DNA-Protected Silver Clusters for Nanophotonics
Abstract
:1. Introduction
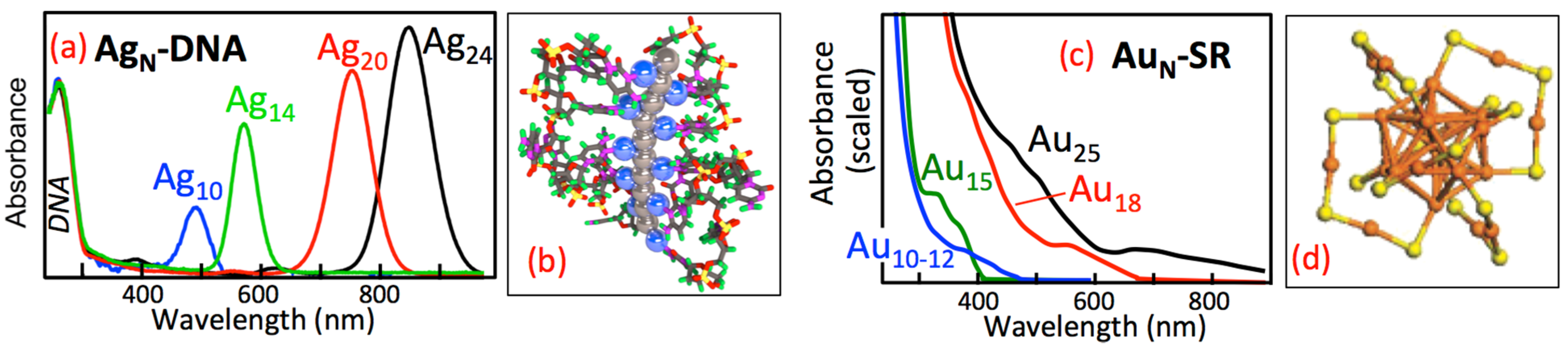
2. Results and Discussion
2.1. Isolation of Pure AgN-DNA and Identification of Composition
2.1.1. HPLC Separation of AgN-DNA and Sizing by in-Line Mass Spectrometry

2.1.2. High Resolution ESI-MS of AgN-DNA Reveals both Neutral and Cationic Silver Content
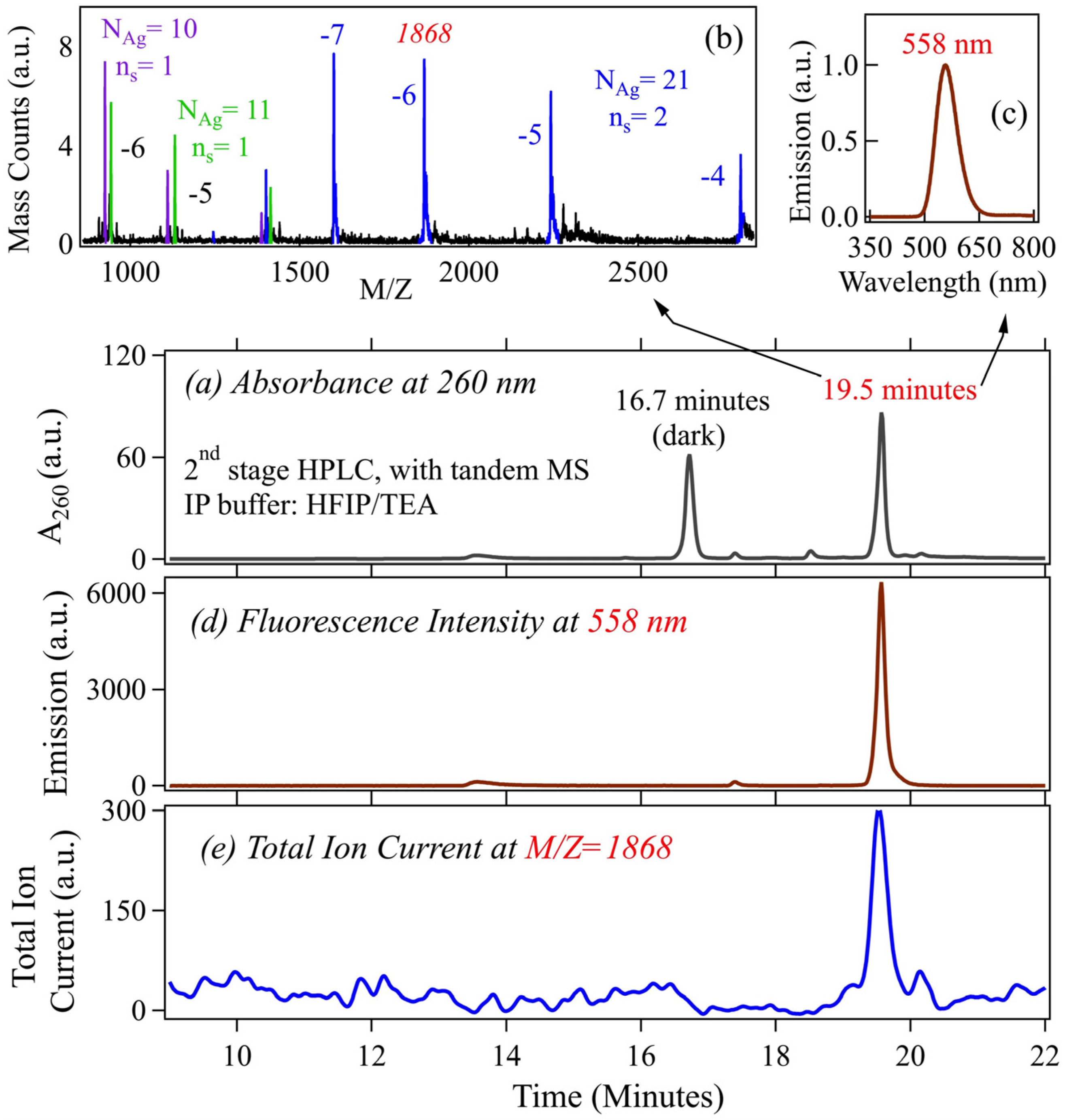
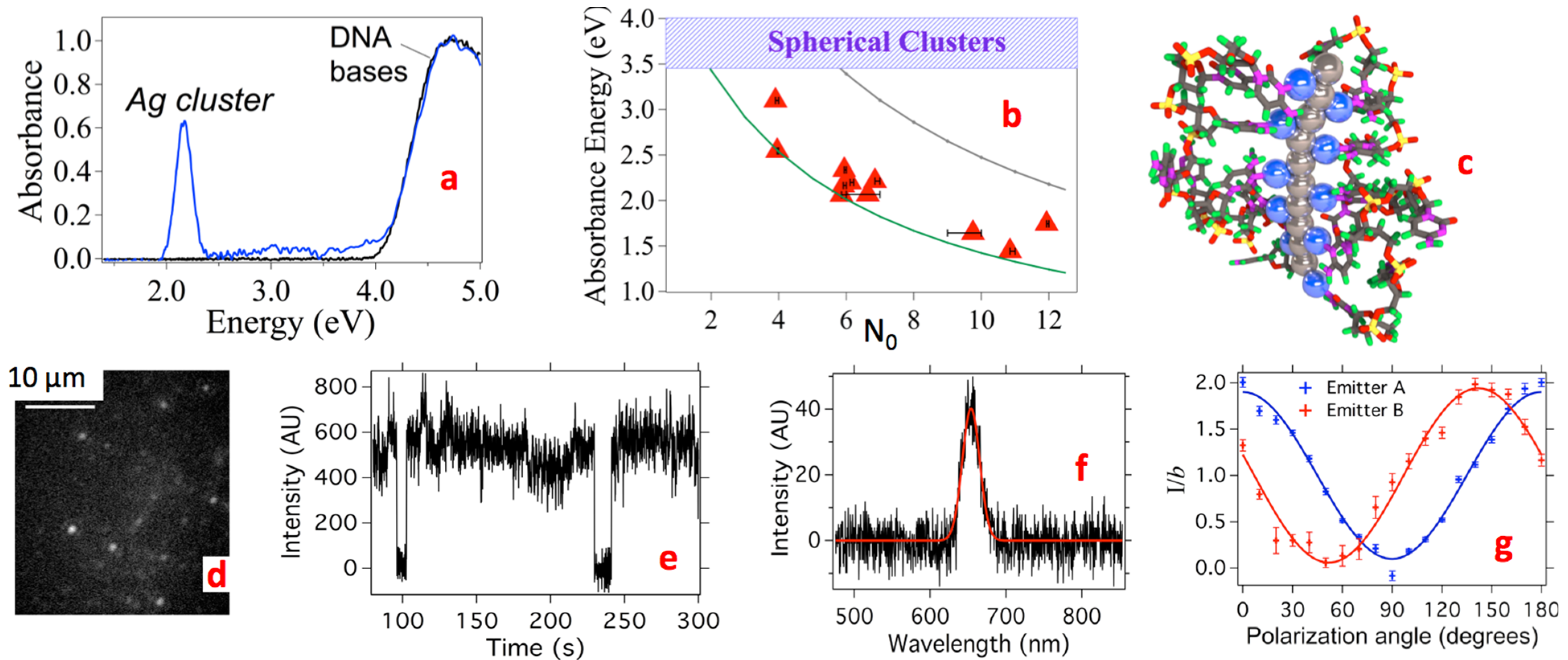
2.1.3. Universal Excitation of AgN-DNA via the Bases
2.1.4. Native Secondary Structure of DNA and AgN-DNA
2.2. Optical Properties of Pure AgN-DNA and Comparison to Other Ligand-Stabilized Metal Clusters
2.2.1. Sensitivity of Metal Cluster Optical Absorbance Spectra to Cluster Shape, Size and Composition

2.2.2. Evidence for Rod-Shaped Clusters in Fluorescent AgN-DNA
2.2.3. Magic Colors from Magic Number Cluster Sizes in AgN-DNA
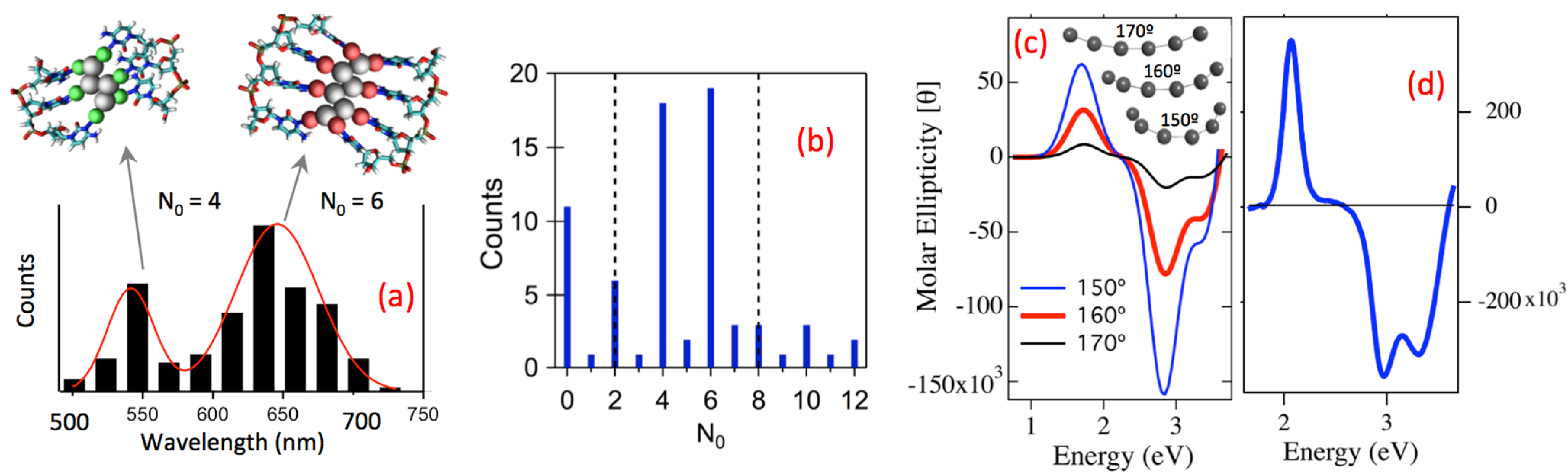
2.2.4. Chiroptical Properties of Pure AgN-DNA
2.2.5. Equilibrium between Dark and Fluorescent forms of AgN-DNA

2.2.6. Effects of Copper and Gold Reduction on Fluorescent Silver-DNA Solutions
2.2.7. The Sequence-Color Code for AgN-DNA
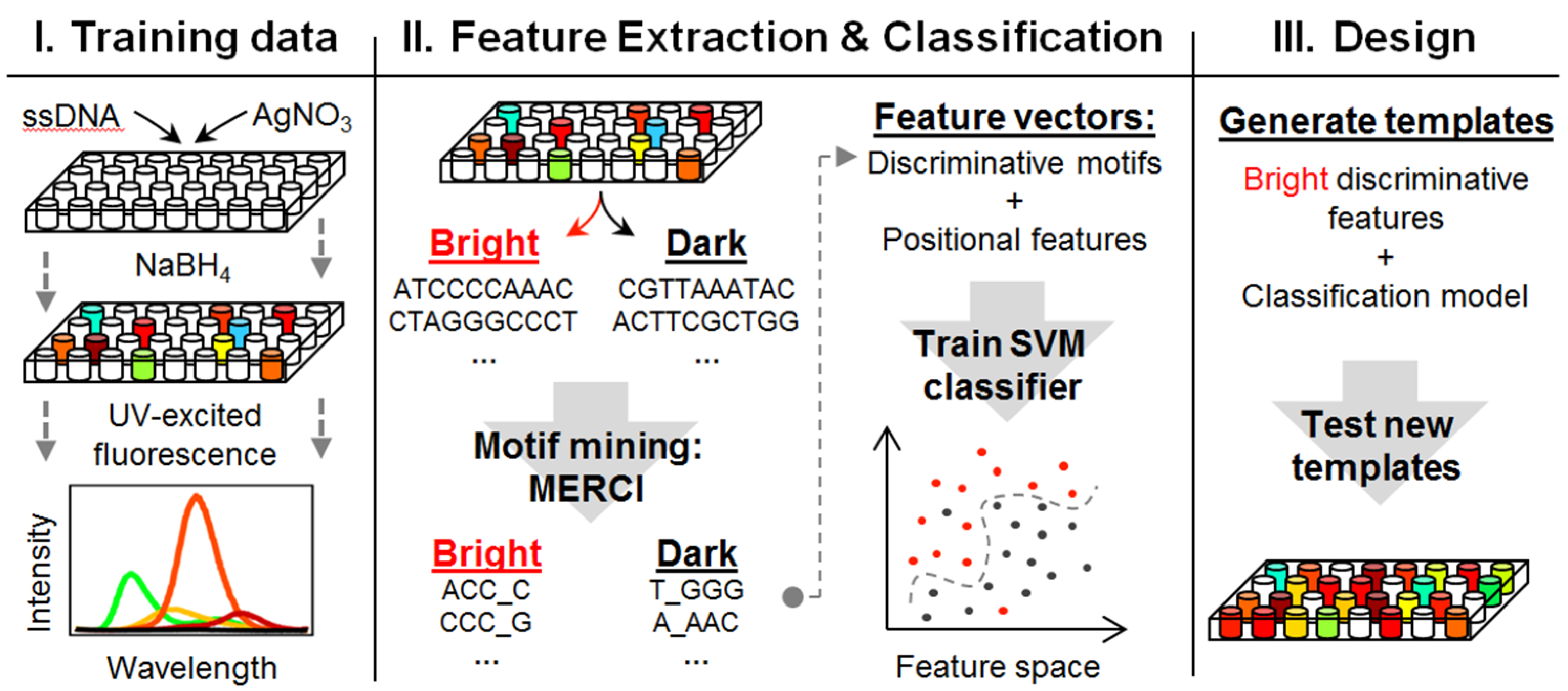
2.2.8. Stabilities of AgN-DNA
2.2.9. Electronic Properties of the Bases and AgN-DNA
2.3. AgN-DNA Assemblies
2.3.1. Bi-Color, Dual Cluster AgN-DNA Assemblies
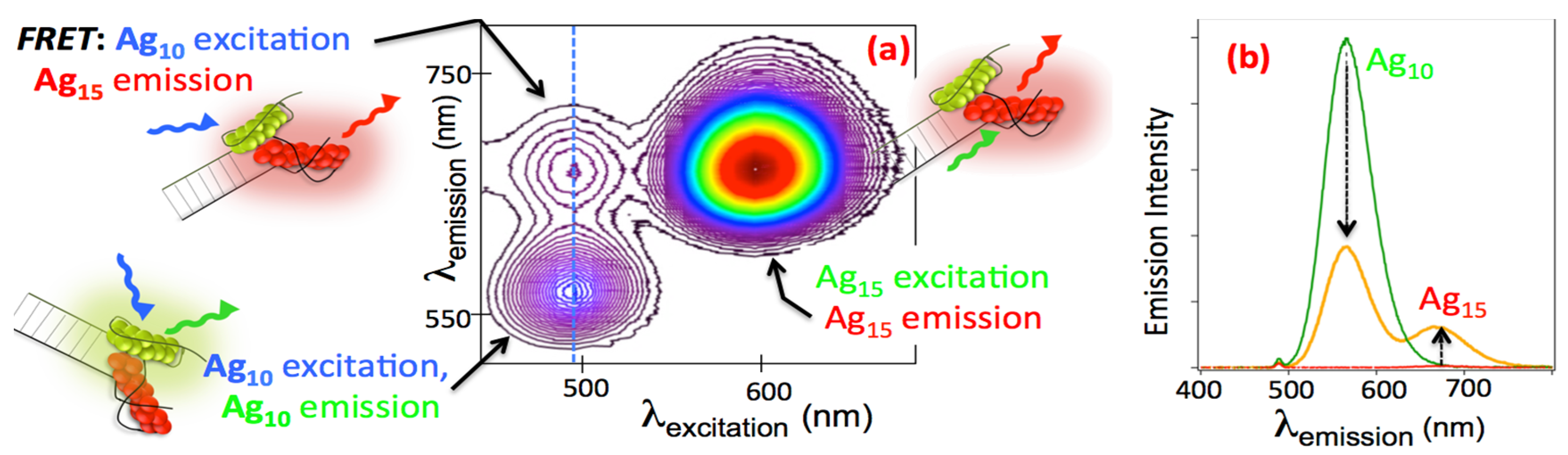
2.3.2. AgN-DNA on DNA scaffolds

3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Petty, J.T.; Zheng, J.; Hud, N.V.; Dickson, R.M. DNA-templated Ag nanocluster formation. J. Am. Chem. Soc. 2004, 126, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Choi, S.; Richards, C.I.; Antoku, Y.; Dickson, R.M. Live cell surface labeling with fluorescent Ag nanocluster composites. Photochem. Photobiol. 2008, 84, 1435. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, L.B.; Ai, J.; Dong, S.; Wang, E.K. Ion-tuned DNA/Ag fluorescent nanoclusters as versatile logic device. ACS Nano 2011, 5, 6334–6338. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yuan, J.; Wang, E.K. Strand exchange reaction modulated fluorescence “on-off” switching of hybridized DNA duplex stabilized silver nanoclusters. Chem. Comm. 2011, 47, 10930–10933. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Yuan, J.P.; Dong, Q.Z.; Wang, E.K. Highly sequence dependent formation of fluorescent silver nanoclusters in hybridized DNA duplexes for single nucleotide mutation identification. J. Am. Chem. Soc. 2010, 132, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Cui, Q.H.; Liu, G.Y.; Wu, F.; Xu, S.J.; Shao, Y. DNA abasic site-directed formation of fluorescent silver nanoclusters for selective nucleobase recognition. Nanotechnology 2011, 22, 305502–305507. