%) | Graphitic N (%) | Pyrrolic N (%) | N+-O− (%) | ||
| CNN-1-9-700 | 16.1 | 69.8 | 39.7 | 30.1 | 22.2 | 8.0 |
| CNN-1-9-800 | 13.7 | 72.7 | 34.2 | 38.5 | 20.8 | 6.5 |
| CNN-1-9-900 | 9.9 | 85.0 | 32.3 | 52.7 | 8.4 | 6.6 |
| CNN-1-9-1000 | 7.1 | 82.1 | 27.2 | 54.9 | 8.1 | 9.8 |
References
- Li, H.; Qin, F.; Yang, Z.; Cui, X.; Wang, J.; Zhang, L. New Reaction Pathway Induced by Plasmon for Selective Benzyl Alcohol Oxidation on BiOCl Possessing Oxygen Vacancies. J. Am. Chem. Soc. 2017, 139, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Yang, K.; Fu, X.; Yuan, R. Photocatalytic Oxidation of Benzyl Alcohol by Homogeneous CuCl2/Solvent: A Model System to Explore the Role of Molecular Oxygen. ACS Catal. 2015, 5, 3760–3766. [Google Scholar] [CrossRef]
- Mazumder, T.; Dandapat, S.; Baidya, T.; Likhar, P.R.; Clark, A.H.; Bera, P.; Tiwari, K.; Payra, S.; Srinivasa Rao, B.; Roy, S.; et al. Dual-Site Cooperation for High Benzyl Alcohol Oxidation Activity of MnO2 in Biphasic MnOx–CeO2 Catalyst Using Aerial O2 in the Vapor Phase. J. Phys. Chem. C 2021, 125, 20831–20844. [Google Scholar] [CrossRef]
- Luong, G.K.T.; Ku, Y. Selective Oxidation of Benzyl Alcohol in the Aqueous Phase by TiO2-Based Photocatalysts: A Review. Chem. Eng. Technol. 2021, 44, 2178–2190. [Google Scholar] [CrossRef]
- Lou, J.D.; Xu, Z.N. Selective Solvent-free Oxidation of Alcohols with Potassium Dichromate. Tetrahedron Lett. 2002, 43, 8843–8844. [Google Scholar] [CrossRef]
- Jose, N.; Sengupta, S.; Basu, J.K. Selective Production of Benzaldehyde by Permanganate Oxidation of Benzyl Alcohol Using 18-crown-6 as Phase Transfer Catalyst. J. Mol. Catal. A Chem. 2009, 309, 153–158. [Google Scholar] [CrossRef]
- Correia, L.M.M.; Kuznetsov, M.L.; Alegria, E.C.B.A. Core–Shell Catalysts for Conventional Oxidation of Alcohols: A Brief Review. Catalysts 2023, 13, 1137. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, R.; Tu, Y.; Gong, X.; Cao, X.; Xu, Q.; Li, Y.; Ye, B.; Ye, Y.; Zhu, J. Revealing the Crystal Facet Effect of Ceria in Pd/CeO2 Catalysts Toward the Selective Oxidation of Benzyl Alcohol. ACS Catal. 2023, 13, 2202–2213. [Google Scholar] [CrossRef]
- Marelli, M.; Jouve, A.; Villa, A.; Psaro, R.; Balerna, A.; Prati, L.; Evangelisti, C. Hybrid Au/CuO Nanoparticles: Effect of Structural Features for Selective Benzyl Alcohol Oxidation. J. Phys Chem C 2019, 123, 2864–2871. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; An, Y.; Wang, X.; Xu, G.; Chen, Y.; Dai, L. Promotional Synergistic Effect of Sn doping into a Novel Bimetallic Sn-W oxides/Graphene Catalyst for Selective Oxidation of Alcohols using Aqueous H2O2 without Additives. Appl. Catal. A 2018, 558, 26–33. [Google Scholar] [CrossRef]
