Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Physical Chemical Characterizations
2.3. Electrochemical Measurements
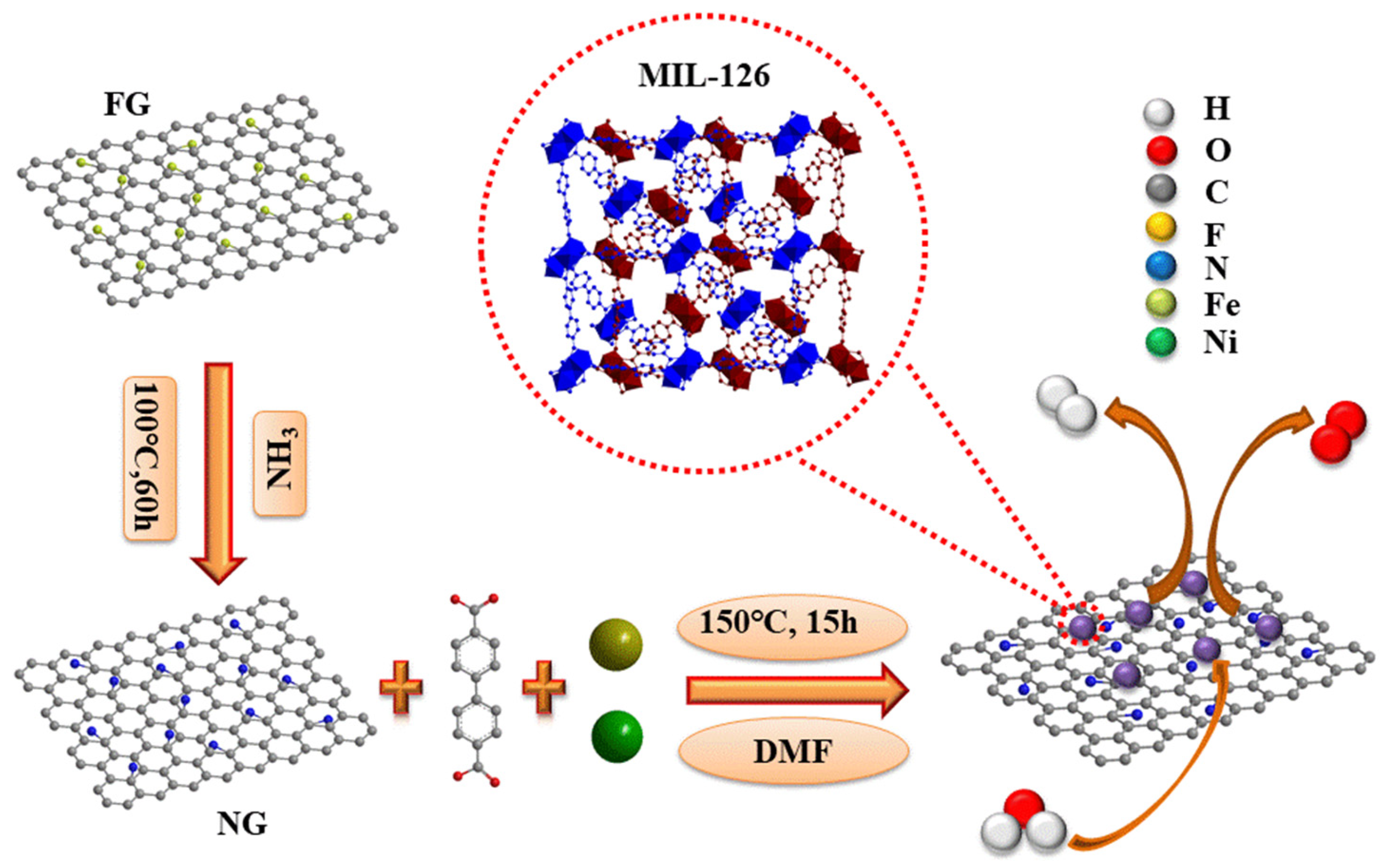
2.4. Synthesis of NG
2.5. Synthesis of Bimetallic (Fe,Ni)-MIL-126
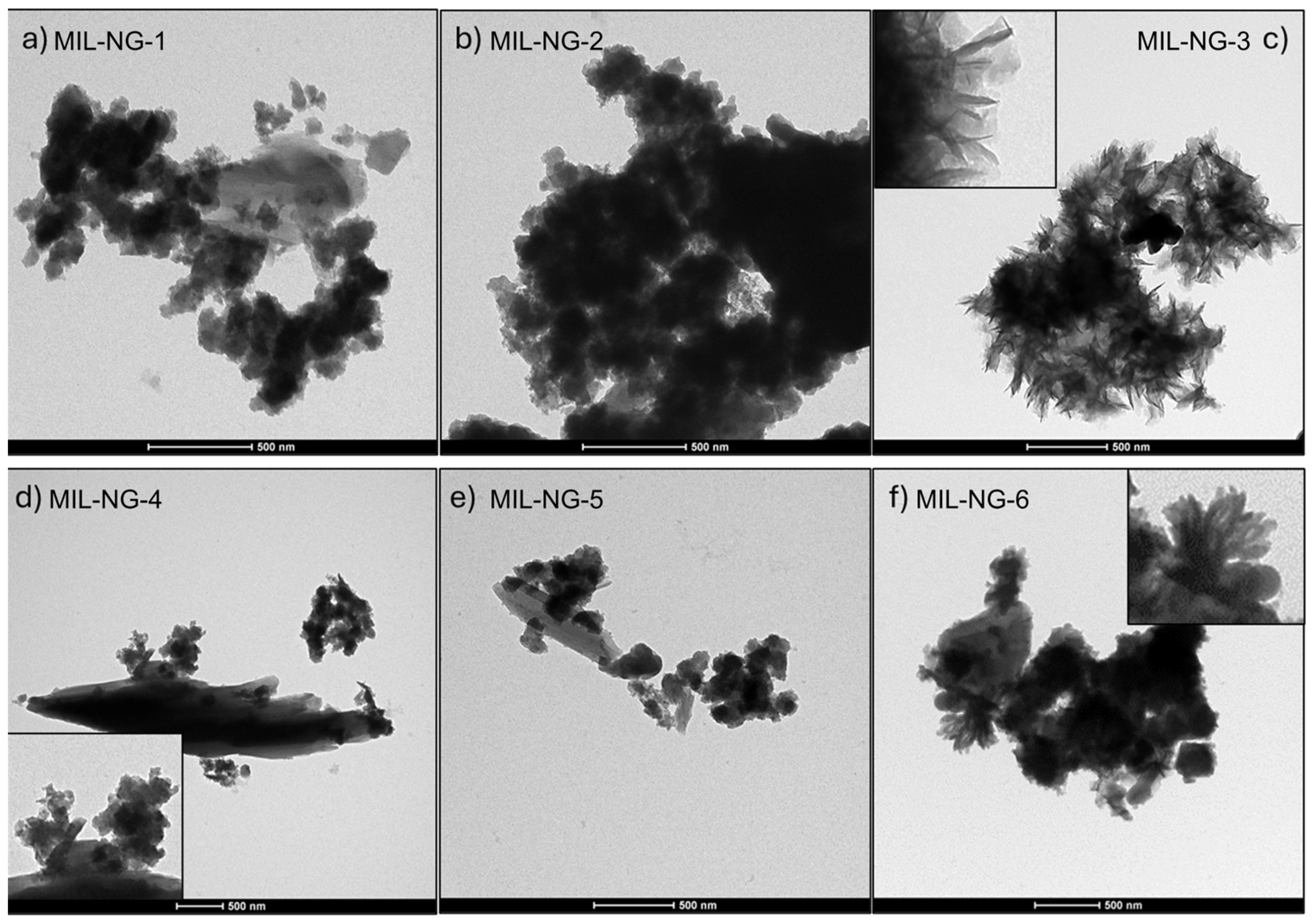
2.6. Synthesis of MIL-NG-n Hybrid
2.7. Synthesis of Bimetallic (Fe,Ni)-NG
2.8. Synthesis of (Fe,Ni)-MIL-126-NG-mix
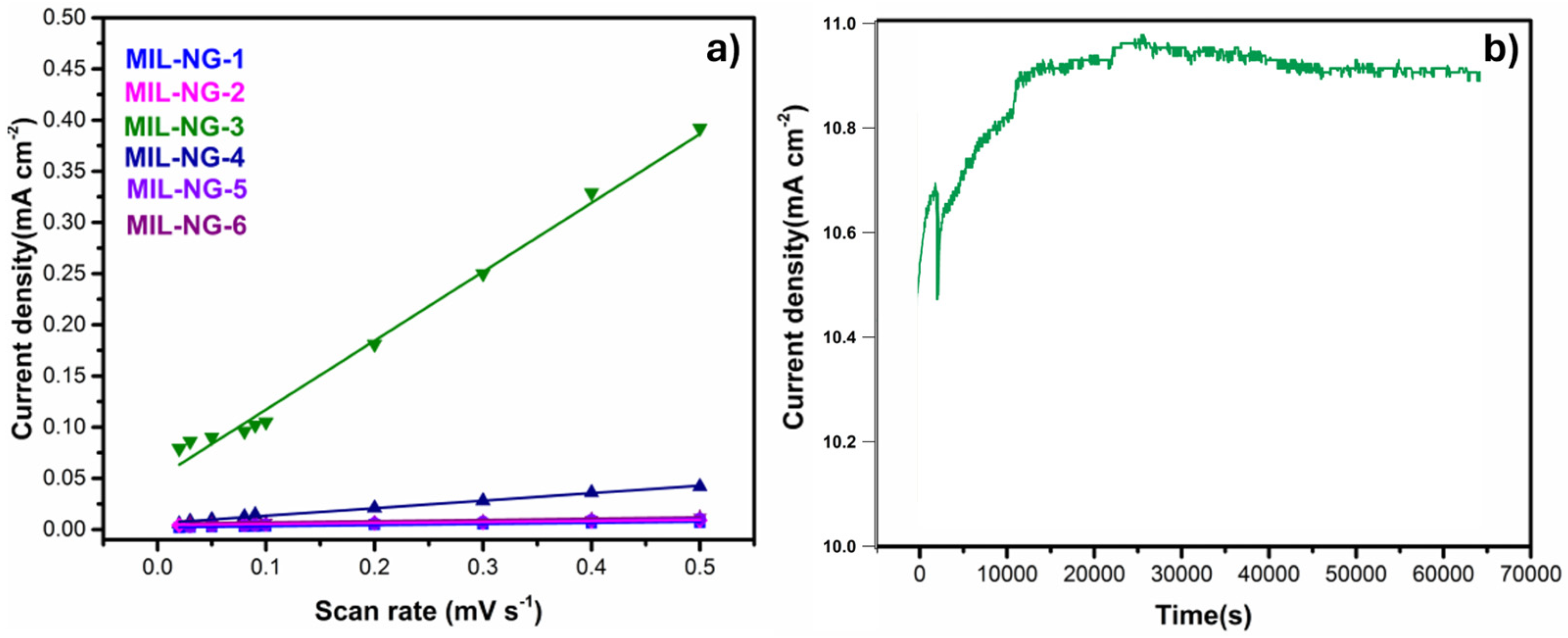
3. Results and Discussion
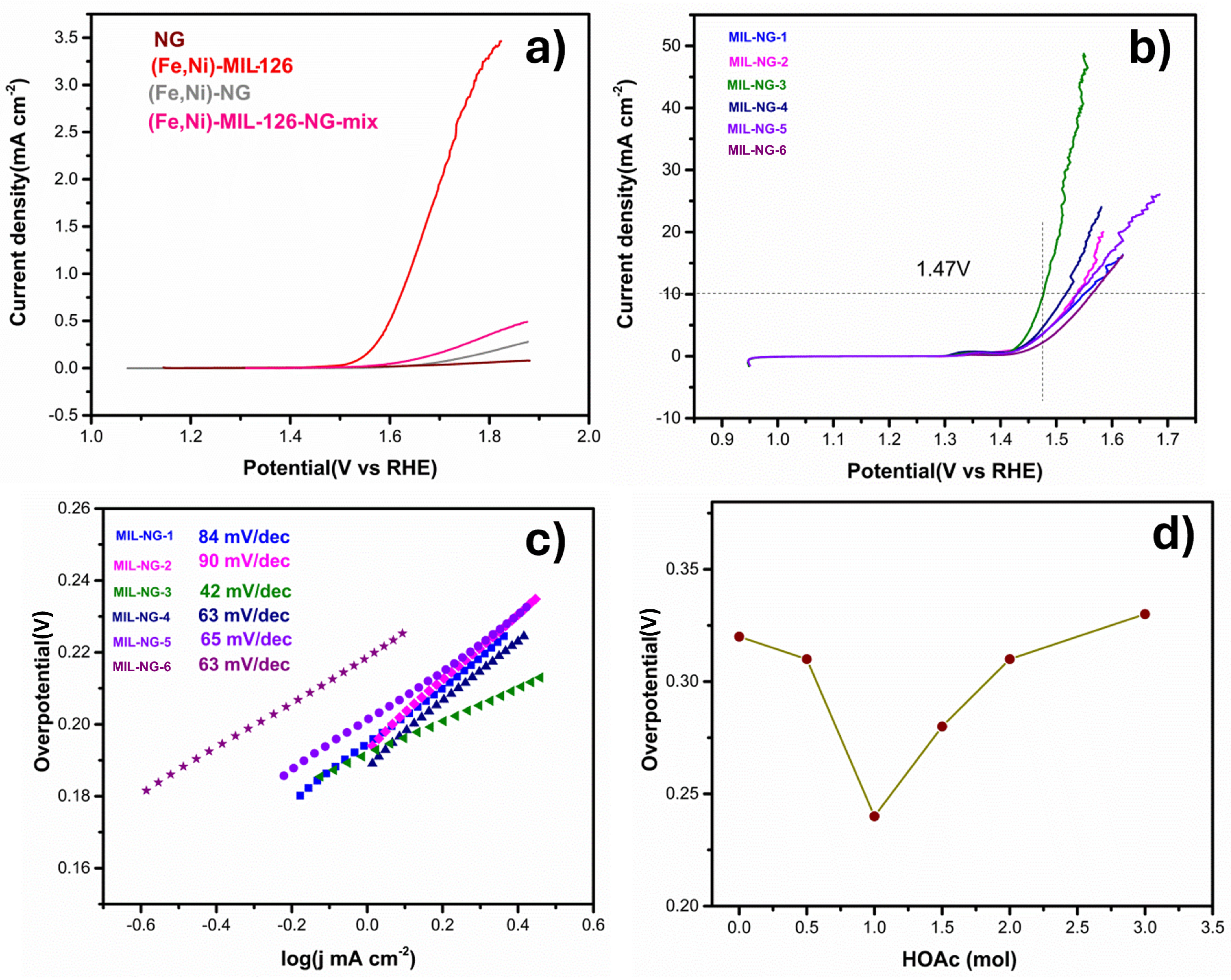
| Sample | Tafel Slope/mV dec−1 | Overpotential at 10 mA cm−2/mV | Cdl/mF cm−2 |
|---|---|---|---|
| MIL-NG-1 | 84 | 320 | 0.01 |
| MIL-NG-2 | 90 | 310 | 0.009 |
| MIL-NG-3 | 42 | 240 | 0.67 |
| MIL-NG-4 | 63 | 280 | 0.07 |
| MIL-NG-5 | 65 | 310 | 0.012 |
| MIL-NG-6 | 63 | 330 | 0.015 |
| Components | EOER (V) at 10 mA cm−2 | Tafel mV·dec−1 | Refs. |
|---|---|---|---|
| MIL-NG-3 | 1.47 | 42 | / |
| MIL-126(FeNi)-700 | 2.0 | [61] | |
| Fe1Ni2-BDC | 1.49 | 35 | [26] |
| Fe/Ni/Co(Mn)-MIL-53/NF | 1.45 | 53.5 | [35] |
| Fe2+-NiFe LDH | 1.479 | 40.4 | [62] |
| NiFeV LDHs | 1.422 | 42 | [63] |
| NiFe LDHs Nanosheets | 1.459 | 62.9 | [64] |
| flame-engraved NiFe-LDH | 1.48 | 69 | [65] |
| Fe−Ni−P/rGO-400 | 1.47 | 63 | [66] |
| Ni2P@C/GO (NiBTC) | 1.515 | 44 | [67] |
| NGO/Ni7S6 (Ni-MOF-74) | 1.61 | 45 | [68] |
| Ni-NiO/N-rGO | 1.47 | 43 | [28] |
| Ni-MOF-600 | 1.6 | 66 | [69] |
| CoP/rGO-400 (ZIF 67) | 1.57 | 66 | [70] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. A Realizable Renewable Energy Future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Lunardon, M.; Kosmala, T.; Durante, C.; Agnoli, S.; Granozzi, G. Atom-by-Atom Identification of Catalytic Active Sites in Operando Conditions by Quantitative Noise Detection. Joule 2022, 6, 617–635. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the Role of Hydrogen in the 21st Century Energy Transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Kosmala, T.; Baby, A.; Lunardon, M.; Perilli, D.; Liu, H.; Durante, C.; Di Valentin, C.; Agnoli, S.; Granozzi, G. Operando Visualization of the Hydrogen Evolution Reaction with Atomic-Scale Precision at Different Metal–Graphene Interfaces. Nat. Catal. 2021, 4, 850–859. [Google Scholar] [CrossRef]
