Enhancing the Photocatalytic Activity of Halide Perovskite Cesium Bismuth Bromide/Hydrogen Titanate Heterostructures for Benzyl Alcohol Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
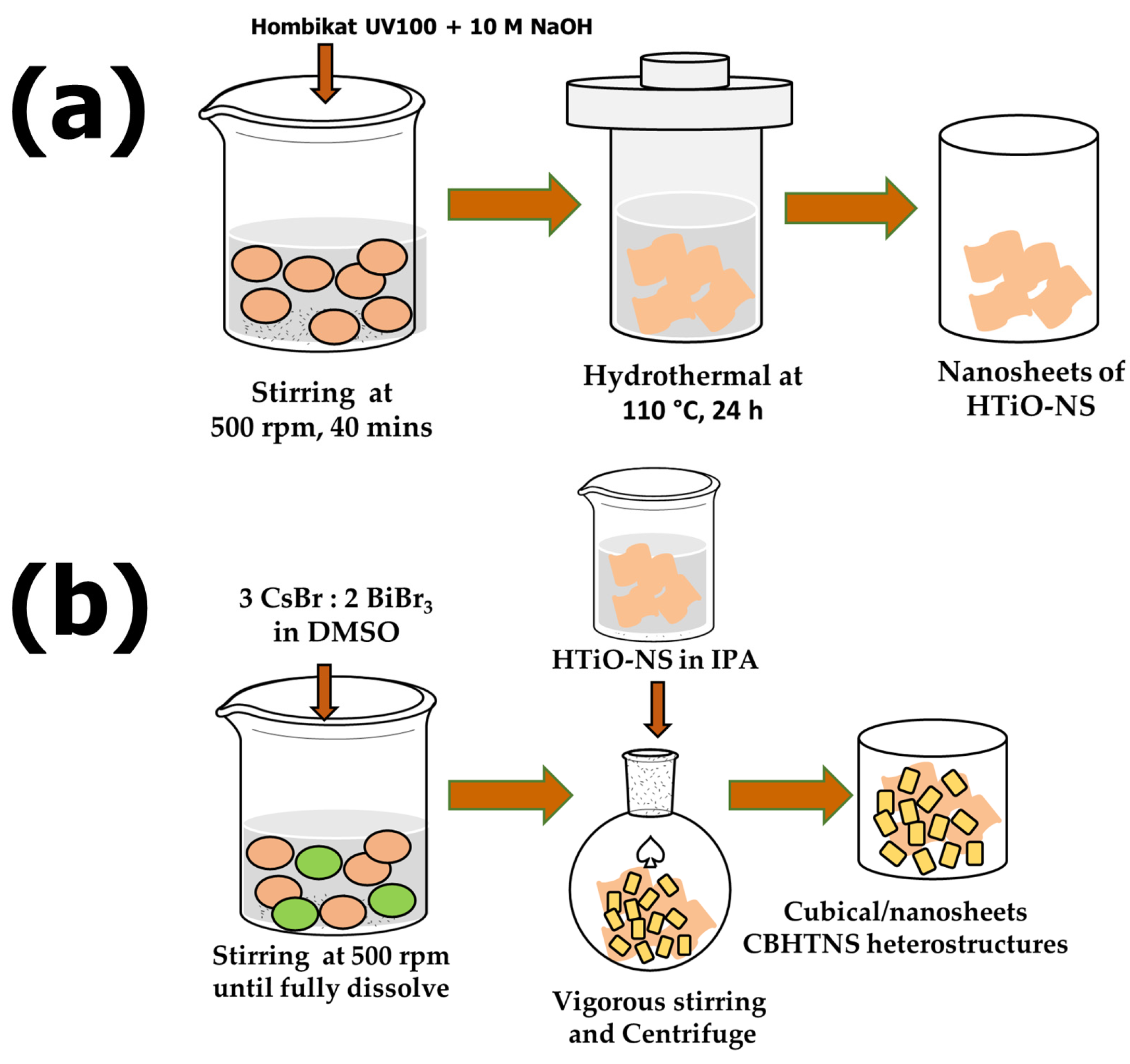
2.2. Synthesis of HTiO-NS
2.3. Synthesis of Cs3Bi2Br9 (CBB)
2.4. Synthesis of Cs3Bi2Br9/HTiO-NS (CBHTNS) Heterostructures
2.5. Characterization Techniques
2.6. Evaluation of Photocatalytic Benzyl Alcohol (BnOH) Oxidation
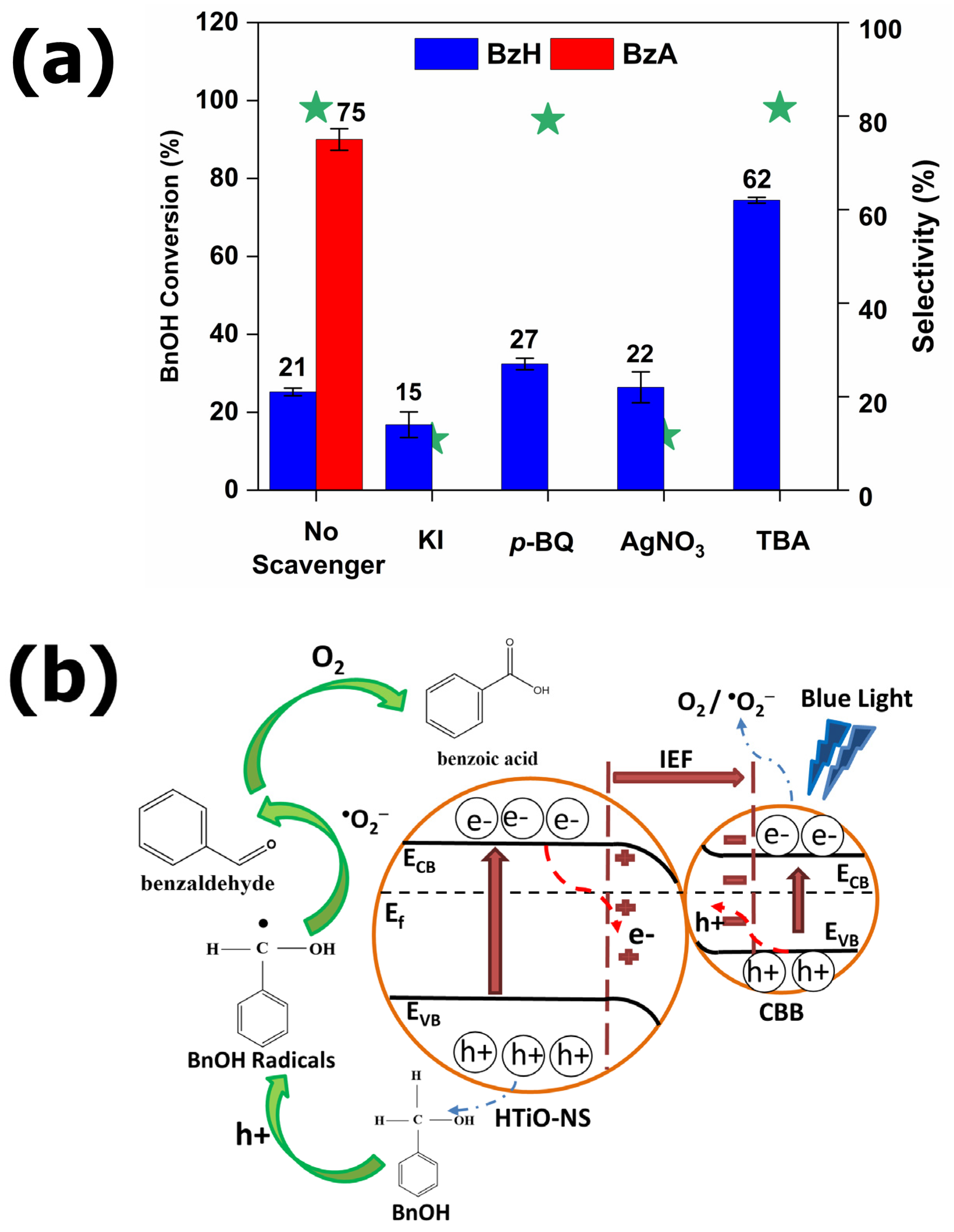
2.7. Reactive Oxygen Species Monitoring (Scavenger Tests)
3. Results and Discussion
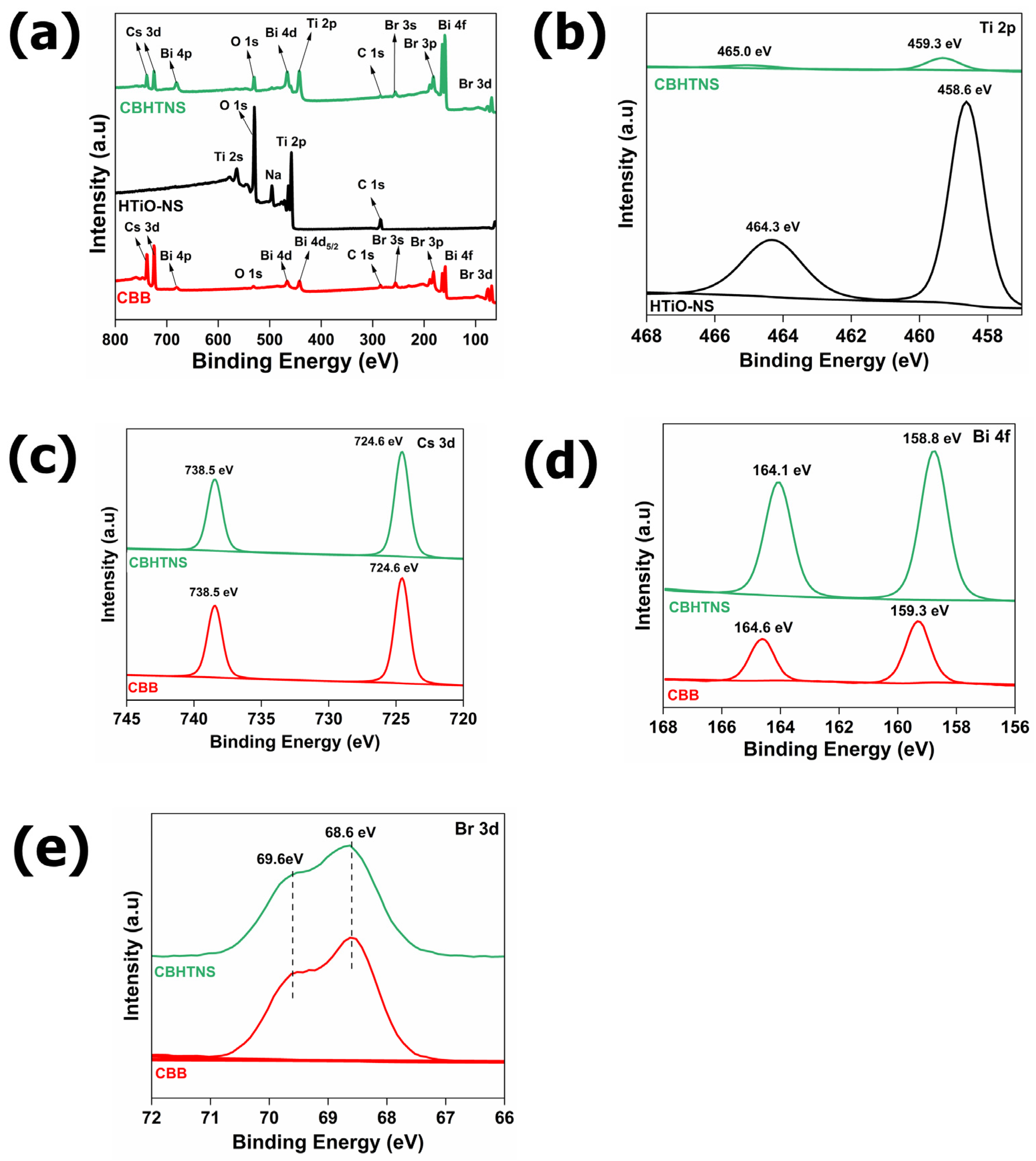
3.1. Elemental Analysis
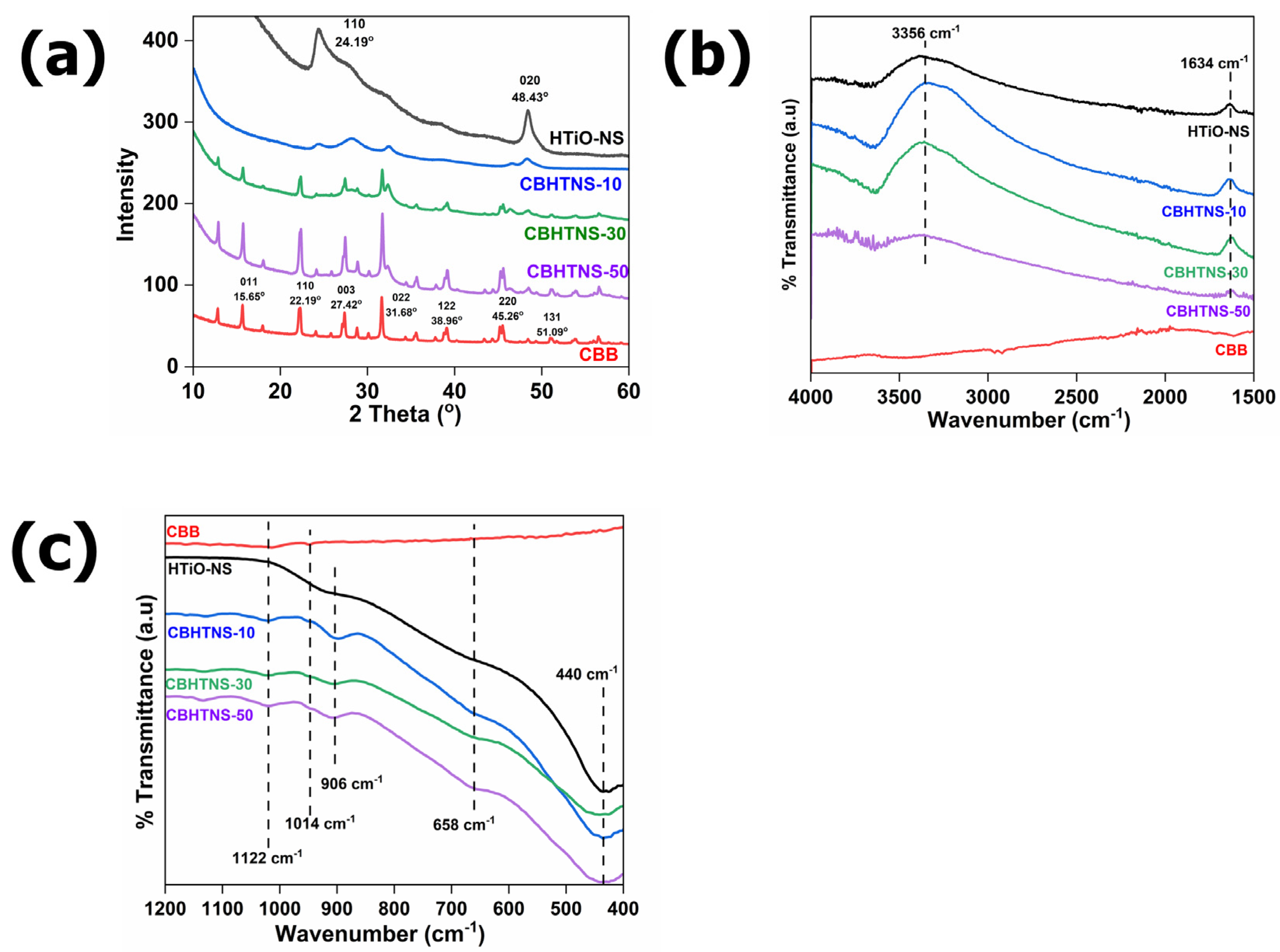
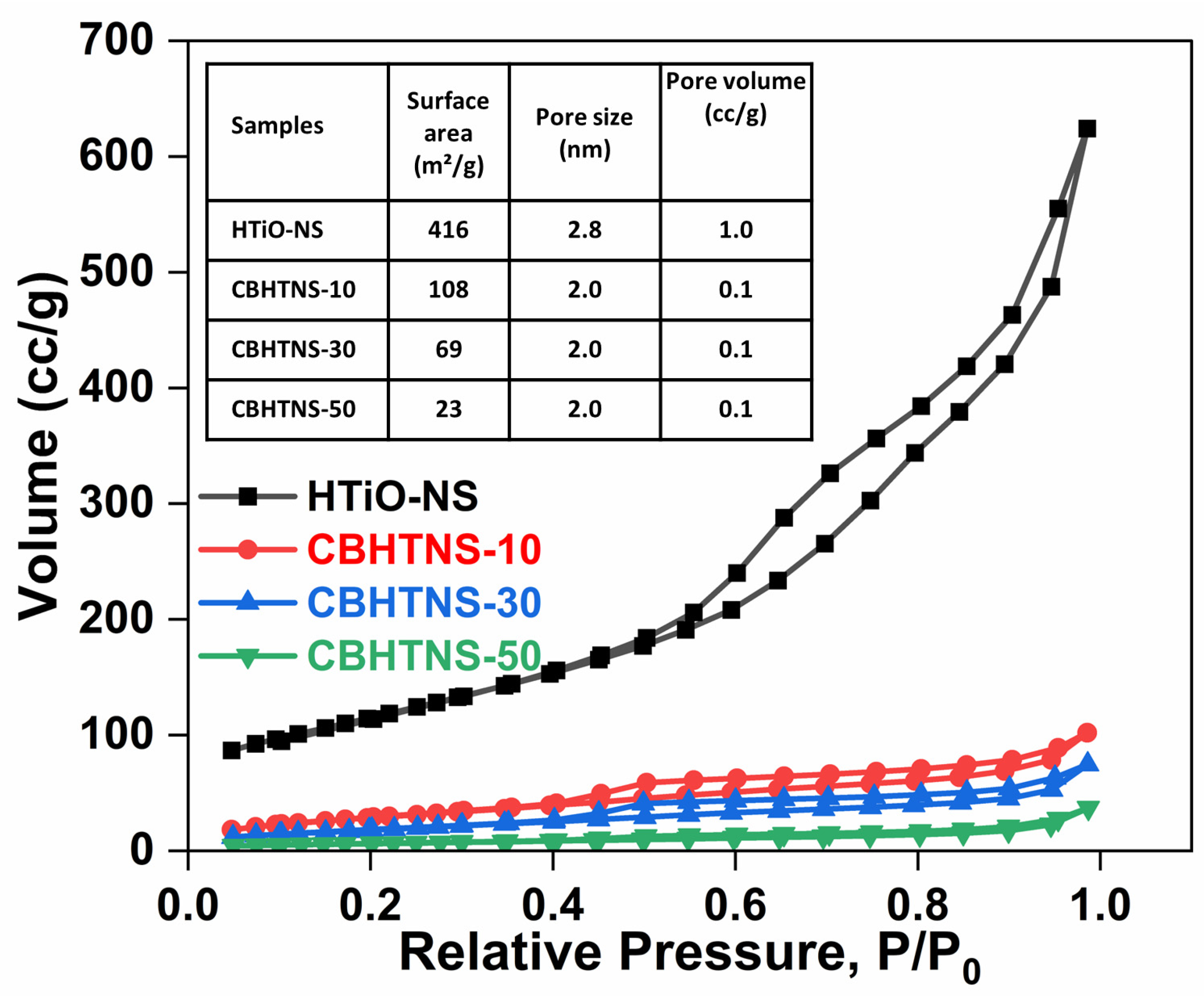
3.2. Morphology and Structure Characterization
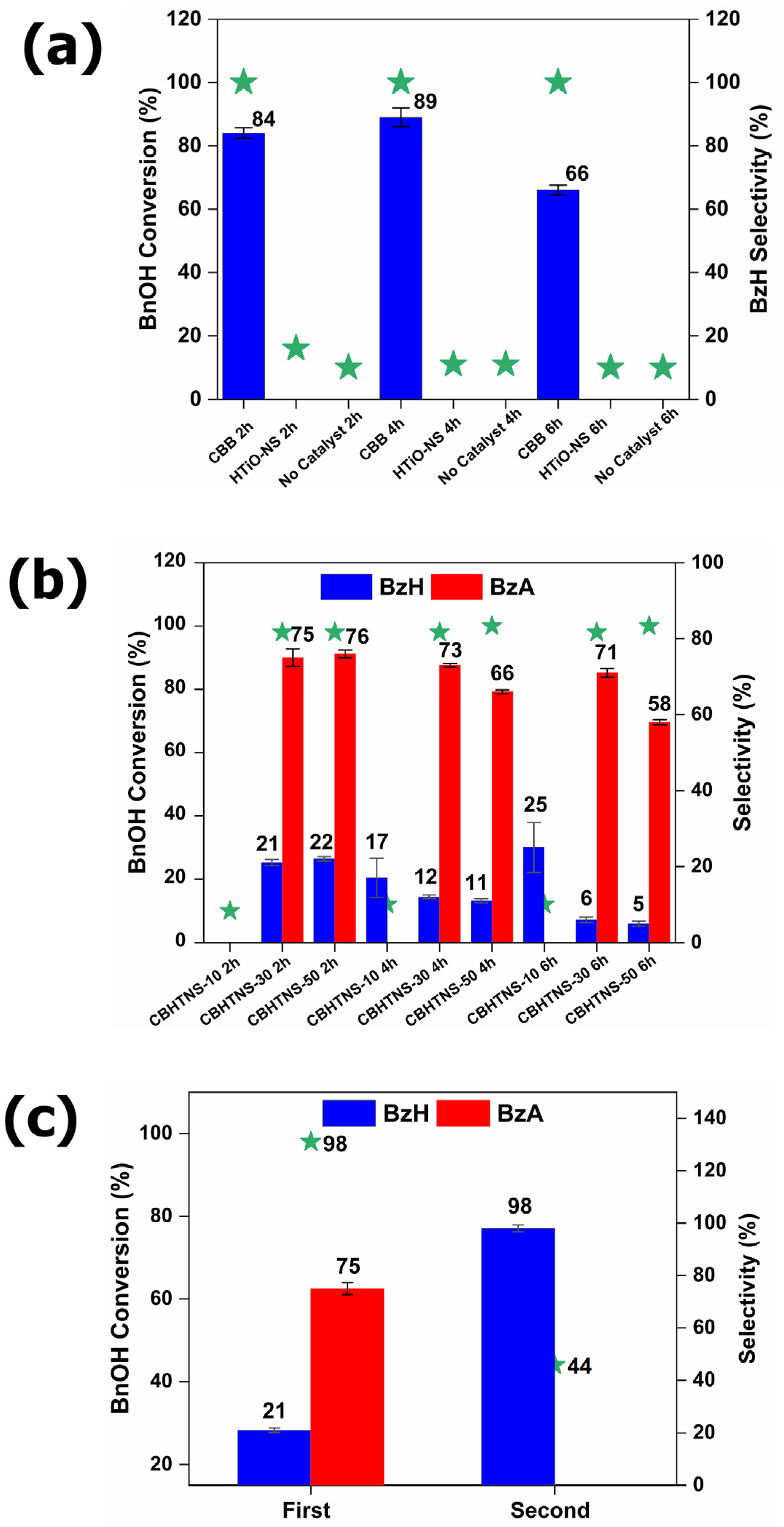
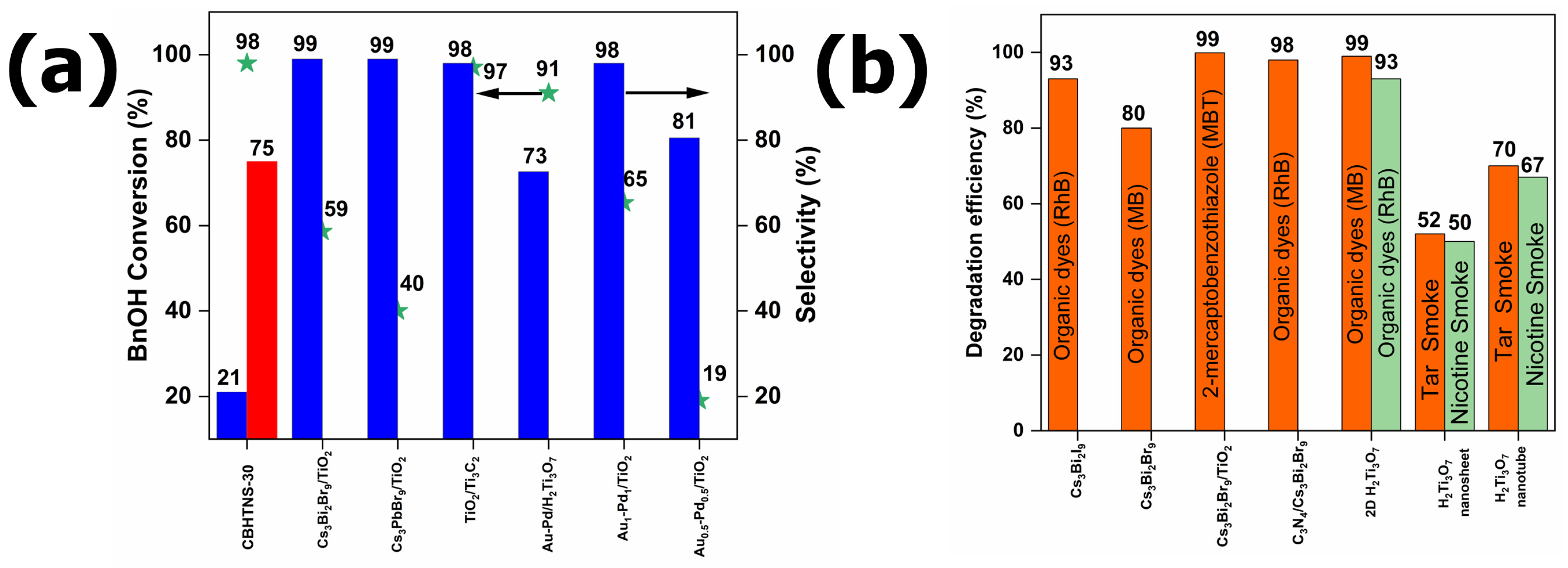
3.3. Evaluation of Photocatalytic Activity—BnOH Oxidation
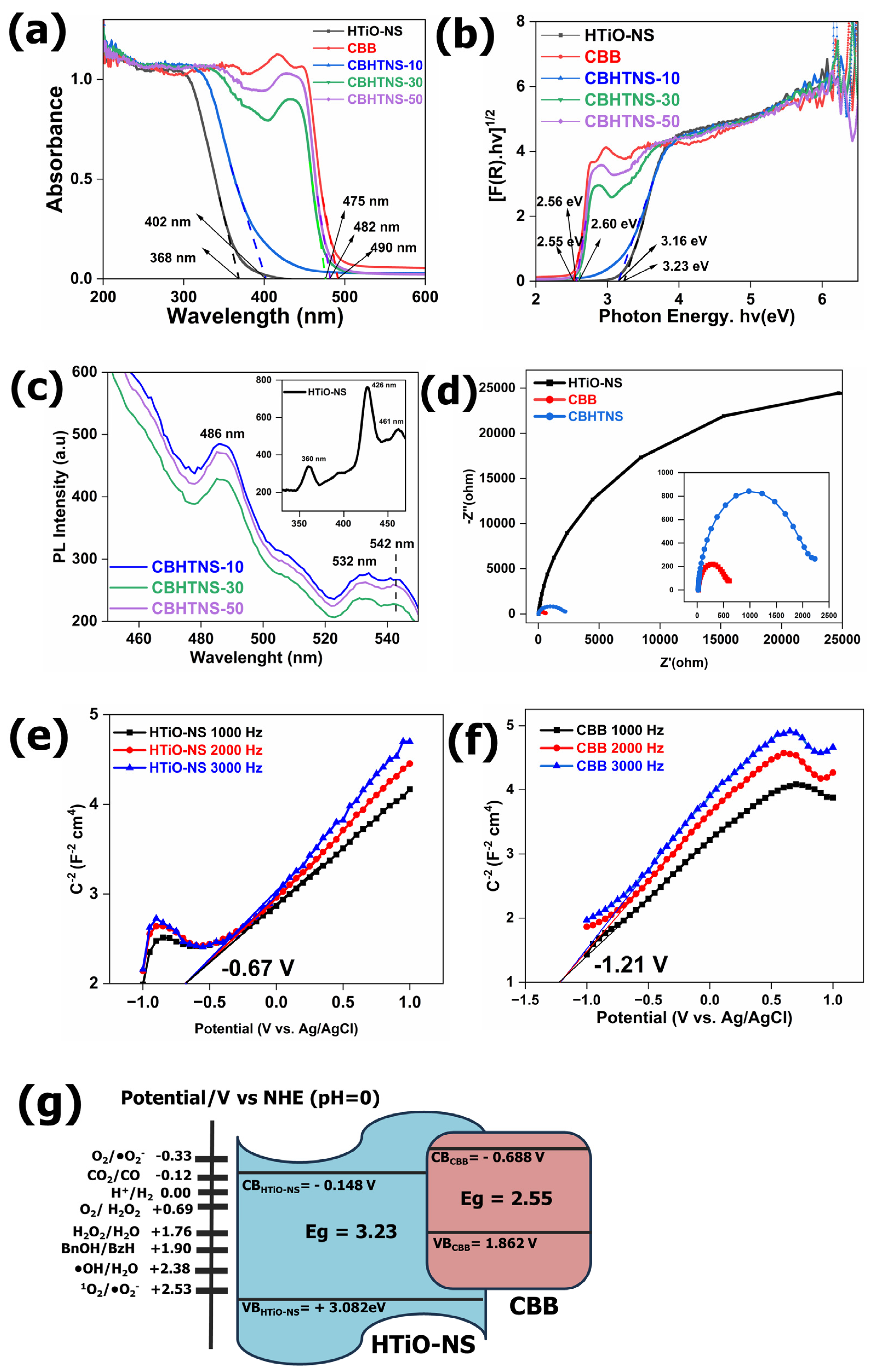
3.4. Mechanism of the Enhanced Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Awang, H.; Peppel, T.; Strunk, J. Photocatalytic Degradation of Diclofenac by Nitrogen-Doped Carbon Quantum Dot-Graphitic Carbon Nitride (CNQD). Catalysts 2023, 13, 735. [Google Scholar] [CrossRef]
- Augugliaro, V.; Palmisano, L. Green Oxidation of Alcohols to Carbonyl Compounds by Heterogeneous Photocatalysis. ChemSusChem 2010, 3, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, H.; Ren, Y.; Qu, Z. Effect of alkaline earth metal promoter on catalytic activity of MnO2 for the complete oxidation of toluene. J. Environ. Sci. 2021, 104, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, Z.; Lin, R.; Cheong, W.-C.M.; Liu, S.; Zhang, J.; Peng, Q.; Chen, C.; Han, T.; Tong, X.; et al. A photochromic composite with enhanced carrier separation for the photocatalytic activation of benzylic C–H bonds in toluene. Nat. Catal. 2018, 1, 704–710. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, S.; Zhou, H.; Ding, Z.; Lin, S.; Li, Z.; Zhang, Z.; Xu, C.; Wu, L.; Wang, X.; et al. Chlorine-Radical-Mediated Photocatalytic Activation of C-H Bonds with Visible Light. Angew. Chem. Int. Ed. 2013, 52, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Somekh, M.; Khenkin, A.M.; Herman, A.; Neumann, R. Selective Visible Light Aerobic Photocatalytic Oxygenation of Alkanes to the Corresponding Carbonyl Compounds. ACS Catal. 2019, 9, 8819–8824. [Google Scholar] [CrossRef]
- Song, L.-N.; Ding, F.; Yang, Y.-K.; Ding, D.; Chen, L.; Au, C.-T.; Yin, S.-F. Synthesis of TiO2/Bi2MoO6 Composite for Partial Oxidation of Aromatic Alkanes under Visible-Light Illumination. ACS Sustain. Chem. Eng. 2018, 6, 17044–17050. [Google Scholar] [CrossRef]
