Application of L-Aspartic Acid-Capped ZnS:Mn Colloidal Nanocrystals as a Photosensor for the Detection of Copper (II) Ions in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of the ZnS:Mn-Asp NCs
2.2. Surface Properties of the ZnS:Mn-Asp NCs
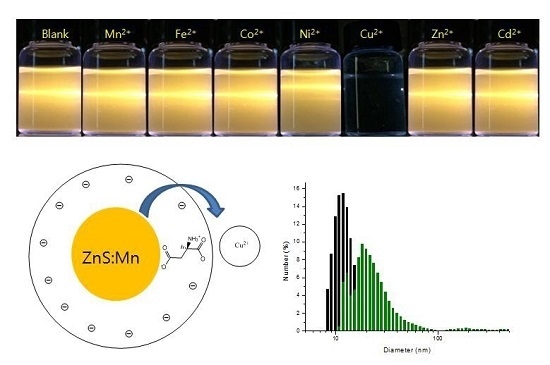
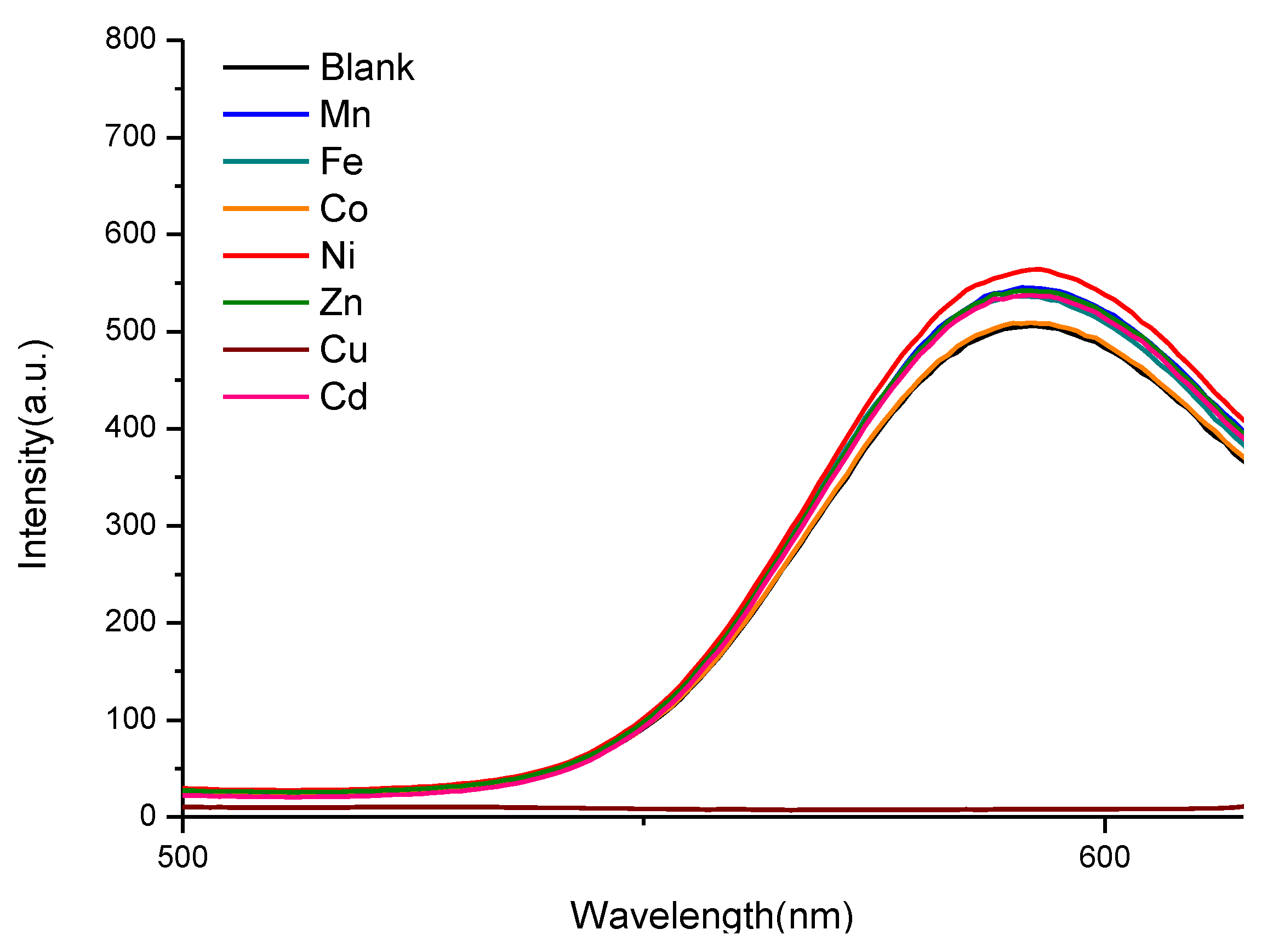
2.3. Photosensor Activity of the ZnS:Mn-Asp NCs
3. Experiemntal
3.1. Instrumentation
3.2. Synthesis of the ZnS:Mn-Asp NCs
3.3. PL Efficiency Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schimpf, A.M.; Knowles, K.E.; Carroll, G.M.; Gamelin, D.R. Electronic Doping and Redox-Potential Tuning in Colloidal Semiconductor Nanocrystals. Acc. Chem. Res. 2015, 48, 1929–1927. [Google Scholar] [CrossRef] [PubMed]
- Senden, T.; Rabouw, F.T.; Meijerink, A. Photonic Effects on the Radiative Decay Rate and Luminescence Quantum Yield of Doped Nanocrystals. ACS Nano 2015, 9, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, S.; Lienau, C.; Grunwald, R. Progress in Nonliear. Nano-Optics; Springer: New York, NY, USA, 2015. [Google Scholar]
- Elumalai, N.K.; Vijila, C.; Jose, R.; Uddin, A.; Ramakrishina, S. Metal oxide semiconducting interfacial layers for photovoltaic and photocatalytic applications. Mater. Renew. Sustain. Energy 2015, 4, 11–25. [Google Scholar] [CrossRef]
- Bhakta, S.A.; Evans, E.; Beavidez, T.E.; Garcia, D. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta 2015, 872, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Chang, E.; Drezek, R.; Colvin, V.L. Water-soluble quantum dots for biomedical applications. Biochem. Biophys. Res. Commun. 2006, 348, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Chandrakar, R.K.; Baghel, R.N.; Chandra, B.P. Synthesis, characterization and thermoluminescence studies of Mn-doped ZnS nanoparticles. Luminescence 2015, 31, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Ananan, S.; Masten, S.J.; Wu, J.J. Photocatalytic hydrogen evolution from water splitting using Cu doped ZnS microspheres under visible light irradiation. Renew. Energy 2015, 89, 18–26. [Google Scholar] [CrossRef]
- Tiwari, N.; Mehto, V.R.; Nishad, K.K.; Singh, P.P.; Pandey, R.K. Vertically Aligned ZnO and ZnS Quantum Dot Based Hybrid White LED. J. Nanoelectron. Optoelectron. 2015, 7, 28–34. [Google Scholar] [CrossRef]
- Lakshmi, P.V.B.; Raj, K.S.; Ramachandran, K. Synthesis and characterization of nano ZnS doped with Mn. Cryst. Res. Technol. 2009, 44, 153–158. [Google Scholar] [CrossRef]