- Lunardon, M.; Cattelan, M.; Agnoli, S.; Granozzi, G. Toward Sustainable and Effective HER Electrocatalysts: Strategies for the Basal Plane Site Activation of Transition Metal Dichalcogenides. Curr. Opin. Electrochem. 2022, 34, 101025. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen Society: From Present to Future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeon, K.-J.; Show, P.L.; Lee, I.H.; Jung, S.-C.; Choi, Y.J.; Rhee, G.H.; Lin, K.-Y.A.; Park, Y.-K. Mini Review on H2 Production from Electrochemical Water Splitting According to Special Nanostructured Morphology of Electrocatalysts. Fuel 2022, 308, 122048. [Google Scholar] [CrossRef]
- Lv, X.-W.; Tian, W.-W.; Yuan, Z.-Y. Recent Advances in High-Efficiency Electrocatalytic Water Splitting Systems. Electrochem. Energy Rev. 2023, 6, 23. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.-Z. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2016, 117, 3717–3797. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured Nonprecious Metal Catalysts for Oxygen Reduction Reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Lunardon, M.; Kosmala, T.; Ghorbani-Asl, M.; Krasheninnikov, A.V.; Kolekar, S.; Durante, C.; Batzill, M.; Agnoli, S.; Granozzi, G. Catalytic Activity of Defect-Engineered Transition Metal Dichalcogenides Mapped with Atomic-Scale Precision by Electrochemical Scanning Tunneling Microscopy. ACS Energy Lett. 2023, 8, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Hess, F. Corrosion Mechanism and Stabilization Strategies for RuO2 and IrO2 Catalysts in the Electrochemical Oxygen Evolution Reaction. Curr. Opin. Electrochem. 2023, 41, 101349. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Ge, J.; Liu, C.; Xing, W. Discontinuously Covered IrO2–RuO2@Ru Electrocatalysts for the Oxygen Evolution Reaction: How High Activity and Long-Term Durability can Be Simultaneously Realized in the Synergistic and Hybrid Nano-Structure. J. Mater. Chem. A 2017, 5, 17221–17229. [Google Scholar] [CrossRef]
- Li, X.-P.; Huang, C.; Han, W.-K.; Ouyang, T.; Liu, Z.-Q. Transition Metal-Based Electrocatalysts for Overall Water Splitting. Chin. Chem. Lett. 2021, 32, 2597–2616. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, e2201099. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Perovskite for Electrocatalytic Oxygen Evolution at Elevated Temperatures. ChemSusChem 2024, 4, e202301534. [Google Scholar] [CrossRef]