- Xu, C.; Pan, Y.; Wan, G.; Liu, H.; Wang, L.; Zhou, H.; Yu, S.-H.; Jiang, H.-L. Turning on Visible-Light Photocatalytic C−H Oxidation over Metal–Organic Frameworks by Introducing Metal-to-Cluster Charge Transfer. J. Am. Chem. Soc. 2019, 141, 19110–19117. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, Y.; Zhang, S.; Fu, H.; Chen, G.; Lou, Z.; Liu, X.; Zhang, Q.; Wang, Z.; Zheng, Z.; et al. Insight into a strategy to improve charge carrier migration in lead-free bismuth-based halide perovskite for efficient selective oxidation of thioanisole under visible light. Chem. Eng. J. 2023, 451, 138927. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, P.; Wu, Y.; Liu, X.; Chen, G.; Gao, P.; Zhang, Q.; Wang, Z.; Zheng, Z.; Cheng, H.; et al. Space-confined growth of lead-free halide perovskite Cs3Bi2Br9 in MCM-41 molecular sieve as an efficient photocatalyst for CO2 reduction at the gas−solid condition under visible light. Appl. Catal. B Environ. 2022, 310, 121375. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W. Lead-Free Organic-Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives. Adv. Mater. 2017, 29, 1605005. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Lee, J.I.; Cho, J.H.; Kang, M.S. Lead-Free Perovskite Nanocrystals for Light-Emitting Devices. J. Phys. Chem. Lett. 2018, 9, 1573–1583. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, Y.; Huang, H.; Liu, X.; Li, Q.; Chen, L.; Xu, D. Stable and Highly Efficient Photocatalysis with Lead-Free Double-Perovskite of Cs2AgBiBr6. Angew. Chem. Int. Ed. 2019, 58, 7263–7267. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Hong, F.; Mao, X.; Zheng, K.; Yang, S.; Li, Y.; Pullerits, T.; Deng, W.; Han, K. Lead-Free, Air-Stable All-Inorganic Cesium Bismuth Halide Perovskite Nanocrystals. Angew. Chem. Int. Ed. Engl. 2017, 56, 12471–12475. [Google Scholar] [CrossRef]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.-Y.; Nam, K.T. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat. Energy 2016, 2, 16185. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, M.; Cui, K.; Li, X.; Zhou, Y.; Li, Y.; Wu, X. Mechanochemical synthesis of MAPbBr3/carbon sphere composites for boosting carrier-involved superoxide species. J. Env. Sci. (China) 2021, 104, 399–414. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Jiang, Y.; Xu, J. Step-Scheme Photocatalyst of CsPbBr3 Quantum Dots/BiOBr Nanosheets for Efficient CO2 Photoreduction. Inorg. Chem. 2022, 61, 3351–3360. [Google Scholar] [CrossRef]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-Driven Metal Halide Perovskite Photocatalysis: Design, Stability, and Performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Liu, R.-R.; Mu, Y.-F.; Feng, Y.-X.; Dong, G.-X.; Zhang, M.; Lu, T.-B. In Situ Construction of Lead-Free Perovskite Direct Z-Scheme Heterojunction Cs3Bi2I9/Bi2WO6 for Efficient Photocatalysis of CO2 Reduction. Sol. RRL 2021, 5, 2000691. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, H.; Zhao, J.; Solís-Fernández, G.; Zhou, C.; Seo, J.W.; Hendrix, J.; Debroye, E.; Steele, J.A.; Hofkens, J.; et al. C(sp3)–H Bond Activation by Perovskite Solar Photocatalyst Cell. ACS Energy Lett. 2019, 4, 203–208. [Google Scholar] [CrossRef]