- Iraqui, S.; Kashyap, S.S.; Rashid, M.H. NiFe2O4 Nanoparticles: An Efficient and Reusable Catalyst for the Selective Oxidation of Benzyl Alcohol to Benzaldehyde Under Mild Conditions. Nanoscale Adv. 2020, 2, 5790–5802. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ke, Y.; Zhang, X.; Wu, S.; Chen, Y.; Xie, Z. Guanine-derived Nitrogen-doped Carbon Nanosheets for Selective Oxidation of Benzyl alcohol. Diam. Relat. Mater. 2023, 132, 109642. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Z.; Niu, Y.; Zhang, B.; Lu, Q.; Wu, S.; Centi, G.; Perathoner, S.; Heumann, S.; Yu, L.; et al. Highly Efficient Metal-Free Nitrogen-Doped Nanocarbons with Unexpected Active Sites for Aerobic Catalytic Reactions. ACS Nano 2019, 13, 13995–14004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, N.; Wang, C.; Song, L.; Yu, R.; Wang, D. Boosting Hydrogen Evolution Reaction on Few-layer Graphdiyne by sp-N and B Co-doping. APL Mater. 2021, 9, 071102. [Google Scholar] [CrossRef]
- Yuan, Y. Preparation of Nitrogen Doped Carbon Materials and Analysis of Their Electrochemical Performance. Int. J. Electrochem. Sci. 2022, 17, 220825. [Google Scholar] [CrossRef]
- Goyenola, C.; Lai, C.-C.; Näslund, L.-Å.; Lu, J.; Högberg, H.; Hultman, L.; Rosen, J.; Gueorguiev, G.K. Theoretical Prediction and Synthesis of CSxFy Thin Films. J. Phys. Chem. C 2016, 120, 9527–9534. [Google Scholar] [CrossRef]
- He, H.; Huang, D.; Tang, Y.; Wang, Q.; Ji, X.; Wang, H.; Guo, Z. Tuning Nitrogen Species in Three-dimensional Porous Carbon Via Phosphorus Doping for Ultra-fast Potassium Storage. Nano Energy 2019, 57, 728–736. [Google Scholar] [CrossRef]
- Broitman, E.; Gueorguiev, G.K.; Furlan, A.; Son, N.T.; Gellman, A.J.; Stafström, S.; Hultman, L. Water Adsorption on Fullerene-like Carbon Nitride Overcoats. Thin Solid Films 2008, 517, 1106–1110. [Google Scholar] [CrossRef]
- Hu, X.; Long, Y.; Fan, M.; Yuan, M.; Zhao, H.; Ma, J.; Dong, Z. Two-dimensional Covalent Organic Frameworks as Self-template Derived Nitrogen-doped Carbon Nanosheets for Eco-friendly Metal-free Catalysis. Appl. Catal. B 2019, 244, 25–35. [Google Scholar] [CrossRef]
- Kiciński, W.; Dyjak, S. Nitrogen-Doped Carbons Derived from Imidazole-Based Cross-Linked Porous Organic Polymers. Molecules 2021, 26, 668. [Google Scholar] [CrossRef]
- Cai, A.; He, H.; Zhang, Q.; Xu, Y.; Li, X.; Zhang, F.; Fan, X.; Peng, W.; Li, Y. Synergistic Effect of N-Doped sp2 Carbon and Porous Structure in Graphene Gels toward Selective Oxidation of C-H Bond. ACS Appl. Mater. Interfaces 2021, 13, 13087–13096. [Google Scholar] [CrossRef]
- Rangraz, Y.; Heravi, M.M.; Elhampour, A. Recent Advances on Heteroatom-Doped Porous Carbon/Metal Materials: Fascinating Heterogeneous Catalysts for Organic Transformations. Chem. Rec. 2021, 21, 1985–2073. [Google Scholar] [CrossRef]
- Watanabe, H.; Asano, S.; Fujita, S.-I.; Yoshida, H.; Arai, M. Nitrogen-Doped, Metal-Free Activated Carbon Catalysts for Aerobic Oxidation of Alcohols. ACS Catal. 2015, 5, 2886–2894. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The Recent Progress of Nitrogen-doped Carbon Nanomaterials for Electrochemical Batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, W.; Gao, J.; Sun, F.; Zhao, G. H2O2 Electrogeneration from O2 Electroreduction by N-Doped Carbon Materials: A Mini-Review on Preparation Methods, Selectivity of N Sites, and Prospects. Adv. Mater. Interfaces 2021, 8, 2002091. [Google Scholar] [CrossRef]