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Sharma, J.; Shih, I.-M.; Vu, D.M.; Martinez, J.S.; Werner, J.H. A fluorescence light-up Ag nanocluster probe that discriminates single-nucleotide variants by emission color. J. Am. Chem. Soc. 2012, 134, 11550–11558. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Vosch, T. Rapid detection of microRNA by a silver nanocluster DNA probe. Anal. Chem. 2011, 83, 6935–6939. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Sharma, J.; Han, J.J.; Martinez, J.S.; Werner, J.H. A DNA-silver nanocluster probe that fluoresces upon hybridization. Nano Lett. 2010, 10, 3106–3110. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, Y.-C.; Li, H.-W.; Chang, H.-T. Fluorescent silver nanoclusters stabilized by DNA scaffolds. Chem. Comm. 2014, 50, 8900–9815. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Winfree, E.; Liu, F.; Wenzler, L.A.; Seeman, N.C. Design and self-assembly of two-dimensional DNA crystals. Nature 1998, 394, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.; Gardner, K.; Oemrawsingh, S.S.R.; Markešević, N.; Olsson, K.; Debord, M.; Bouwmeester, D.; Gwinn, E. Evidence for rod-shaped DNA-stabilized silver nanocluster emitters. Adv. Mater. 2013, 25, 2797–2803. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Kamat, P.V. Size-dependent excited state behavior of glutathione-capped gold clusters and their light-harvesting capacity. J. Am. Chem. Soc. 2014, 136, 11093–11099. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, H. Atomic and electronic structure of gold clusters: Understanding flakes, cages and superatoms from simple concepts. Chem. Soc. Rev. 2008, 37, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Stein, I.H.; Schüller, V.; Böhm, P.; Tinnefeld, P.; Liedl, T. Single-molecule FRET ruler based on rigid DNA origami blocks. Chemphyschem 2011, 12, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, A.; Schreiber, R.; Fan, Z.; Pardatscher, G.; Roller, E.-M.; Högele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Asenjo-Garcia, A.; Liu, Q.; Jiang, Q.; García de Abajo, F.J.; Liu, N.; Ding, B. Three-dimensional plasmonic chiral tetramers assembled by DNA origami. Nano Lett. 2013, 13, 2128–2133. [Google Scholar] [CrossRef] [PubMed]