- Hezam, A.; Peppel, T.; Strunk, J. Pathways towards a systematic development of Z scheme photocatalysts for CO2 reduction. Curr. Opin. Green. Sustain. Chem. 2023, 41, 100789. [Google Scholar] [CrossRef]
- Soontornchaiyakul, W.; Fujimura, T.; Yano, N.; Kataoka, Y.; Sasai, R. Photocatalytic Hydrogen Evolution over Exfoliated Rh-Doped Titanate Nanosheets. ACS Omega 2020, 5, 9929–9936. [Google Scholar] [CrossRef] [PubMed]
- Hareesh, P.; Babitha, K.B.; Shukla, S. Processing fly ash stabilized hydrogen titanate nano-sheets for industrial dye-removal application. J. Hazard. Mater. 2012, 229–230, 177–182. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Qiufeng, H.; He, H.; Liu, Y.; Guo, W.; Hong, M. High-Temperature Formation of Titanate Nanotubes and the Transformation Mechanism of Nanotubes into Nanowires. Cryst. Growth Des. Cryst. Growth. Des. 2009, 9, 3632–3637. [Google Scholar] [CrossRef]
- Schünemann, S.; van Gastel, M.; Tüysüz, H. A CsPbBr3/TiO2 Composite for Visible-Light-Driven Photocatalytic Benzyl Alcohol Oxidation. ChemSusChem 2018, 11, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ye, W.; Wei, J.; Li, L.; Wang, J.; He, J.-H.; Lu, J.-M. Lead-free perovskite Cs3Bi2Br9 heterojunctions for highly efficient and selective photocatalysis under mild conditions. J. Alloys Compd. 2022, 893, 162326. [Google Scholar] [CrossRef]
- Xu, W.-L.; Hu, J.; Yang, Q.; Lian, Y.; Zheng, M.; Du, E. Charge transfer dynamics in C3N4 encapsulated Cs3Bi2Br9 nanocrystals heterojunction for photocatalytic application. J. Alloys Compd. 2024, 988, 174275. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Sheng, J.; He, Y.; Li, J.; Yuan, C.; Huang, H.; Wang, S.; Sun, Y.; Wang, Z.; Dong, F. Identification of Halogen-Associated Active Sites on Bismuth-Based Perovskite Quantum Dots for Efficient and Selective CO2-to-CO Photoreduction. ACS Nano 2020, 14, 13103–13114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.; Wang, K.; Lou, L. Role of primary active species and TiO2 surface characteristic in UV-illuminated photodegradation of Acid Orange 7. J. Photochem. Photobiol. A Chem. 2005, 172, 47–54. [Google Scholar] [CrossRef]