- Du, J.; Yu, Y.; Liu, L.; Lv, H.; Chen, A.; Hou, S. Confined-Space Pyrolysis of Polystyrene/Polyacrylonitrile for Nitrogen-Doped Hollow Mesoporous Carbon Spheres with High Supercapacitor Performance. ACS Appl. Energy Mater. 2019, 2, 4402–4410. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, M.; Ning, G.; Sun, Y.; Wang, H.; Fan, X.; Lu, H.; Zhang, Y.; Wang, H. Co/FeC Core–nitrogen Doped Hollow Carbon Shell Structure with Tunable Shell-thickness for Oxygen Evolution Reaction. J. Colloid Sci. 2020, 580, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Polyaniline-Derived N-Doped Ordered Mesoporous Carbon Thin Films: Efficient Catalysts towards Oxygen Reduction Reaction. Polymer 2020, 12, 2382. [Google Scholar] [CrossRef]
- Sun, R.; Wang, X.; Wang, X.; Tan, B. Three-Dimensional Crystalline Covalent Triazine Frameworks via a Polycondensation Approach. Angew. Chem. Int. Ed. 2022, 61, e202117668. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Q.; Wang, S.; Li, Z.; Li, B.; Jin, S.; Tan, B. Crystalline Covalent Triazine Frameworks by In Situ Oxidation of Alcohols to Aldehyde Monomers. Angew. Chem. Int. Ed. 2018, 57, 11968–11972. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, L.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. MoS2 Quantum Dots-Modified Covalent Triazine-Based Frameworks for Enhanced Photocatalytic Hydrogen Evolution. ChemSusChem 2018, 11, 1108–1113. [Google Scholar] [CrossRef]
- Guan, Y.; Fan, P.; Dong, W.; Shang, D. A Covalent Triazine-based Framework Containing Hydrogen-bonding for Highly Drug Loading and pH-responsive Release. J. Macromol. Sci. Part A Pure Appl. Chem. 2021, 58, 630–635. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Liu, J.; Wang, K.; Jin, S.; Tan, B. Palladium as a Superior Cocatalyst to Platinum for Hydrogen Evolution Using Covalent Triazine Frameworks as a Support. ACS Appl. Mater. Interfaces 2020, 12, 12774–12782. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Sun, R.; Tan, B. Covalent Triazine Frameworks (CTFs): Synthesis, Crystallization, and Photocatalytic Water Splitting. Chem. Eur. J. 2023, 29, e202203077. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Li, M.; Yin, Y.; Chen, J.; Zhong, Q.; Du, R.; Liu, S.; He, Y.; Fu, W.; Zeng, F. Advances in the Synthesis of Covalent Triazine Frameworks. ACS Omega 2023, 8, 4527–4542. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Y.; Sun, F.-X.; Zhu, G.-S. Targeted Synthesis of Novel Porous Aromatic Frameworks with Selective Separation of CO2/CH4 and CO2/N2. Chin. Chem. Lett. 2014, 25, 1407–1410. [Google Scholar] [CrossRef]
- Pang, Z.; Li, G.; Xiong, X.; Ji, L.; Xu, Q.; Zou, X.; Lu, X. Molten Salt Synthesis of Porous Carbon and its Application in Supercapacitors: A review. J. Energy Chem. 2021, 61, 622–640. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. A Green and Scalable Route to Yield Porous Carbon Sheets from Biomass for Supercapacitors with High Capacity. J. Mater. Chem. A 2018, 6, 1244–1254. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, W.; Wang, X.; Tong, W.; Li, P.; Zhou, H.; Wang, Y.; Zhang, Y. Molten Salt Induced Nitrogen-doped Biochar Nanosheets as Highly Efficient Peroxymonosulfate Catalyst for Organic Pollutant Degradation. Environ. Pollut. 2020, 260, 114053. [Google Scholar] [CrossRef]