- Han, S.D.; Singh, K.C.; Lee, H.S.; Cho, T.Y.; Hulme, J.P.; Han, C.H.; Chun, I.S.; Gwak, J. Synthesis and photoluminescence properties of Mn2+ doped ZnS nano-crystals with surface passivation. Mater. Chem. Phys. 2008, 112, 1083–1087. [Google Scholar] [CrossRef]
- Suyver, J.F.; Wuister, S.F.; Kelly, J.J.; Meijerink, A. Synthesis and Photoluminescence of Nanocrystalline ZnS:Mn2+. Nano Lett. 2001, 1, 429–433. [Google Scholar] [CrossRef]
- Somers, R.C.; Bawendi, M.G.; Nocera, D.G. CdSe nanocrystal based chem-/bio- sensors. Chem. Soc. Rev. 2007, 36, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, V.; Chibli, H.; Fiammengo, R.; Galeone, A.; Malvindi, M.A.; Vecchio, G.; Cingolani, R.; Nadeau, J.L.; Pompa, P.P. InP/ZnS as a safer alternative to CdSe/ZnS core/shell quantum dots: In vitro and in vivo toxicity assessment. Nanoscale 2013, 5, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Aldana, J.; Wang, A.; Peng, X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 2001, 123, 8844–8850. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, Y.; Ding, Y.; Lu, Y.; Zhang, F. Application of thioglycolic acid capped nano-ZnS as a fluorescence probe for the determination of nevirapine. Anal. Methods 2012, 4, 4213–4219. [Google Scholar] [CrossRef]
- Kong, H.Y.; Hwang, C.S.; Byun, J. Biological Toxicity Changes of Mercaptoacetic Acid and Mercaptopropionic Acid Upon Coordination onto ZnS:Mn Nanocrystal. Bull. Korean Chem. Soc. 2012, 33, 657–662. [Google Scholar] [CrossRef]
- Park, S.; Song, B.; Kong, H.Y.; Byun, J.; Hwang, C.S. Biological Toxicities and Aggregation Effects of L-Glycine and L-Alanine Capped ZnS:Mn Nanocrystals in Aqueous Solution. Bull. Korean Chem. Soc. 2014, 35, 1169–1176. [Google Scholar] [CrossRef]
- Augustine, M.S.; Anas, A.; Das, A.V.; Sreekanth, S.; Jayalekshmi, S. Cytotoxicity and cellular uptake of ZnS:Mn nanocrystals biofunctionalized with chitosan and aminoacids. Spectrochem. Chim. Acta A 2015, 136, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Trinchi, A.; Hardin, S.G.; Cole, I.S. Fluorescent heavy metal cation sensing with water dispersible 2MPA capped CdSe/ZnS quantum dots. J. Lumin. 2015, 166, 88–92. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Bosco, J.; Rayappan, B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Heo, J.; Hwang, C.S. Application of the Water-Dispersible ZnS:Mn Nanocrystal as an Effective and Convenient Photosensor Material for the Detection of Zn2+ and Cd2+ ions in Aqueous Solution. Bull. Korean Chem. Soc. 2015, 36, 2411–2412. [Google Scholar] [CrossRef]