- Higashimoto, S.; Suetsugu, N.; Azuma, M.; Ohue, H.; Sakata, Y. Efficient and selective oxidation of benzylic alcohol by O2 into corresponding aldehydes on a TiO2 photocatalyst under visible light irradiation: Effect of phenyl-ring substitution on the photocatalytic activity. J. Catal. 2010, 274, 76–83. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B.; Xiao, X.; Gu, F.L.; Zhang, R.-Q. Roles of the active species involved in the photocatalytic oxidation of benzyl alcohol into benzaldehyde on TiO2 under UV light: Experimental and DFT studies. J. Mol. Catal. A Chem. 2016, 420, 82–87. [Google Scholar] [CrossRef]
- Lee, D.; Kim, M.; Woo, H.-Y.; Chae, J.; Lee, D.; Jeon, S.; Oh, S.J.; Paik, T. Heating-up synthesis of cesium bismuth bromide perovskite nanocrystals with tailored composition, morphology, and optical properties. RSC Adv. 2020, 10, 7126–7133. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Zhang, M.; Li, Q.; Yang, J. Construction of 2D/2D TiO2/g-C3N4 nanosheet heterostructures with improved photocatalytic activity. Mater. Res. Bull. 2020, 125, 110765. [Google Scholar] [CrossRef]
- Wu, L.; Yang, X.; Li, J.; Huang, Y.; Li, X. Fabrication of titanium dioxide nanotubes with good morphology at high calcination temperature and their photocatalytic activity. Mater. Chem. Phys. 2017, 202, 136–142. [Google Scholar] [CrossRef]
- Alkanad, K.; Hezam, A.; Al-Zaqri, N.; Bajiri, M.A.; Alnaggar, G.; Drmosh, Q.A.; Almukhlifi, H.A.; Neratur Krishnappagowda, L. One-Step Hydrothermal Synthesis of Anatase TiO2 Nanotubes for Efficient Photocatalytic CO2 Reduction. ACS Omega 2022, 7, 38686–38699. [Google Scholar] [CrossRef]
- Hua, S.; Yu, X.; Li, F.; Duan, J.; Ji, H.; Liu, W. Hydrogen titanate nanosheets with both adsorptive and photocatalytic properties used for organic dyes removal. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 211–218. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, W.; Du, G.H.; Peng, L.M. Trititanate Nanotubes Made via a Single Alkali Treatment. Adv. Mater. 2002, 14, 1208–1211. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Synthesis and characterization of ion-exchangeable titanate nanotubes. Chemistry 2003, 9, 2229–2238. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Chen, Y.; Fang, M.; Zhang, J.; Wang, H. Highly Efficient, Irreversible and Selective Ion Exchange Property of Layered Titanate Nanostructures. Adv. Funct. Mater. 2011, 22, 835–841. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Li, L.; Zhang, J.-R.; Zhu, J.-J. An Improved Strategy for High-Quality Cesium Bismuth Bromine Perovskite Quantum Dots with Remarkable Electrochemiluminescence Activities. Anal. Chem. 2019, 91, 8607–8614. [Google Scholar] [CrossRef]
