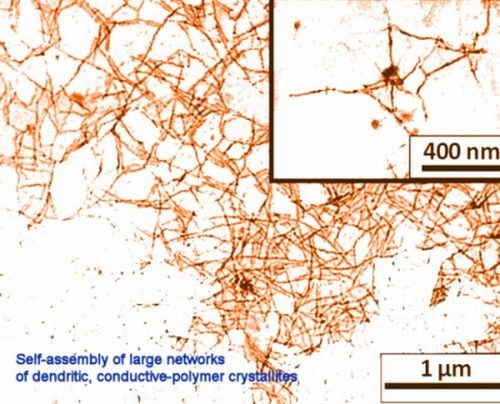
Assembl y of Poly-3-Hexylthiophene Nano-Crystallites into Low Dimensional Structures Using Indandione Derivatives
Abstract
:1. Introduction
2. Methods
2.1. Synthesis
2.2. Measurements
3. Results and Discussion
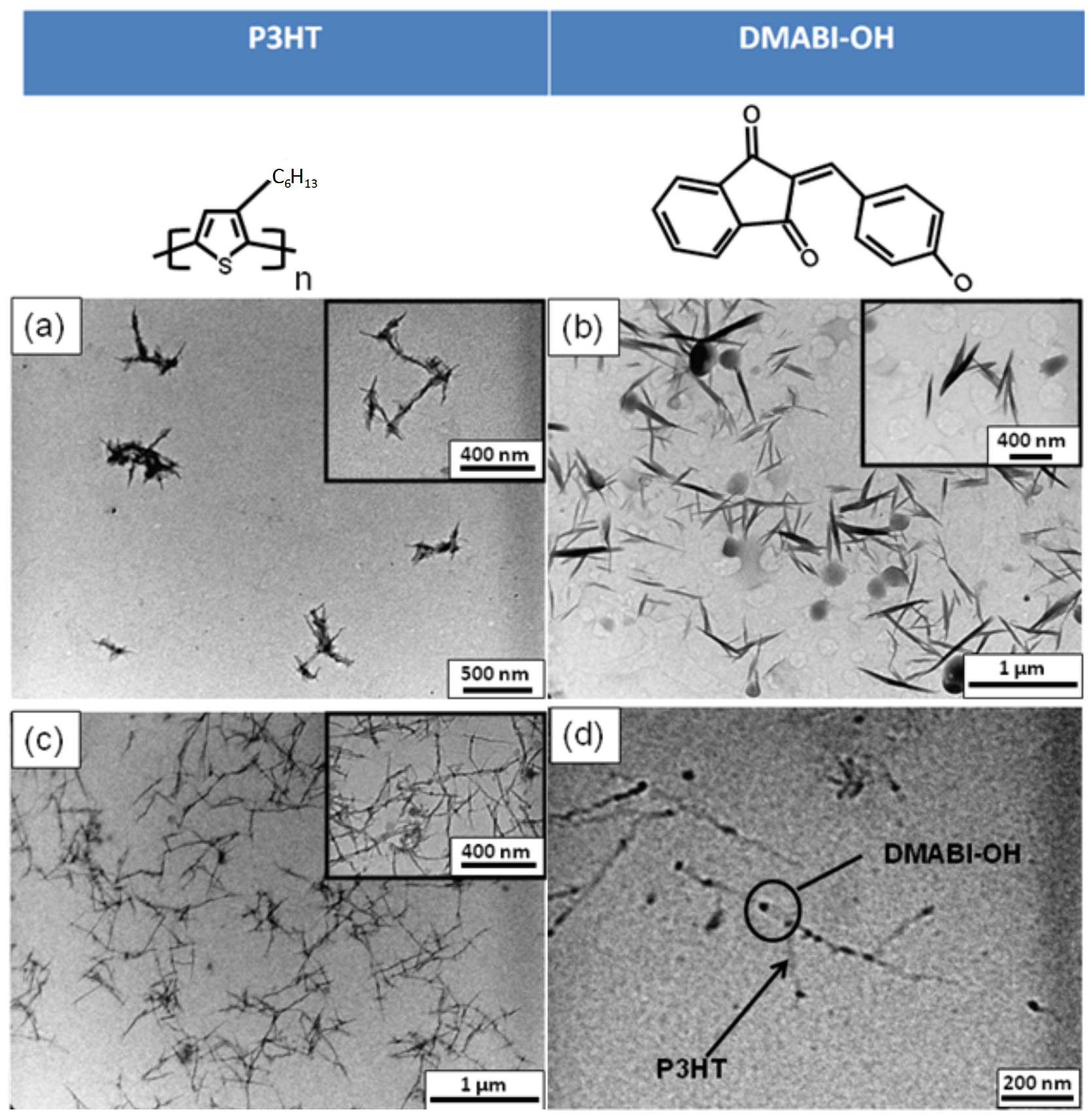

3.1. ATR-FTIR Analysis

| Sample | C=O stretch |
|---|---|
| DMABI-OH | 1669.12 cm−1 |
| P3HT- DMABI-OH | 1666.97 cm−1 |
| DMABI-Ju | 1662.34 cm−1 |
| P3HT- DMABI-Ju | 1665.25 cm−1 |
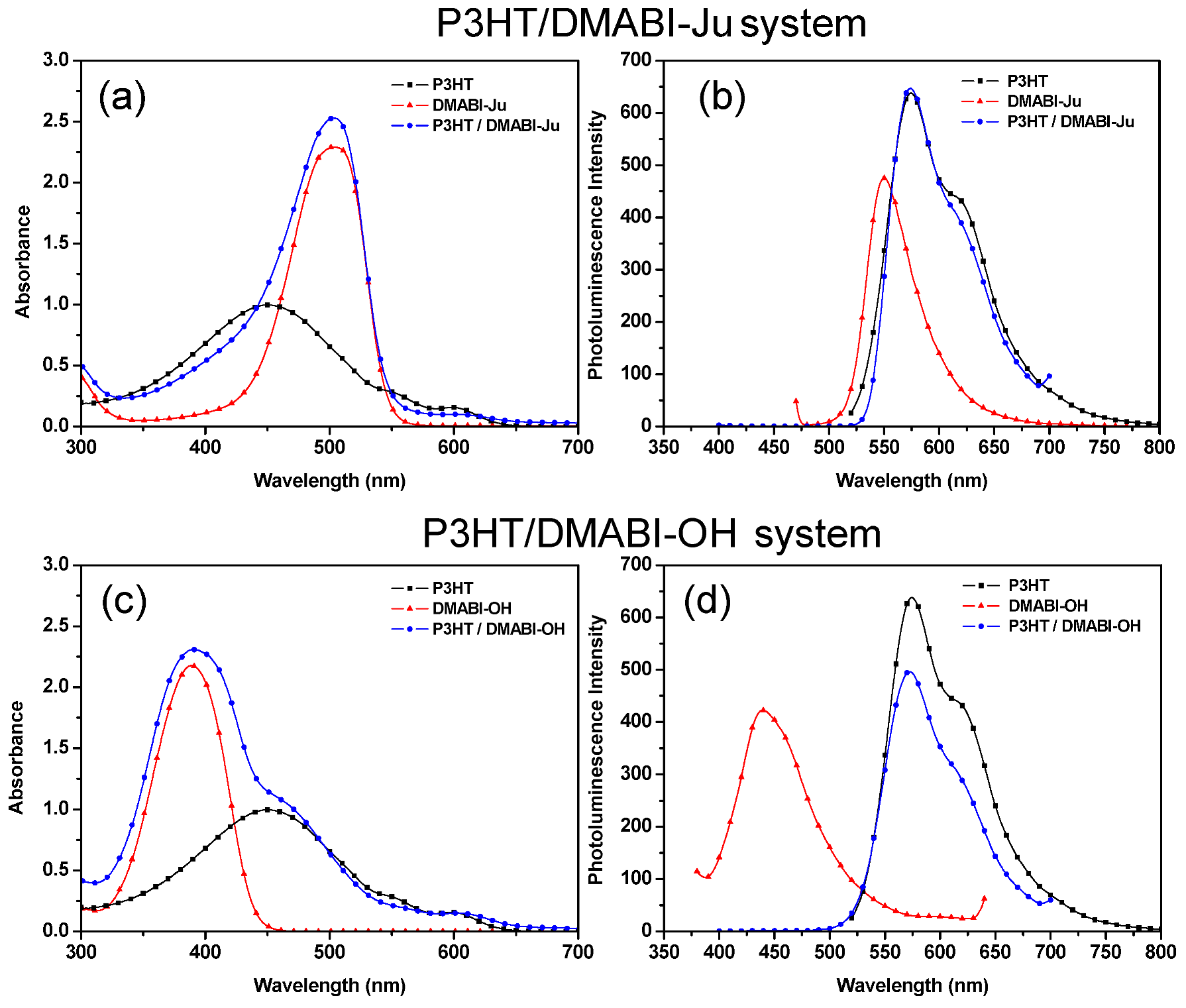
3.2. Optical Spectroscopy
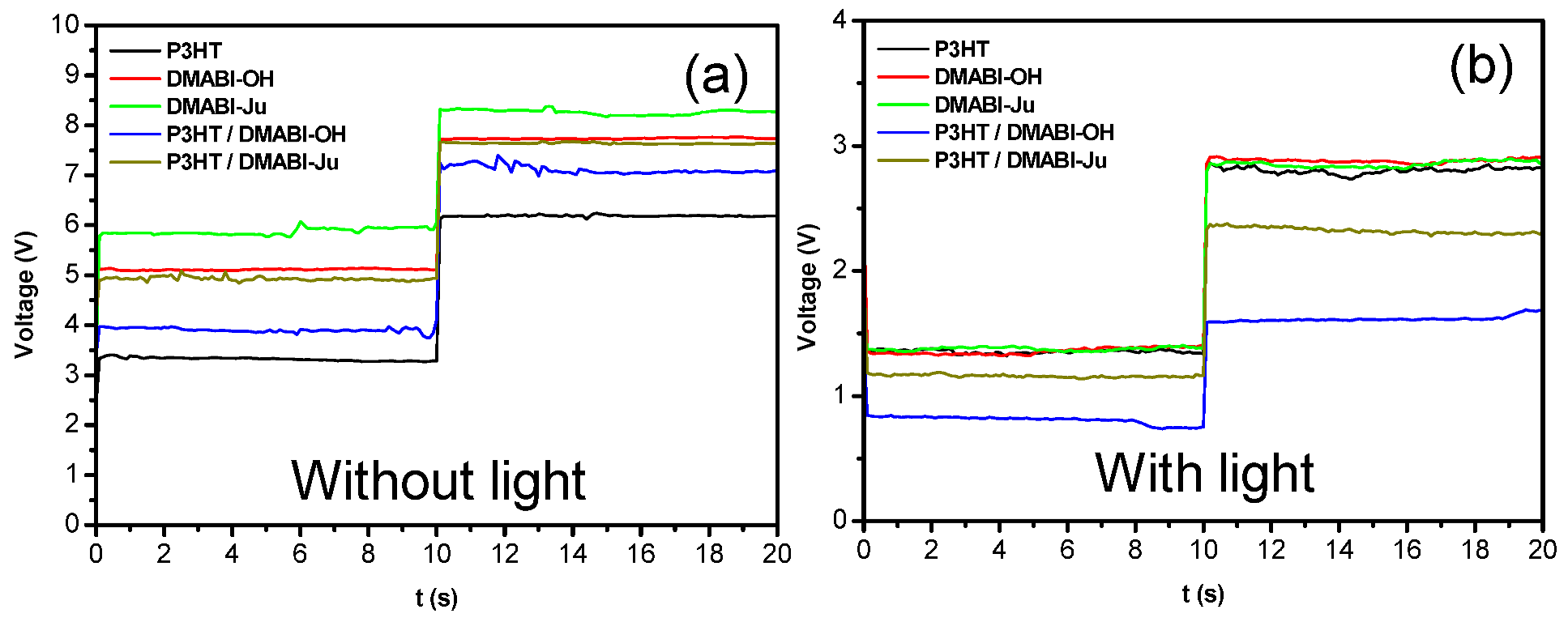
3.3. Electrical Behavior Under Illumination
4. Conclusions
Acknowledgments
Supplementary Files
Supplementary File 1References
- Lesourd, J. Solar photovoltaic systems: The economics of a renewable energy resource. Environ. Model. Softw. 2001, 16, 147–156. [Google Scholar] [CrossRef]
- Berson, S.; de Bettignies, R.; Bailly, S.; Guillerez, S. Poly(3-hexylthiophene) fibers for photovoltaic applications. Adv. Funct. Mater. 2007, 17, 1377–1384. [Google Scholar] [CrossRef]
- Nelles, D.A.; Yeo, G.W. Alternative splicing in stem cell self-renewal and differentiation. Adv. Exp. Med. Biol. 2010, 695, 92–104. [Google Scholar] [CrossRef]
- Mayer, A.C.; Scully, S.R.; Hardin, B.E.; Rowell, M.W.; Mcgehee, M.D. Polymer-based solar cells. Mater. Today 2007, 10, 28–33. [Google Scholar]
- Vanlaeke, P.; Swinnen, A.; Haeldermans, I.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; D’Haen, J.; Heremans, P.; Poortmans, J.; et al. P3HT/PCBM bulk heterojunction solar cells: Relation between morphology and electro-optical characteristics. Sol. Energy Mater. Sol. Cells 2006, 90, 2150–2158. [Google Scholar] [CrossRef]