- Cai, Z.; Bu, X.; Wang, P.; Ho, J.C.; Yang, J.; Wang, X. Recent Advances in Layered Double Hydroxide Electrocatalysts for the Oxygen Evolution Reaction. J. Mater. Chem. A 2019, 7, 5069–5089. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Zhu, S.; Feng, L.; Xing, W. Ni-Based Layered Double Hydroxide Catalysts for Oxygen Evolution Reaction. Mater. Today Phys. 2021, 16, 100292. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; El Hankari, S.; Zou, Y.; Wang, S. Recent Progress on Layered Double Hydroxides and Their Derivatives for Electrocatalytic Water Splitting. Adv. Sci. 2018, 5, 1800064. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Luo, S.; Zhao, Z.; Wang, X.; Luo, Y.; Wang, Z.; Jin, J.; Ma, J. Lateral-Size-Mediated Efficient Oxygen Evolution Reaction: Insights into the Atomically Thin Quantum Dot Structure of NiFe2O4. ACS Catal. 2017, 7, 5557–5567. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Li, W. Electrocatalytic Oxygen Evolution over Supported Small Amorphous Ni–Fe Nanoparticles in Alkaline Electrolyte. Langmuir 2014, 30, 7893–7901. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, X.; Guo, K.; Dong, L.; Miao, X. Alkaline Media Regulated NiFe-LDH-Based Nickel–Iron Phosphides toward Robust Overall Water Splitting. Catalysts 2023, 13, 198. [Google Scholar] [CrossRef]
- Salvò, D.; Mosconi, D.; Neyman, A.; Bar-Sadan, M.; Calvillo, L.; Granozzi, G.; Cattelan, M.; Agnoli, S. Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media. Nanomaterials 2023, 13, 683. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional Hydrogen Storage Achieved by Screening Nearly Half a Million Metal-Organic Frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Jin, S. How to Effectively Utilize MOFs for Electrocatalysis. ACS Energy Lett. 2019, 4, 1443–1445. [Google Scholar] [CrossRef]
- Tang, P.; Paganelli, S.; Carraro, F.; Blanco, M.; Riccò, R.; Marega, C.; Badocco, D.; Pastore, P.; Doonan, C.J.; Agnoli, S. Postsynthetic Metalated MOFs as Atomically Dispersed Catalysts for Hydroformylation Reactions. ACS Appl. Mater. Interfaces 2020, 12, 54798–54805. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Ko, M.; Park, M.; Kim, M.G.; Oh, P.; Chae, S.; Park, S.; Casimir, A.; Wu, G.; et al. Metal (Ni, Co)-Metal Oxides/Graphene Nanocomposites as Multifunctional Electrocatalysts. Adv. Funct. Mater. 