- Pilo-Pais, M.; Watson, A.; Demers, S.; LaBean, T.H.; Finkelstein, G. Surface-enhanced raman scattering plasmonic enhancement using DNA origami-based complex metallic nanostructures. Nano Lett. 2014, 14, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, E.G.; O’Neill, P.; Guerrero, A.; Bouwmeester, D.; Fygenson, D.K. Sequence-dependent fluorescence from DNA-hosted silver nanoclusters. Adv. Mater. 2008, 20, 279–283. [Google Scholar] [CrossRef]
- Richards, C.I.; Choi, S.; Hsiang, J.C.; Antoku, Y.; Vosch, T.; Bongiorno, A.; Teng, Y.L.; Dickson, R.M. Oligonucleotide-stabilized Ag nanocluster fluorophores. J. Am. Chem. Soc. 2008, 130, 5038–5039. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, B.; Ritchie, C.M.; Buckman, J.G.; Johnsen, K.R.; Goodwin, P.M.; Petty, J.T. Base-directed formation of fluorescent silver clusters. J. Phys. Chem. C 2008, 112, 18776–18782. [Google Scholar] [CrossRef]
- Sengupta, B.; Springer, K.; Buckman, J.G.; Story, S.P.; Hasan, Z.W.; Prudowsky, Z.D.; Rudisill, S.E.; Degtyareva, N.N.; Petty, J.T. DNA templates for fluorescent silver clusters and i-motif folding. J. Phys. Chem. C 2009, 113, 19518–19524. [Google Scholar] [CrossRef]
- Schultz, D.; Gwinn, E.G. Stabilization of fluorescent silver clusters by RNA homopolymers and their DNA analogs: C,G versus A,T(U) dichotomy. Chem. Comm. 2011, 47, 4715–4718. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.M.; Schultz, D.; Swasey, S.; Pavlovich, J.; Debord, M.; Chiu, A.; Olsson, K.; Gwinn, E. Magic numbers in DNA-stabilized fluorescent silver clusters lead to magic colors. J. Phys. Chem. Lett. 2014, 5, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Jaswinder, S.; Yeh, H.C.; Yoo, H.; Werner, J.H.; Martinez, J.S. A complementary palette of fluorescent silver nanoclusters. Chem. Comm. 2010, 46, 3280. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.; Gwinn, E.G. Silver atom and strand numbers in fluorescent and dark Ag:DNAs. Chem. Comm. 2012, 48, 5748–5750. [Google Scholar] [CrossRef] [PubMed]
- Vosch, T.; Antoku, Y.; Hsiang, J.C.; Richards, C.I.; Gonzales, J.I.; Dickson, R.M. Strongly emissive individual DNA-encapsulated Ag nanoclusters as single-molecule fluorophores. Proc. Nat. Acad. Sci. USA 2007, 104, 12616–12621. [Google Scholar] [CrossRef]
- Fountain, K.J.; Gilar, M.; Gebler, J.C. Analysis of native and chemically modified oligonucleotides by tandem ion-pair reversed-phase high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Comm. Mass Spec. 2003, 17, 646–653. [Google Scholar] [CrossRef]
- Gilar, M.; Fountain, K.J.; Budman, Y.; Neue, U.D.; Yardley, K.R.; Rainville, P.D.; Russell, R.J.; Gebler, J.C. Ion-pair reversed-phase high-performance liquid chromatography analysis of oligonucleotides: Retention prediction. J. Chromat. A 2002, 958, 167–182. [Google Scholar] [CrossRef]