- Bhargava, R.N.; Gallagher, D.; Hong, X.; Nurmikko, A. Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett. 1994, 72, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Borse, P.H.; Srinivas, D.; Shinde, R.F.; Date, S.K.; Vogel, W.; Kulkarni, S.K. Effect of Mn 2+ concentration in ZnS nanoparticles on photoluminescence and electron-spin-resonance spectra. Phys. Rev. B 1999, 60, 8659–8665. [Google Scholar] [CrossRef]
- Kushida, T.; Tanaka, Y.; Oka, Y. Excited-state absorption spectra of ZnS:Mn. Solid State Commun. 1974, 14, 617–620. [Google Scholar] [CrossRef]
- Yu, I.; Isobe, T.; Senna, M. Optical properties and characteristics of ZnS nano-particles with homogeneous Mn distribution. J. Phys. Chem. Solids 1996, 57, 373–379. [Google Scholar] [CrossRef]
- Karar, N.; Singh, F.; Mehta, B.R. Structure and photoluminescence studies on ZnS:Mn nanoparticles. J. Appl. Phys. 2004, 95, 656–660. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Meech, S.R.; Philips, D. Photophysics of some common fluorescence standards. J. Photochem. 1983, 23, 193–217. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.A.; Kim, K.M.; Huh, Y.D.; Hyun, J.W.; Kim, H.S.; Noh, S.J.; Hwang, C.S. Syntheses and Optical Properties of the Water-Dispersible ZnS:Mn Nanocrystals Surface Capped by L-Aminoacid Ligands: Arginine, Cysteine, Histidine, and Methionine. Bull. Korean Chem. Soc. 2007, 28, 1091–1096. [Google Scholar]
- International Union of Crystallography. International Tables for X-ray Crystallography, Part III; Kynock Press: Dordrecht, The Netherlands, 1985; pp. 318–320. [Google Scholar]
- Jones, F.W. The measurements of particle size by the X-ray method. Proc. R. Soc. Lond. Ser. A 1938, 166, 16–43. [Google Scholar] [CrossRef]
- Navarrete, J.T.L.; Hernandez, B.; Ramirez, F.J. IR and Raman spectra of L-aspartic acid and isotopic derivatives. Biopolymers 1994, 34, 1065–1077. [Google Scholar] [CrossRef]
- Rajkumar, B.J.M.; Ramakrishnan, V.; Rajaram, R.K. Infrared and Raman spectra of dl-aspartic acid nitrate monohydrate. Spectrochim. Acta A 1998, 54, 1527–1532. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Prell, J.S.; Steill, J.D.; Oomens, J.; Williams, E.R. Interactions of Mono- and Divalent Metal Ions with Aspartic and Glutamic Acid Investigated with IR Photodissociation Spectroscopy and Theory. J. Phys. Chem. A 2008, 112, 10823–10830. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; John Wiley & Son: New York, NY, USA, 1997; pp. 350–358. [Google Scholar]