- Masri, M.; Girisha, K.B.; Hezam, A.; Qahtan, T.F.; Alkanad, K.; Masri, F.; Namratha, K.; Udayabhanu; Byrappa, K. Enhanced photocatalytic activity and stability of 2D Cs3Bi2Br9 perovskite nanosheets synthesized via modified antisolvent method. Colloids Surf. C Environ. Asp. 2024, 2, 100024. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wu, Y.; Chen, J.; Zhao, J.; Jin, F.; Na, P. Carbon dots-TiO2 nanosheets composites for photoreduction of Cr(VI) under sunlight illumination: Favorable role of carbon dots. Appl. Catal. B-Environ. 2018, 224, 508–517. [Google Scholar] [CrossRef]
- Kundu, S.; Sain, S.; Choudhury, P.; Sarkar, S.; Das, P.K.; Pradhan, S.K. Microstructure characterization of biocompatible heterojunction hydrogen titanate-Ag2O nanocomposites for superior visible light photocatalysis and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 374–386. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, C.; Chen, Q.; Ni, J. Adsorption of Pb(II) and Cd(II) from aqueous solutions using titanate nanotubes prepared via hydrothermal method. J. Hazard. Mater. 2011, 189, 741–748. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lo, S.-L.; Kuo, J. Pb(II) adsorption capacity and behavior of titanate nanotubes made by microwave hydrothermal method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 126–131. [Google Scholar] [CrossRef]
- Milanović Marija, S.I.; Nikolić Ljubica, M. Preparation and photocatalytic activity of the layered titanates. Process. Appl. Ceram. 2010, 4, 69–73. [Google Scholar] [CrossRef]
- Nazari, P.; Dowlatabadi-Bazaz, R.; Mofid, M.R.; Pourmand, M.R.; Daryani, N.E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Shahverdi, A.R. The Antimicrobial Effects and Metabolomic Footprinting of Carboxyl-Capped Bismuth Nanoparticles Against Helicobacter pylori. Appl. Biochem. Biotechnol. 2014, 172, 570–579. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Ayati, A.; Baharara, J.; Jafari, A.M.; Ebrahimi, M. Acidic cesium salt of Preyssler nanoparticles: A new, green and recyclable nanocatalyst for the synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones. J. Nanostructure Chem. 2014, 4, 93. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Sarma, D.; Malliakas, C.D.; Subrahmanyam, K.S.; Islam, S.M.; Kanatzidis, M.G. K2xSn4−xS8−x (x = 0.65–1): A new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO22+ ions. Chem. Sci. 2016, 7, 1121–1132. [Google Scholar] [CrossRef]
- Liu, C.; Qian, X.; Wei, Q.; Chen, Z.; Chen, J.; Wang, W.; Chen, X.; Gao, J.; Liu, Y.; Xie, L. Construction of hydrostable cesium lead bromide-titania for visible-light degradation of tetracycline hydrochloride in water. J. Clean. Prod. 2022, 365, 132830. [Google Scholar] [CrossRef]
- Yang, F.; Elnabawy, A.O.; Schimmenti, R.; Song, P.; Wang, J.; Peng, Z.; Yao, S.; Deng, R.; Song, S.; Lin, Y.; et al. Bismuthene for highly efficient carbon dioxide electroreduction reaction. Nat. Commun. 2020, 11, 1088. [Google Scholar] [CrossRef]