- Petty, J.T.; Fan, C.Y.; Story, S.P.; Sengupta, B.; Iyer, A.S.; Prudowsky, Z.; Dickson, R.M. DNA encapsulation of 10 silver atoms producing a bright, modulatable, near-infrared-emitting cluster. J. Phys. Chem. Lett. 2010, 1, 2524–2529. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.T.; Nicholson, D.A.; Sergev, O.O.; Graham, S.K. Near-infrared silver cluster optically signaling oligonucleotide hybridization and assembling two DNA hosts. Anal. Chem. 2014, 86, 9220–9228. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.T.; Giri, B.; Miller, I.C.; Nicholson, D.A.; Sergev, O.O.; Banks, T.M.; Story, S.P. Silver clusters as both chromophoric reporters and DNA ligands. Anal. Chem. 2013, 85, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y. Determination of the binding stoichiometry by the continuous variation method: The job plot. Meth. Enzymol. 1982, 87, 509–525. [Google Scholar] [PubMed]
- Ma, K.; Shao, Y.; Ciu, Q.; Wu, F.; Xu, S.; Liu, G. Base-stacking determined fluorescence emission of DNA abasic site-templated silver clusters. Langmuir 2012, 28, 15313–15322. [Google Scholar] [CrossRef] [PubMed]
- Neidig, M.L.; Sharma, J.; Yeh, H.-C.; Martinez, J.S.; Conradson, S.D.; Shreve, A.P. Ag K-edge EXAFS analysis of DNA-templated fluorescent silver nanoclusters: Insight into the structural origins of emission tuning by DNA sequence variations. J. Am. Chem. Soc. 2011, 133, 11837–11839. [Google Scholar] [CrossRef] [PubMed]
- Greig, M.J. Detection of oligonucleotide-ligand complexes by ESI-MS (DOLCE-MS) as a component of high throughput screening. J. Biomol. Screen. 2000, 5, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Hofstadler, S.; Sannes-Lowry, K. Applications of ESI-MS in drug discovery: Interrogation of non-covalent complexes. Nat. Rev. Drug Discov. 2006, 5, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Rosu, F.; Gabelica, V.; Houssier, C.; de Pauw, E. Determination of affinity, stoichiometry and sequence selectivity of minor groove binder complexes with double-stranded oligodeoxy-nucleotides by electrospray ionization mass spectrometry. Nucleic Acids Res. 2002, 30, e82. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.R.; Gwinn, E.G.; Fygenson, D.K. UV Excitation of DNA-stabilized Ag-cluster fluorescence via the DNA bases. J. Phys. Chem. C 2011, 115, 24061–24066. [Google Scholar] [CrossRef]