- Roddick-Lanzilotta, A.D.; McQuillan, A.J. An in situ Infrared Spectroscopic Study of Glutamic Acid and of Aspartic Acid Adsorbed on TiO2: Implications for the Biocompatibility of Titanium. J. Colloid Interface Sci. 2000, 227, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, R. Particle Characterization: Light Scattering Methods; Kluwar Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 289–340. [Google Scholar]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering; Dover Publications Inc.: New York, NY, USA, 2000; pp. 223–276. [Google Scholar]
- Heo, J.; Hwang, C.S. Surface Properties and Photocatalytic Activities of the Colloidal ZnS:Mn Nanocrystals Prepared at Various pH Conditions. Nanomaterials 2015, 5, 1955–1970. [Google Scholar] [CrossRef]
- Bo, C.; Ping, Z. A new determining method of copper(II) ions at ng·mL−1 levels based on quenching of the water-soluble nanocrystals fluorescence. Anal. Bioanal. Chem. 2005, 381, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Keizer, J. Nonlinear fluorescence quenching and the origin of positive curvature in Stern-Volmer plots. J. Am. Chem. Soc. 1983, 105, 1494–1498. [Google Scholar] [CrossRef]
- Desilets, D.J.; Kissinger, P.T.; Lytle, F.E. Improved method for determination of Stern-Volmer quenching constants. Anal. Chem. 1987, 59, 1244–1246. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, T.; Wang, S.; Hou, X. Semiconductor quantum dots-based metal ion probes. Nanoscale 2014, 6, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Isarov, A.V.; Chrysochoos, J. Optical and photochemical properties of nonstoichiometric cadmium sulfide nanoparticles: Surface modification with copper (II) ions. Langmuir 1997, 13, 3142–3149. [Google Scholar] [CrossRef]
- Chen, J.L.; Zheng, A.F.; Gao, Y.C.; He, C.Y.; Wu, G.H.; Chen, Y.C.; Kai, X.M.; Zhu, C.Q. Functionalized CdS quantum dots-based luminescence probe for detection of heavy and transition metal ions in aqueous solution. Spectrochim. Acta A 2008, 69, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Irving, H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Xie, H.-Y.; Liang, J.-G.; Zhang, Z.-L.; Liu, Y.; He, Z.-K.; Pang, D.-W. Luminescent CdSe-ZnS quantum dots as selective Cu2+ probe. Spectrochim. Acta A 2004, 60, 2527–2530. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Z.; Chi, X.; Gao, J. Facile, sensitive, and ratiometric detection of mercuric ions using GSH-capped semiconductor quantum dots. Analyst 2013, 138, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rozenweig, Z. Luminescent CdS quantum dot as selective ion probes. Anal. Chem. 2002, 74, 5132–5138. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Lee, N.; Kim, Y.A.; Park, Y.B. Synthesis of the Water Dispersible L-Valine Capped ZnS:Mn Nanocrystal and the Crystal Structure of the Precursor Complex: [Zn(Val)2(H2O)]. Bull. Korean Chem. Soc. 2006, 27, 1809–1814. [Google Scholar]
- Antolini, L.; Marcotrigiano, G.; Menabue, L.; Pellacani, G.C.; Saldini, M. Thermal, spectroscopic, magnetic, and structural properties of mixed-ligand complexes of copper(II) with L-aspartic acid and amines. Crystal and molecular structure of (L-aspartato)(imidazole)copper(II) dihydrate. Inorg. Chem. 1982, 21, 2263–2267. [Google Scholar] [CrossRef]









| ZnS:Mn-Asp | Free L-Asp [33] | Assignments |
|---|---|---|
| Not appeared | 479 | δ(N–H) |
| Not appeared | 525 | δ(C–C–O) |
| 658 | 643 | δ(O–C–O) |
| 874 | 876 | ω(C–C) |
| 899 | 894 | ρ(C–C) |
| 1047 | 1046 | ν(C–N) |
| 1152 | 1153 | ρ(N–H) |
| 1248 | 1257 | ν(C–H) |
| 1310 | 1312 | δ(O–H) |
| 1420 | 1422 | ρ(C–H) |
| 1504 | 1514 | ν(OCO) + δ(C–H) |
| 1641 | Not appeared | ν(C=O–M) |
| 1717 | 1736 | ν(C=O) |
| 2950 | 3013 | ν(N–H) |
| 3350 | 3434 | ν(O–H, H2O) |
| Methods | Data |
|---|---|
| UV-VIS absorption (λmax, nm) | 320 |
| PL emission (λmax, nm)PL efficiency (%) | 5909.81 |
| concentration Mn dopant, ICP-AES (%) | 1.70 |
| Average Particle Size, HR-TEM (nm) | 5.25 |
| Average Particle Size, XRD (nm) | 5.41 |
| Zeta Potential, Zeta-PSA (mV) | −4.58 |
| Average size of aggregates in water, DLS (nm) | 19.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, J.; Hwang, C.-S. Application of L-Aspartic Acid-Capped ZnS:Mn Colloidal Nanocrystals as a Photosensor for the Detection of Copper (II) Ions in Aqueous Solution. Nanomaterials 2016, 6, 82. https://doi.org/10.3390/nano6050082
Heo J, Hwang C-S. Application of L-Aspartic Acid-Capped ZnS:Mn Colloidal Nanocrystals as a Photosensor for the Detection of Copper (II) Ions in Aqueous Solution. Nanomaterials. 2016; 6(5):82. https://doi.org/10.3390/nano6050082
Chicago/Turabian StyleHeo, Jungho, and Cheong-Soo Hwang. 2016. "Application of L-Aspartic Acid-Capped ZnS:Mn Colloidal Nanocrystals as a Photosensor for the Detection of Copper (II) Ions in Aqueous Solution" Nanomaterials 6, no. 5: 82. https://doi.org/10.3390/nano6050082