- Li, Q.; Song, T.; Zhang, Y.; Wang, Q.; Yang, Y. Boosting Photocatalytic Activity and Stability of Lead-Free Cs3Bi2Br9 Perovskite Nanocrystals via In Situ Growth on Monolayer 2D Ti3C2Tx MXene for C–H Bond Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 27323–27333. [Google Scholar] [CrossRef]
- Rakibuddin, M.; Kim, H. Reduced graphene oxide supported C3N4 nanoflakes and quantum dots as metal-free catalysts for visible light assisted CO2 reduction. Beilstein J. Nanotechnol. 2019, 10, 448–458. [Google Scholar] [CrossRef]
- Enli, L.; Lin, X.; Hong, Y.; Yang, L.; Luo, B.; Shi, W.; Shi, J. Rational copolymerization strategy engineered C self-doped g-C3N4 for efficient and robust solar photocatalytic H2 evolution. Renew. Energy 2021, 178, 757–765. [Google Scholar] [CrossRef]
- Wang, C.; Fan, H.; Ren, X.; Fang, J.; Ma, J.; Zhao, N. Porous graphitic carbon nitride nanosheets by pre-polymerization for enhanced photocatalysis. Mater. Charact. 2018, 139, 89–99. [Google Scholar] [CrossRef]
- Yusslee, E.; Beskhyroun, S. The effect of water-to-binder ratio (W/B) on pore structure of one-part alkali activated mortar. Heliyon 2023, 9, e12983. [Google Scholar] [CrossRef] [PubMed]
- Bresolin, B.-M.; Balayeva, N.O.; Granone, L.I.; Dillert, R.; Bahnemann, D.W.; Sillanpää, M. Anchoring lead-free halide Cs3Bi2I9 perovskite on UV100–TiO2 for enhanced photocatalytic performance. Sol. Energy Mater. Sol. Cells 2020, 204, 110214. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.-D.; Xu, S.; Li, J.-X.; Chen, J.-S. Synthesis of uranium oxide nanoparticles and their catalytic performance for benzyl alcohol conversion to benzaldehyde. J. Mater. Chem. 2008, 18, 1146–1152. [Google Scholar] [CrossRef]
- Du, M.; Zeng, G.; Huang, J.; Sun, D.; Li, Q.; Wang, G.; Li, X. Green Photocatalytic Oxidation of Benzyl Alcohol over Noble-Metal-Modified H2Ti3O7 Nanowires. ACS Sustain. Chem. Eng. 2019, 7, 9717–9726. [Google Scholar] [CrossRef]
- JamJam, N.M.; Taufiq Yap, Y.H.; Muhamad, E.N.; Izham Saiman, M.; Saleh, T.A. Free solvent oxidation of molecular benzyl alcohol by newly synthesized AuPd/titania catalysts. Inorg. Chem. Commun. 2019, 107, 107471. [Google Scholar] [CrossRef]
- Leng, M.; Chen, Z.; Yang, Y.; Li, Z.; Zeng, K.; Li, K.; Niu, G.; He, Y.; Zhou, Q.; Tang, J. Lead-Free, Blue Emitting Bismuth Halide Perovskite Quantum Dots. Angew. Chem. Int. Ed. 2016, 55, 15012–15016. [Google Scholar] [CrossRef] [PubMed]
- Marcì, G.; Addamo, M.; Augugliaro, V.; Coluccia, S.; García-López, E.; Loddo, V.; Martra, G.; Palmisano, L.; Schiavello, M. Photocatalytic oxidation of toluene on irradiated TiO2: Comparison of degradation performance in humidified air, in water and in water containing a zwitterionic surfactant. J. Photochem. Photobiol. A Chem. 2003, 160, 105–114. [Google Scholar] [CrossRef]