- Canetti, M.; Bertini, F.; Scavia, G.; Porzio, W. Structural investigation on bulk poly(3-hexylthiophene): Combined SAXS, WAXD, and AFM studies. Eur. Polym. J. 2009, 45, 2572–2579. [Google Scholar] [CrossRef]
- Wu, Z.; Petzold, A.; Henze, T.; Thurn-Albrecht, T.; Lohwasser, R.H.; Sommer, M.; Thelakkat, M. Temperature and molecular weight dependent hierarchical equilibrium structures in semiconducting poly(3-hexylthiophene). Macromolecules 2010, 43, 4646–4653. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Ferenczi, T.; Agostinelli, T.; Etchegoin, P.G.; Kim, Y.; Anthopoulos, T.D.; Stavrinou, P.N.; Bradley, D.D.C.; Nelson, J. Morphology evolution via self-organization and lateral and vertical diffusion in polymer: Fullerene solar cell blends. Nat. Mater. 2008, 7, 158–164. [Google Scholar] [CrossRef]
- Yang, B.H.; Shin, T.J.; Yang, L.; Cho, K.; Ryu, C.Y.; Bao, Z. Effect of mesoscale crystalline structure on the field-effect mobility of regioregular poly(3-hexyl thiophene) in thin-film transistors. Adv. Funct. Mater. 2005, 15, 671–676. [Google Scholar] [CrossRef]
- Ravirajan, P.; Peiró, A.M.; Nazeeruddin, M.K.; Graetzel, M.; Bradley, D.D.C.; Durrant, J.R.; Nelson, J. Hybrid polymer/zinc oxide photovoltaic devices with vertically oriented ZnO nanorods and an amphiphilic molecular interface layer. J. Phys. Chem. B 2006, 110, 7635–7639. [Google Scholar]