- Ruse, E.; Larboni, M.; Lavi, A.; Pyrikov, M.; Leibovitch, Y.; Ohayon-Lavi, A.; Vradman, L.; Regev, O. Molten Salt In-situ Exfoliation of Graphite to Graphene Nanoplatelets Applied for Energy Storage. Carbon 2021, 176, 168–177. [Google Scholar] [CrossRef]
- Zhu, Y.-N.; Cao, C.-Y.; Jiang, W.-J.; Yang, S.-L.; Hu, J.-S.; Song, W.-G.; Wan, L.-J. Nitrogen, Phosphorus and Sulfur Co-doped Ultrathin Carbon Nanosheets as a Metal-free Catalyst for Selective Oxidation of Aromatic Alkanes and the Oxygen Reduction Reaction. J. Mater. Chem. A 2016, 4, 18470–18477. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Xu, X.; Chen, L.; Lu, T.; Hill, J.P.; Pan, L.; Yamauchi, Y. Embedding Metal–Organic Frameworks for the Design of Flexible Hybrid Supercapacitors by Electrospinning: Synthesis of Highly Graphitized Carbon Nanofibers Containing Metal Oxide Nanoparticles. Small Struct. 2022, 3, 2200015. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Z.; Zhao, X.; Chen, X.; Zhu, Y.; Chu, H. Electrochemical Performance of Porous Carbons Derived from Needle Coke with Different Textures for Supercapacitor Electrode Materials. Carbon Lett. 2020, 31, 57–65. [Google Scholar] [CrossRef]
- Supriya, S.; Sriram, G.; Ngaini, Z.; Kavitha, C.; Kurkuri, M.; De Padova, I.P.; Hegde, G. The Role of Temperature on Physical–Chemical Properties of Green Synthesized Porous Carbon Nanoparticles. Waste Biomass Valorization 2019, 11, 3821–3831. [Google Scholar] [CrossRef]
- Yang, T.; Lin, H.; Loh, K.P.; Jia, B. Fundamental Transport Mechanisms and Advancements of Graphene Oxide Membranes for Molecular Separation. Chem. Mater. 2019, 31, 1829–1846. [Google Scholar] [CrossRef]
- Picheau, E.; Impellizzeri, A.; Rybkovskiy, D.; Bayle, M.; Mevellec, J.-Y.; Hof, F.; Saadaoui, H.; Noé, L.; Torres Dias, A.C.; Duvail, J.-L.; et al. Intense Raman D Band without Disorder in Flattened Carbon Nanotubes. ACS Nano 2021, 15, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A.; Saito, R. Raman Spectroscopy for Carbon Nanotube Applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Wang, J.; Jin, S.; Tan, B. A Facile Approach to Prepare Multiple Heteroatom-Doped Carbon Materials from Imine-Linked Porous Organic Polymers. Sci. Rep. 2018, 8, 4200. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, Y.; Chen, G. Highly Crinkled and Interconnected N, O and S Co-doped Carbon Nanosheet Modified Separators for Efficient Li-S Batteries. Mater. Chem. Front. 2023, 7, 1072–1081. [Google Scholar] [CrossRef]
- Mel’gunov, M.S. Application of the Simple Bayesian Classifier for the N2 (77 K) Adsorption/Desorption Hysteresis Loop Recognition. Adsorption 2022, 29, 199–208. [Google Scholar] [CrossRef]
- Li, J.; Sun, H.; Wang, S.; Dong, Y.; Liu, S. Selective Oxidation of Alcohols by Graphene-like Carbon with Electrophilic Oxygen and Integrated Pyridinic Nitrogen Active Sites. Nanoscale 2021, 13, 12979–12990. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, L.; Xu, H.; Yang, X.; Wang, H.; Lu, X.; Yang, P.; Wu, P.; Liao, L. Molten Salt-mediated Synthesis of Porous N-doped Carbon as an Efficient ORR Electrocatalyst for Zinc–Air Batteries. New J. Chem. 2023, 47, 2279–2285. [Google Scholar] [CrossRef]