- Swasey, S.M.; Karimova, N.; Aikens, C.M.; Schultz, D.E.; Simon, A.J.; Gwinn, E.G. Chiral electronic transitions in fluorescent silver clusters stabilized by DNA. ACS Nano 2014, 8, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Cao, S.; Togashi, H.; Tashiro, M.; Fujimoto, T.; Machinami, T.; Oda, S.; Miyake, Y.; Okamoto, I.; Tanaka, Y. Specific interactions between silver (I) ions and cytosine-cytosine pairs in DNA duplexes. Chem. Comm. 2008, 39, 4825–4827. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.R.; Velazquez, L.R.; Dunn, D.G.; Gwinn, E.G.; Fygenson, D.K. Hairpins with poly-C loops stabilize four types of fluorescent Agn:DNA. J. Phys. Chem. C 2009, 113, 4229–4233. [Google Scholar] [CrossRef]
- Driehorst, T.; O’Neill, P.R.; Goodwin, P.J.; Pennathur, S.; Fygenson, D.K. Distinct conformations of DNA-stabilized fluorescent silver nanoclusters revealed by electrophoretic mobility and diffusivity measurements. Langmuir 2011, 77, 8923–8933. [Google Scholar] [CrossRef]
- Shah, P.; Rorvig-Lund, A.; Ben Chaabane, S.; Thulstrup, P.W.; Kjaergaard, H.G.; Fron, E.; Hofkens, J.; Yang, S.W.; Vosch, T. Design aspects of bright red emissive silver nanoclusters/DNA probes for microRNA detection. ACS Nano 2012, 6, 8803–8814. [Google Scholar] [CrossRef] [PubMed]
- De Heer, W.A. The physics of simple metal clusters: Experimental aspects and simple models. Rev. Mod. Phys. 1993, 65, 611–676. [Google Scholar] [CrossRef]
- Walter, M.; Akola, J.; Lopez-Acevedo, O.; Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Whetten, R.L.; Grönbeck, H.; Häkkinen, H. A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 9157–9162. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; Mohamed, M.B.; El-Sayed, M.A. Simulation of the optical absorption spectra of gold nanorods as a function of their aspect ratio and the effect of the medium dielectric constant. J. Phys. Chem. B 1999, 103, 3073–3077. [Google Scholar] [CrossRef]
- Yan, J.; Gao, S. Plasmon resonances in linear atomic chains: Free-electron behavior and anisotropic screening of d electrons. Phys. Rev. B 2008, 78, 235413. [Google Scholar] [CrossRef]
- Baishya, K.; Idrobo, J.; Öğüt, S.; Yang, M.; Jackson, K.; Jellinek, J. Optical absorption spectra of intermediate-size silver clusters from first principles. Phys. Rev. B 2008, 78, 075439. [Google Scholar] [CrossRef]
- Desireddy, A.; Conn, B.E.; Guo, J.; Yoon, B.; Barnett, R.N.; Monahan, B.M.; Kirschbaum, K.; Griffith, W.P.; Whetten, R.L.; Landman, U.; et al. Ultrastable silver nanoparticles. Nature 2013, 501, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Guidez, E.B.; Aikens, C.M. Theoretical analysis of the optical excitation spectra of silver and gold nanowires. Nanoscale 2012, 4, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, Z.; Chevrier, D.M.; Leong, D.T.; Zhang, P.; Jiang, D.; Xie, J. Identification of a highly luminescent Au22(SG)18 nanocluster. J. Am. Chem. Soc. 2014, 22, 5–8. [Google Scholar]