- Lin, Y.; Lee, Y.; Chang, L.; Wu, J.; Chen, C. The influence of interface modifier on the performance of nanostructured ZnO/polymer hybrid solar cells. Appl. Phys. Lett. 2009, 94, 063308. [Google Scholar]
- Kim, Y.G.; Thompson, B.C.; Ananthakrishnan, N.; Padmanaban, G. Variable band gap conjugated polymers for optoelectronic and redox applications. J. Mater. Res. 2005, 20, 3188–3198. [Google Scholar] [CrossRef]
- Wua, M.; Liaoa, H.; Loa, H.; Chena, S.; Lina, Y.; Yenb, W.; Zenga, T.; Chena, C.; Chenc, Y.; Su, W. Nanostructured polymer blends (P3HT/PMMA): Inorganic titania hybrid photovoltaic devices. Sol. Energy Mater. Sol. Cells 2009, 93, 961–965. [Google Scholar] [CrossRef]
- Rutkis, M.; Jurgis, A.; Kampars, V.; Vembris, A.; Tokmakovs, A.; Kokars, V. Toward device applicable second order NLO polymer materials: Definition of the chromophore figure of merit. J. Phys. 2007, 93, 012028. [Google Scholar]
- Jursenas, S.; Gulbinas, V.; Kuprionis, Z.; Kananavicius, R.; Kodis, G.; Gustavsson, T.; Mialocq, J.C.; Valkunas, L. Femtosecond excited-state dynamics in N,N-dimethylaminobenzylidene-1,3-indandione (DMABI) films. Synth. Met. 2000, 109, 169–172. [Google Scholar] [CrossRef]
- Gulbinas, V.; Karpicz, R.; Muzikante, I.; Valkunas, L. Fluorescence quenching by trapped charge carriers in N,N-dimethylaminobenzylidene 1,3-indandione films. Thin Solid Films 2010, 518, 3299–3304. [Google Scholar] [CrossRef]
- Gulbinas, V.; Kodis, G.; Jursenas, S.; Valkunas, L.; Gruodis, A.; Mialocq, J.-C.; Pommeret, S.; Gustavsson, T. Charge transfer induced excited state twisting of N,N-Dimethylaminobenzylidene-1,3-indandione in solution. J. Phys. Chem. A 1999, 103, 3969–3980. [Google Scholar] [CrossRef]