- Jin, J.; Huang, H.; Chen, C.; Smith, P.W.; Folgueras, M.C.; Yu, S.; Zhang, Y.; Chen, P.-C.; Seeler, F.; Schaefer, B.; et al. Benzyl Alcohol Photo-oxidation Based on Molecular Electronic Transitions in Metal Halide Perovskites. ACS Photonics 2023, 10, 772–779. [Google Scholar] [CrossRef]
- Bao, X.; Li, H.; Wang, Z.; Tong, F.; Liu, M.; Zheng, Z.; Wang, P.; Cheng, H.; Liu, Y.; Dai, Y.; et al. TiO2/Ti3C2 as an efficient photocatalyst for selective oxidation of benzyl alcohol to benzaldehyde. Appl. Catal. B Environ. 2021, 286, 119885. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, Z.; Zhong, Y.; Qiu, J.; Zhang, X.; Gao, Y.; Gu, X.; Yao, J. TiO2 nanorods loaded with AuPt alloy nanoparticles for the photocatalytic oxidation of benzyl alcohol. J. Phys. Chem. Solids 2019, 126, 27–32. [Google Scholar] [CrossRef]
- Bresolin, B.-M.; Günnemann, C.; Bahnemann, D.W.; Sillanpää, M. Pb-Free Cs3Bi2I9 Perovskite as a Visible-Light-Active Photocatalyst for Organic Pollutant Degradation. Nanomaterials 2020, 10, 763. [Google Scholar] [CrossRef]
- Deng, Q.; Huang, C.; Xie, W.; Zhang, J.; Zhao, Y.; Hong, Z.; Pang, A.; Wei, M. Significant reduction of harmful compounds in tobacco smoke by the use of titanate nanosheets and nanotubes. Chem. Commun. 2011, 47, 6153–6155. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc Plot Method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- You, Q.; Zhang, Q.; Gu, M.; Du, R.; Chen, P.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Self-assembled graphitic carbon nitride regulated by carbon quantum dots with optimized electronic band structure for enhanced photocatalytic degradation of diclofenac. Chem. Eng. J. 2022, 431, 133927. [Google Scholar] [CrossRef]
- Leelavathi, A.; Madras, G.; Ravishankar, N. New Insights into Electronic and Geometric Effects in the Enhanced Photoelectrooxidation of Ethanol Using ZnO Nanorod/Ultrathin Au Nanowire Hybrids. J. Am. Chem. Soc. 2014, 136, 14445–14455. [Google Scholar] [CrossRef]
- Seng, R.X.; Tan, L.-L.; Lee, W.P.C.; Ong, W.-J.; Chai, S.-P. Nitrogen-doped carbon quantum dots-decorated 2D graphitic carbon nitride as a promising photocatalyst for environmental remediation: A study on the importance of hybridization approach. J. Environ. Manag. 2020, 255, 109936. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chang, K.; Huabin, Z.; Hai, X.; Yang, L.; Wang, T.; Ye, J. Drastic Enhancement of Photocatalytic Activities over Phosphoric Acid Protonated Porous g-C3N4 Nanosheets under Visible Light. Small 2016, 12, 4431–4439. [Google Scholar] [CrossRef]
- Xiong, L.; Tang, J. Strategies and Challenges on Selectivity of Photocatalytic Oxidation of Organic Substances. Adv. Energy Mater. 2021, 11, 2003216. [Google Scholar] [CrossRef]