- Oemrawsingh, S.S.R.; Markešević, N.; Gwinn, E.G.; Eliel, E.R.; Bouwmeester, D. Spectral properties of individual DNA-hosted silver nanoclusters at low temperatures. J. Phys. Chem. C 2012, 116, 25568–25575. [Google Scholar] [CrossRef]
- Lermé, J.; Lyon, D.; Kastler, A. Size evolution of the surface plasmon resonance damping in silver nanoparticles : Confinement and dielectric effects. J. Phys. Chem. C 2011, 115, 14098–14110. [Google Scholar] [CrossRef]
- Weick, G.; Molina, R.; Weinmann, D.; Jalabert, R. Lifetime of the first and second collective excitations in metallic nanoparticles. Phys. Rev. B 2005, 72, 115410. [Google Scholar] [CrossRef]
- Markešević, N.; Oemrawsingh, S.S.R.; Schultz, D.; Gwinn, E.G.; Bouwmeester, D. Polarization resolved measurements of individual DNA-stabilized silver clusters. Adv. Opt. Mater. 2014, 2, 765–770. [Google Scholar] [CrossRef]
- Walter, M.; Whetten, R.L.; Ha, H.; Gro, H. On the Structure of Thiolate-Protected Au 25. J. Am. Chem. Soc. 2008, 130, 3756–3757. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Rocha, R.C.; Phipps, M.L.; Yeh, H.C.; Balatsky, K.A.; Vu, D.M.; Shreve, A.P.; Werner, J.H.; Martinez, J.S. A DNA-templated fluorescent silver nanocluster with enhanced stability. Nanoscale 2012, 4, 4107–4110. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.Y.; Chen, W.Y.; Chang, H.T. One-pot synthesis of fluorescent oligonucleotide Ag nanoclusters for specific and sensitive detection of DNA. Biosens. Bioelectron. 2011, 26, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Dolamic, I.; Knoppe, S.; Dass, A.; Bürgi, T. First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands. Nat. Commun. 2012, 3, 798. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.T.; Sergev, O.O.; Nicholson, D.A.; Goodwin, P.M.; Giri, B.; McMullan, D.R. A silver cluster-DNA equilibrium. Anal. Chem. 2013, 85, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.Y.; Chen, W.Y.; Chang, H.T. Characterization and application to the detection of single-stranded DNA binding protein of fluorescent DNA-templated copper/silver nanoclusters. Analyst 2011, 136, 3623–3628. [Google Scholar] [CrossRef] [PubMed]