- Valkunas, L.; Juodzbalis, D.; Urbas, A.; Gruodis, A.; Durandin, D.; Silinsh, E.; Klimkans, A.; Larsson, S. Visible fluorescence on IR excitation of polar dimethylaminobenzylidene 1,3-indandione crystals. Adv. Mater. Opt. Electr. 1993, 2, 221–232. [Google Scholar]
- Kodis, G.; Gulbinas, V.; Valkūnas, L.; Juršėnas, S. Non-linear luminescence of dimethylaminobenzylidene-1,3-indandione solids. Adv. Mater. Opt. Electr. 1996, 6, 391–394. [Google Scholar] [CrossRef]
- Castellano, O.; Herna, J.; Soscu, H. The topology of the charge distribution of the silanol-thiophene van der Waals complex: Ab initio and DFT study. J. Mol. Struct. 2000, 531, 315–321. [Google Scholar] [CrossRef]
- Yu, S.Y.; Garcia-Martinez, J.; Li, W.; Meitzner, G.D.; Iglesia, E. Temperature programmed desorption and infrared and X-ray absorption studies of thiophene adsorption, desorption and reactions on H-ZSM5 and Co/H-ZSM5. Phys. Chem. Chem. Phys. 2002, 4, 1241–1251. [Google Scholar] [CrossRef]
- Kucsman, A.; Kapovits, I.; Czugler, M.; Parkanyi, L.; Kalman, A. Intramolacular sulfur oxygen interaction in organosulfur compounds with different valence states- an X-ray study of methyl-2-nitrobenzene sulfenate, methyl-2-N sulfinate, M sulfonate and 2-nitrobenzenesulfenyl chloride. J. Mol. Struct. 1989, 198, 339–353. [Google Scholar] [CrossRef]
- Aida, G.-A.; Larrubia, M.A.; Ramirez, J.; Busca, G. FT-IR evidence of the interaction of benzothiophene with the hydroxyl groups of H-MFI and H-MOR zeolites. Vib. Spectrosc. 2006, 41, 42–47. [Google Scholar] [CrossRef]