2015, 25, 5799–5808. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating Metal–Organic Frameworks for Catalyzing Acidic Oxygen Evolution for Proton Exchange Membrane Water Electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Horcajada, P.; Salles, F.; Wuttke, S.; Devic, T.; Heurtaux, D.; Maurin, G.; Vimont, A.; Daturi, M.; David, O.; Magnier, E.; et al. How Linker’s Modification Controls Swelling Properties of Highly Flexible Iron(III) Dicarboxylates MIL-88. J. Am. Chem. Soc. 2011, 133, 17839–17847. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Chevreau, H.; Devic, T.; Horcajada, P.; Maurin, G.; Férey, G.; Popov, D.; Riekel, C.; Wuttke, S.; Lavalley, J.-C.; et al. How Interpenetration Ensures Rigidity and Permanent Porosity in a Highly Flexible Hybrid Solid. Chem. Mater. 2012, 24, 2486–2492. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zeng, G.; Cheng, M.; Huang, D.; Liu, Y.; Zhou, C.; Xiong, W.; Yang, Y.; Wang, W.; et al. Materials Institute Lavoisier (MIL) Based Materials for Photocatalytic Applications. Coord. Chem. Rev. 2021, 438, 213874. [Google Scholar] [CrossRef]
- Taffa, D.H.; Balkenhohl, D.; Amiri, M.; Wark, M. Minireview: Ni–Fe and Ni–Co Metal–Organic Frameworks for Electrocatalytic Water-Splitting Reactions. Small Struct. 2022, 4, 263. [Google Scholar] [CrossRef]
- Sun, F.; Wang, G.; Ding, Y.; Wang, C.; Yuan, B.; Lin, Y. NiFe-Based Metal–Organic Framework Nanosheets Directly Supported on Nickel Foam Acting as Robust Electrodes for Electrochemical Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 584. [Google Scholar] [CrossRef]
- Li, F.; Shao, Q.; Huang, X.; Lang, J. Nanoscale Trimetallic Metal–Organic Frameworks Enable Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2017, 57, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhang, W.; Jin, Z.; An, W.; Gao, Y.; Zhang, X.; Liu, J. Electrocatalytically Active MOF/Graphite Oxide Hybrid for Electrosynthesis of Dimethyl Carbonate. Electrochim. Acta 2014, 144, 1–6. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H. Synthesis of Iron(iii)-Based Metal–Organic Framework/Graphene Oxide Composites with Increased Photocatalytic Performance for Dye Degradation. RSC Adv. 2014, 4, 40435–40438. [Google Scholar] [CrossRef]