- Jiang, T.; Jia, C.; Zhang, L.; He, S.; Sang, Y.; Li, H.; Li, Y.; Xu, X.; Liu, H. Gold and gold–palladium alloy nanoparticles on heterostructured TiO2 nanobelts as plasmonic photocatalysts for benzyl alcohol oxidation. Nanoscale 2015, 7, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.R.; Chaudhari, P.A.; Narkhede, V.S. Solvent-free liquid phase oxidation of benzyl alcohol to benzaldehyde by molecular oxygen using non-noble transition metal containing hydrotalcite-like solid catalysts. Catal. Commun. 2003, 4, 171–175. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Rodríguez-Pereira, J.; Amado-González, E.; Rueda-P, J.; Ospina, R.; Masi, S.; Yoon, S.J.; Tirado, J.; Jaramillo, F.; Agouram, S.; et al. Unravelling the Photocatalytic Behavior of All-Inorganic Mixed Halide Perovskites: The Role of Surface Chemical States. ACS Appl. Mater. Interfaces 2020, 12, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W.; Robben, L.; Yarovyi, V.; Wark, M. Palladium Doped Porous Titania Photocatalysts: Impact of Mesoporous Order and Crystallinity. Chem. Mater. 2010, 22, 108–116. [Google Scholar] [CrossRef]

| Photocatalysts | Ti (Calc.) | Cs (Calc.) | Bi (Calc.) | Br (Calc.) | Na (Calc.) | C (Calc.) | H (Calc.) |
|---|---|---|---|---|---|---|---|
| CBB | - | 21.5 | 27.1 | 35.6 | - | - | - |
| (26.0) | (27.2) | (46.8) | - | - | - | ||
| HTiO-NS | 51.5 | - | - | - | 1.4 | - | 0.5 |
| (55.7) | - | - | - | (0.0) | - | (0.7) | |
| CBHTNS-10 | 45.8 | 2.4 | 2.2 | 3.6 | <0.5 | <1.0 | 0.2 |
| (46.5) | (4.3) | (4.5) | (7.8) | (0.0) | (0.0) | (0.6) | |
| CBHTNS-30 | 35.3 | 6.9 | 8.1 | 12.2 | <0.5 | <1.0 | 0.1 |
| (34.9) | (9.7) | (10.2) | (17.5) | (0.0) | (0.0) | (0.5) | |
| CBHTNS-50 | 26.0 | 9.0 | 13.1 | 18.2 | <0.5 | <1.0 | 0.1 |
| (28.0) | (13.0) | (13.6) | (22.3) | (0.0) | (0.0) | (0.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awang, H.; Hezam, A.; Peppel, T.; Strunk, J. Enhancing the Photocatalytic Activity of Halide Perovskite Cesium Bismuth Bromide/Hydrogen Titanate Heterostructures for Benzyl Alcohol Oxidation. Nanomaterials 2024, 14, 752. https://doi.org/10.3390/nano14090752
Awang H, Hezam A, Peppel T, Strunk J. Enhancing the Photocatalytic Activity of Halide Perovskite Cesium Bismuth Bromide/Hydrogen Titanate Heterostructures for Benzyl Alcohol Oxidation. Nanomaterials. 2024; 14(9):752. https://doi.org/10.3390/nano14090752
Chicago/Turabian StyleAwang, Huzaikha, Abdo Hezam, Tim Peppel, and Jennifer Strunk. 2024. "Enhancing the Photocatalytic Activity of Halide Perovskite Cesium Bismuth Bromide/Hydrogen Titanate Heterostructures for Benzyl Alcohol Oxidation" Nanomaterials 14, no. 9: 752. https://doi.org/10.3390/nano14090752