- Van Bavel, S.S.; Bärenklau, M.; de With, G.; Hoppe, H.; Loos, J. P3HT/PCBM bulk heterojunction solar cells: Impact of blend composition and 3D morphology on device performance. Adv. Funct. Mater. 2010, 20, 1458–1463. [Google Scholar] [CrossRef]
- Yazawa, K.; Inoue, Y.; Shimizu, T.; Tansho, M.; Asakawa, N. Molecular dynamics of regioregular poly(3-hexylthiophene) investigated by NMR relaxation and an interpretation of temperature dependent optical absorption. J. Phys. Chem. B 2010, 114, 1241–1248. [Google Scholar]
- Xu, J.; Hu, J.; Liu, X.; Qiu, X.; Wei, Z. Stepwise self-assembly of P3HT/CdSe hybrid nanowires with enhanced photoconductivity. Macromol. Rapid Commun. 2009, 30, 1419–1423. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheval, N.; Kampars, V.; Fowkes, C.; Shirtcliffe, N.; Fahmi, A. Assembl y of Poly-3-Hexylthiophene Nano-Crystallites into Low Dimensional Structures Using Indandione Derivatives. Nanomaterials 2013, 3, 107-116. https://doi.org/10.3390/nano3010107
Cheval N, Kampars V, Fowkes C, Shirtcliffe N, Fahmi A. Assembl y of Poly-3-Hexylthiophene Nano-Crystallites into Low Dimensional Structures Using Indandione Derivatives. Nanomaterials. 2013; 3(1):107-116. https://doi.org/10.3390/nano3010107
Chicago/Turabian StyleCheval, Nicolas, Valdis Kampars, Clifford Fowkes, Neil Shirtcliffe, and Amir Fahmi. 2013. "Assembl y of Poly-3-Hexylthiophene Nano-Crystallites into Low Dimensional Structures Using Indandione Derivatives" Nanomaterials 3, no. 1: 107-116. https://doi.org/10.3390/nano3010107