- Lalitha, A.; Shin, J.E.; Bonakala, S.; Oh, J.Y.; Park, H.B.; Maurin, G. Unraveling the Enhancement of the Interfacial Compatibility between Metal–Organic Framework and Functionalized Graphene Oxide. J. Phys. Chem. C 2019, 123, 4984–4993. [Google Scholar] [CrossRef]
- Favaro, M.; Agnoli, S.; Cattelan, M.; Moretto, A.; Durante, C.; Leonardi, S.; Kunze-Liebhäuser, J.; Schneider, O.; Gennaro, A.; Granozzi, G. Shaping Graphene Oxide by Electrochemistry: From Foams to Self-Assembled Molecular Materials. Carbon 2014, 77, 405–415. [Google Scholar] [CrossRef]
- Mosconi, D.; Kosmala, T.; Lunardon, M.; Neyman, A.; Bar-Sadan, M.; Agnoli, S.; Granozzi, G. One-Pot Synthesis of MoS2(1−x)Se2x on N-Doped Reduced Graphene Oxide: Tailoring Chemical and Structural Properties for Photoenhanced Hydrogen Evolution Reaction. Nanoscale Adv. 2020, 2, 4830–4840. [Google Scholar] [CrossRef]
- Favaro, M.; Carraro, F.; Cattelan, M.; Colazzo, L.; Durante, C.; Sambi, M.; Gennaro, A.; Agnoli, S.; Granozzi, G. Multiple Doping of Graphene Oxide Foams and Quantum Dots: New Switchable Systems for Oxygen Reduction and Water Remediation. J. Mater. Chem. A 2015, 3, 14334–14347. [Google Scholar] [CrossRef]
- Carraro, F.; Cattelan, M.; Favaro, M.; Calvillo, L. Aerosol Synthesis of N and N-S Doped and Crumpled Graphene Nanostructures. Nanomaterials 2018, 8, 406. [Google Scholar] [CrossRef]
- Lunardon, M.; Ran, J.; Mosconi, D.; Marega, C.; Wang, Z.; Xia, H.; Agnoli, S.; Granozzi, G. Hybrid Transition Metal Dichalcogenide/Graphene Microspheres for Hydrogen Evolution Reaction. Nanomaterials 2020, 10, 2376. [Google Scholar] [CrossRef]
- Lyu, S.; Guo, C.; Wang, J.; Li, Z.; Yang, B.; Lei, L.; Wang, L.; Xiao, J.; Zhang, T.; Hou, Y. Exceptional Catalytic Activity of Oxygen Evolution Reaction via Two-Dimensional Graphene Multilayer Confined Metal-Organic Frameworks. Nat. Commun. 2022, 13, 6171. [Google Scholar] [CrossRef] [PubMed]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef] [PubMed]
- Pasarakonda, S.L.; Ponnada, S.; Gorle, D.B.; Chandra Bose, R.S.; Palariya, A.; Kiai, M.S.; Gandham, H.B.; Kathiresan, M.; Sharma, R.K.; Nowduri, A. On the Role of Graphene Oxide in Bifunctional Ni/MOF/RGO Composites in Electrochemical Nitrate Detection and Oxygen Evolution Reaction. New J. Chem. 2023, 47, 725–736. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically Active Graphene–Porphyrin MOF Composite for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Yu, Q.; Luo, Y.; Zhang, Z.; Zhang, C.; Qiu, L.; Liu, B. Controllable Structure Reconstruction of Nickel–Iron Compounds toward Highly Efficient Oxygen Evolution. Nanoscale 2020, 12, 10751–10759. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A Review Unveiling the Critical Roles of Fe in Enhancing OER Activity of Ni and Co Based Catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Furukawa, S.; Takashima, Y.; Yoshida, K.; Isoda, S.; Kitagawa, S. Nanoporous Nanorods Fabricated by Coordination Modulation and Oriented Attachment Growth. Angew. Chem. Int. Ed. 2009, 48, 4739–4743. [Google Scholar] [CrossRef] [PubMed]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled Multiscale Synthesis of Porous Coordination Polymer in Nano/Micro Regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Bagherzadeh, E.; Zebarjad, S.M.; Hosseini, H.R.M. Morphology Modification of the Iron Fumarate MIL-88A Metal–Organic Framework Using Formic Acid and Acetic Acid as Modulators. Eur. J. Inorg. Chem. 2018, 2018, 1909–1915. [Google Scholar] [CrossRef]
- Bara, D.; Wilson, C.; Mörtel, M.; Khusniyarov, M.M.; Ling, S.; Slater, B.; Sproules, S.; Forgan, R.S. Kinetic Control of Interpenetration in Fe–Biphenyl-4,4′-Dicarboxylate Metal–Organic Frameworks by Coordination and Oxidation Modulation. J. Am. Chem. Soc. 2019, 141, 8346–8357. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hui, K.N.; San Hui, K.; Peng, S.; Xu, Y. Recent Progress in Metal–Organic Framework/Graphene-Derived Materials for Energy Storage and Conversion: Design, Preparation, and Application. Chem. Sci. 2021, 12, 5737–5766. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel Hydroxides and Related Materials: A Review of Their Structures, Synthesis and Properties. Proc. Math. Phys. Eng. Sci. 2015, 471, 20140792. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Dai, H. A Mini Review of NiFe-Based Materials as Highly Active Oxygen Evolution Reaction Electrocatalysts. Nano Res. 2014, 8, 23–39. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Tang, Z. Insight into Structural Evolution, Active Sites, and Stability of Heterogeneous Electrocatalysts. Angew. Chem. Int. Ed. 2022, 61, e202110186. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, J.; Cai, B.; Huang, Z.; Garcia-Esparza, A.T.; Sokaras, D.; Zhang, Y.; Giordano, L.; Akkiraju, K.; Zhu, Y.G.; et al. Tunable Metal Hydroxide–Organic Frameworks for Catalysing Oxygen Evolution. Nat. Mater. 2022, 21, 673–680. [Google Scholar] [CrossRef]