- Shaikh, A.; Singh, B.K.; Mohapatra, D.; Parida, S. Nitrogen-Doped Carbon Nano-Onions as a Metal-Free Electrocatalyst. Electrocatalysis 2019, 10, 222–231. [Google Scholar] [CrossRef]
- Sibul, R.; Kibena-Põldsepp, E.; Ratso, S.; Kook, M.; Käärik, M.; Merisalu, M.; Paiste, P.; Leis, J.; Sammelselg, V.; Tammeveski, K. Nitrogen-doped Carbon-based Electrocatalysts Synthesised by Ball-milling. Electrochem. Commun. 2018, 93, 39–43. [Google Scholar] [CrossRef]
- Broitman, E.; Furlan, A.; Gueorguiev, G.K.; Czigány, Z.; Högberg, H.; Hultman, L. Structural and Mechanical Properties of CNX and CPX Thin Solid Films. Key Eng. Mater. 2011, 488-489, 581–584. [Google Scholar]
- Wang, W.; Jiang, X.; Diao, J.; He, Z.-H.; Wang, K.; Yang, Y.; Liu, Z.-T.; Nan, J.; Qiao, C. Quinone-amine Polymers Derived N and O Dual Doped Carbocatalyst for Metal-free Benzyl Alcohol Aerobic Oxidation. Mol. Catal. 2020, 498, 111257. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Gao, X.; Lv, Z.; He, Y.; Wang, J.; Fan, W. Kraft Lignin Derived S and O Co-doped Porous Graphene for Metal-free Benzylic Alcohol Oxidation. Catal. Sci. Technol. 2020, 10, 2786–2796. [Google Scholar] [CrossRef]
- Cai, C.; Chen, Y.; Hu, P.; Zhu, T.; Li, X.; Yu, Q.; Zhou, L.; Yang, X.; Mai, L. Regulating the Interlayer Spacings of Hard Carbon Nanofibers Enables Enhanced Pore Filling Sodium Storage. Small 2021, 18, 2105303. [Google Scholar] [CrossRef]
- Anji Reddy, M.; Helen, M.; Groß, A.; Fichtner, M.; Euchner, H. Insight into Sodium Insertion and the Storage Mechanism in Hard Carbon. ACS Energy Lett. 2018, 3, 2851–2857. [Google Scholar] [CrossRef]





| Entry | Substrate | Product | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 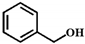 | 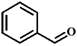 | 6 | 57.3 |
| 2 | 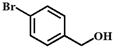 | 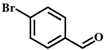 | 6 | 66.2 |
| 3 | 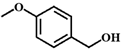 | 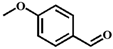 | 6 | 38.7 |
| 4 |  | 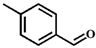 | 6 | 30.0 |
| 5 | 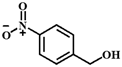 | 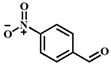 | 6 | 41.9 |
| 6 |  | 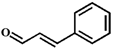 | 6 | 37.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Zhu, Y.; Yang, Y.; Zhu, Q. Nitrogen-Doped Porous Carbon Derived from Covalent Triazine Framework for Catalytic Oxidation of Benzyl Alcohol. Nanomaterials 2024, 14, 744. https://doi.org/10.3390/nano14090744
Pan X, Zhu Y, Yang Y, Zhu Q. Nitrogen-Doped Porous Carbon Derived from Covalent Triazine Framework for Catalytic Oxidation of Benzyl Alcohol. Nanomaterials. 2024; 14(9):744. https://doi.org/10.3390/nano14090744
Chicago/Turabian StylePan, Xin, Yanan Zhu, Yongchang Yang, and Qianqian Zhu. 2024. "Nitrogen-Doped Porous Carbon Derived from Covalent Triazine Framework for Catalytic Oxidation of Benzyl Alcohol" Nanomaterials 14, no. 9: 744. https://doi.org/10.3390/nano14090744