- Son, I.; Shek, Y.L.; Dubins, D.N.; Chalikian, T.V. Hydration changes accompanying helix-to-coil DNA transitions. J. Am. Chem. Soc. 2014, 136, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Spink, C.H.; Garbett, N.; Chaires, J.B. Enthalpies of DNA melting in the presence of osmolytes. Biophys. Chem. 2007, 126, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Lan, G.-Y.; Chang, H.-T. Use of fluorescent DNA-templated gold/silver nanoclusters for the detection of sulfide ions. Anal. Chem. 2011, 83, 9450–9455. [Google Scholar] [CrossRef] [PubMed]
- Berti, L.; Burley, G.A. Nucleic acid and nucleotide-mediated synthesis of inorganic nanoparticles. Nat. Nanotechnol. 2008, 3, 81–87. [Google Scholar] [CrossRef]
- Wang, S.X.; Meng, X.M.; Das, A.; Li, T.; Song, Y.B.; Cao, T.T.; Zhu, X.Y.; Zhu, M.Z.; Jin, R.C. A 200-fold quantum yield boost in the photoluminescence of silver-doped Ag(x)Au(25 − x) nanoclusters: The 13th silver atom matters. Angew. Chem. Int. Ed. 2014, 53, 2376–2380. [Google Scholar] [CrossRef]
- Copp, S.M.; Bogdanov, P.; Debord, M.; Singh, A.; Gwinn, E. Base motif recognition and design of DNA templates for fluorescent silver clusters by machine learning. Adv. Mater. 2014, 26, 5839–5845. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar]
- Bradford, J.R.; Westhead, D.R. Improved prediction of protein-protein binding sites using a support vector machines approach. Bioinformatics 2005, 21, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.P.S.; Grundy, W.N.; Lin, D.; Cristianini, N.; Sugnet, C.W.; Furey, T.S.; Ares, M.; Haussler, D. Knowledge-based analysis of microarray gene expression by using support vector machines. Proc. Natl. Acad. Sci. USA 2000, 97, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Vens, C.; Rosso, M.N.; Danchin, E.G.J. Identifying discriminative classification-based motifs in biological sequences. Bioinformatics 2011, 27, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Walczak, S.; Morishita, K.; Ahmed, M.; Liu, J. Towards understanding of poly-guanine activated fluorescent silver nanoclusters. Nanotechnology 2014, 25, 155501–155510. [Google Scholar] [CrossRef]
- Morishita, K.; MacLean, J.L.; Liu, B.; Jiang, H.; Liu, J. Correlation of photobleaching, oxidation and metal induced fluorescence quenching of DNA-templated silver nanoclusters. Nanoscale 2013, 5, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, R.; Kononov, A. Excitation spectra argue for threadlike shape of DNA-stabilized silver fluorescent clusters. J. Phys. Chem. C 2013, 117, 18681–18687. [Google Scholar] [CrossRef]
- Guo, R.; Murray, R.W.; Hill, C.; Carolina, N. Substituent effects on redox potentials and optical gap energies of molecule-like Au38(SPhX)24 nanoparticles. J. Am. Chem. Soc. 2005, 38, 12140–12143. [Google Scholar] [CrossRef]