- Lionet, Z.; Nishijima, S.; Kim, T.-H.; Horiuchi, Y.; Lee, S.W.; Matsuoka, M.; Persidis, A. Bimetallic MOF-Templated Synthesis of Alloy Nanoparticle-Embedded Porous Carbons for Oxygen Evolution and Reduction Reactions. Nature 1999, 17, 229. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, D.; Wang, M.; Bak, S.; Wu, Y.; Wu, Z.; Tian, Y.; Xiong, X.; Li, Y.; Liu, W.; et al. Introducing Fe2+ into Nickel–Iron Layered Double Hydroxide: Local Structure Modulated Water Oxidation Activity. Angew. Chem. 2018, 130, 9536–9540. [Google Scholar] [CrossRef]
- Li, P.; Duan, X.; Kuang, Y.; Li, Y.; Zhang, G.; Liu, W.; Sun, X. Tuning Electronic Structure of NiFe Layered Double Hydroxides with Vanadium Doping toward High Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2018, 8, 3341. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, M.; Li, Y.; Wang, S. Tuning Surface Electronic Configuration of NiFe LDHs Nanosheets by Introducing Cation Vacancies (Fe or Ni) as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Small 2018, 14, 1800136. [Google Scholar] [CrossRef]
- Zhou, D.; Xiong, X.; Cai, Z.; Han, N.; Jia, Y.; Xie, Q.; Duan, X.; Xie, T.; Zheng, X.; Sun, X.; et al. Flame-Engraved Nickel–Iron Layered Double Hydroxide Nanosheets for Boosting Oxygen Evolution Reactivity. Small Methods 2018, 2, 83. [Google Scholar] [CrossRef]
- Fang, X.; Jiao, L.; Zhang, R.; Jiang, H.-L. Porphyrinic Metal–Organic Framework-Templated Fe–Ni–P/Reduced Graphene Oxide for Efficient Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2017, 9, 23852–23858. [Google Scholar] [CrossRef]
- Wang, M.; Lin, M.; Li, J.; Huang, L.; Zhuang, Z.; Lin, C.; Zhou, L.; Mai, L. Metal–Organic Framework Derived Carbon-Confined Ni2P Nanocrystals Supported on Graphene for an Efficient Oxygen Evolution Reaction. Chem. Commun. 2017, 53, 8372–8375. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Masa, J.; Tomanec, O.; Peeters, D.; Ranc, V.; Schneemann, A.; Zboril, R.; Schuhmann, W.; Fischer, R.A. Nanoporous Nitrogen-Doped Graphene Oxide/Nickel Sulfide Composite Sheets Derived from a Metal-Organic Framework as an Efficient Electrocatalyst for Hydrogen and Oxygen Evolution. Adv. Funct. Mater. 2017, 27, 451. [Google Scholar] [CrossRef]