- Yin, P.; Hariadi, R.F.; Sahu, S.; Choi, H.M. T.; Park, S.H.; Labean, T.H.; Reif, J.H. Programming DNA tube circumferences. Science 2008, 321, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Schade, N.B.; Sun, L.; Fan, J.A.; Bae, D.R.; Mariscal, M.M.; Lee, G.; Capasso, F.; Sacanna, S.; Manoharan, V.N.; et al. Ultrasmooth, highly spherical monocrystalline gold particles for precision plasmonics. ACS Nano 2013, 7, 11064–11070. [Google Scholar] [CrossRef] [PubMed]
- Guticrez, M.; Henglein, A. Formation of colloidal silver by “push-pull” reduction of Ag+. J. Phys. Chem. 1993, 97, 11368–11370. [Google Scholar] [CrossRef]
- Schultz, D.; Copp, S.M.; Markešević, N.; Gardner, K.; Oemrawsingh, S.S.R.; Bouwmeester, D.; Gwinn, E. Dual-color nanoscale assemblies of structurally stable, few-atom silver clusters, as reported by fluorescence resonance energy transfer. ACS Nano 2013, 7, 9798–9807. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.R.; Young, K.; Schiffels, D.; Fygenson, D.K. Few-atom fluorescent silver clusters assemble at programmed sites on DNA nanotubes. Nano Lett. 2012, 12, 5464–5469. [Google Scholar] [CrossRef] [PubMed]
- Pilo-Pais, M.; Goldberg, S.; Samano, E.; Labean, T.H.; Finkelstein, G. Connecting the nanodots: Programmable nanofabrication of fused metal shapes on DNA templates. Nano Lett. 2011, 11, 3489–3492. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Guo, W.W.; Wang, F.; Lioubashevski, O.; Wilner, I. Self-assembly of luminescent Ag nanocluster-functionalized nanowires. Langmuir 2013, 29, 13066–13071. [Google Scholar] [CrossRef]
- Guo, W.; Orbach, R.; Mironi-Harpaz, I.; Seliktar, D.; Wilner, I. Fluorescent hydrogels composed of nucleic acid-stabilized silver nanoclusters. Small 2013, 9, 3748–3752. [Google Scholar] [CrossRef] [PubMed]
- Nilius, N.; Wallis, T.M.; Ho, W. Development of one-dimensional band structure in artificial gold chains. Science 2002, 297, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwinn, E.; Schultz, D.; Copp, S.M.; Swasey, S. DNA-Protected Silver Clusters for Nanophotonics. Nanomaterials 2015, 5, 180-207. https://doi.org/10.3390/nano5010180
Gwinn E, Schultz D, Copp SM, Swasey S. DNA-Protected Silver Clusters for Nanophotonics. Nanomaterials. 2015; 5(1):180-207. https://doi.org/10.3390/nano5010180
Chicago/Turabian StyleGwinn, Elisabeth, Danielle Schultz, Stacy M. Copp, and Steven Swasey. 2015. "DNA-Protected Silver Clusters for Nanophotonics" Nanomaterials 5, no. 1: 180-207. https://doi.org/10.3390/nano5010180