- Ai, L.; Tian, T.; Jiang, J. Ultrathin Graphene Layers Encapsulating Nickel Nanoparticles Derived Metal–Organic Frameworks for Highly Efficient Electrocatalytic Hydrogen and Oxygen Evolution Reactions. ACS Sustain. Chem. Eng. 2017, 5, 4771–4777. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Chemical Science Metal-Organic Framework-Based CoP/Reduced Graphene Oxide: High-Performance Bifunctional Electrocatalyst for Overall Water Splitting Metal-Organic Framework-Based CoP/Reduced Graphene Oxide: High-Performance Bifunctional Electrocatalyst for Overall Water Splitting. Chem. Sci. 2016, 7, 1615–2442. [Google Scholar] [CrossRef]

| MIL-NG-1 | MIL-NG-2 | MIL-NG-3 | MIL-NG-4 | MIL-NG-5 | MIL-NG-6 | |
|---|---|---|---|---|---|---|
| HOAc (mmol) | / | 0.5 | 1 | 1.5 | 2 | 3 |
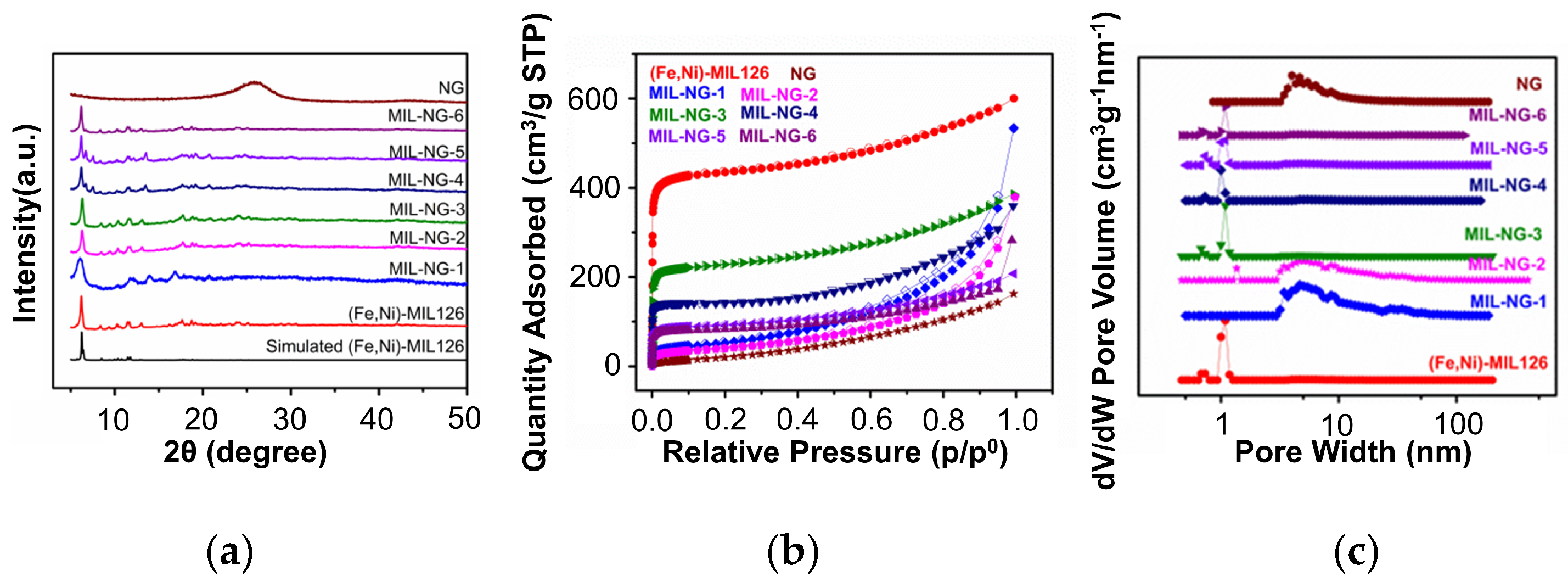
| Sample | Type of Porosity | Specific Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|---|
| NG | IV type and hysteresis loop, mesoporous | 92 | 5 |
| (Fe,Ni)-MIL-126 | I type, microporous | 1720 | 1 |
| MIL-NG-1 | IV type and hysteresis loop, mesoporous | 148 | 5 |
| MIL-NG-2 | IV type and hysteresis loop, mesoporous | 196 | 5 |
| MIL-NG-3 | I type, microporous | 884 | 1 |
| MIL-NG-4 | I type, microporous | 595 | 1 |
| MIL-NG-5 | I type, microporous | 352 | 1 |
| MIL-NG-6 | I type, microporous | 285 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.; Di Vizio, B.; Yang, J.; Patil, B.; Cattelan, M.; Agnoli, S. Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction. Nanomaterials 2024, 14, 751. https://doi.org/10.3390/nano14090751
Tang P, Di Vizio B, Yang J, Patil B, Cattelan M, Agnoli S. Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction. Nanomaterials. 2024; 14(9):751. https://doi.org/10.3390/nano14090751
Chicago/Turabian StyleTang, Panjuan, Biagio Di Vizio, Jijin Yang, Bhushan Patil, Mattia Cattelan, and Stefano Agnoli. 2024. "Fe,Ni-Based Metal–Organic Frameworks Embedded in Nanoporous Nitrogen-Doped Graphene as a Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction" Nanomaterials 14, no. 9: 751. https://doi.org/10.3390/nano14090751






