Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating
Abstract
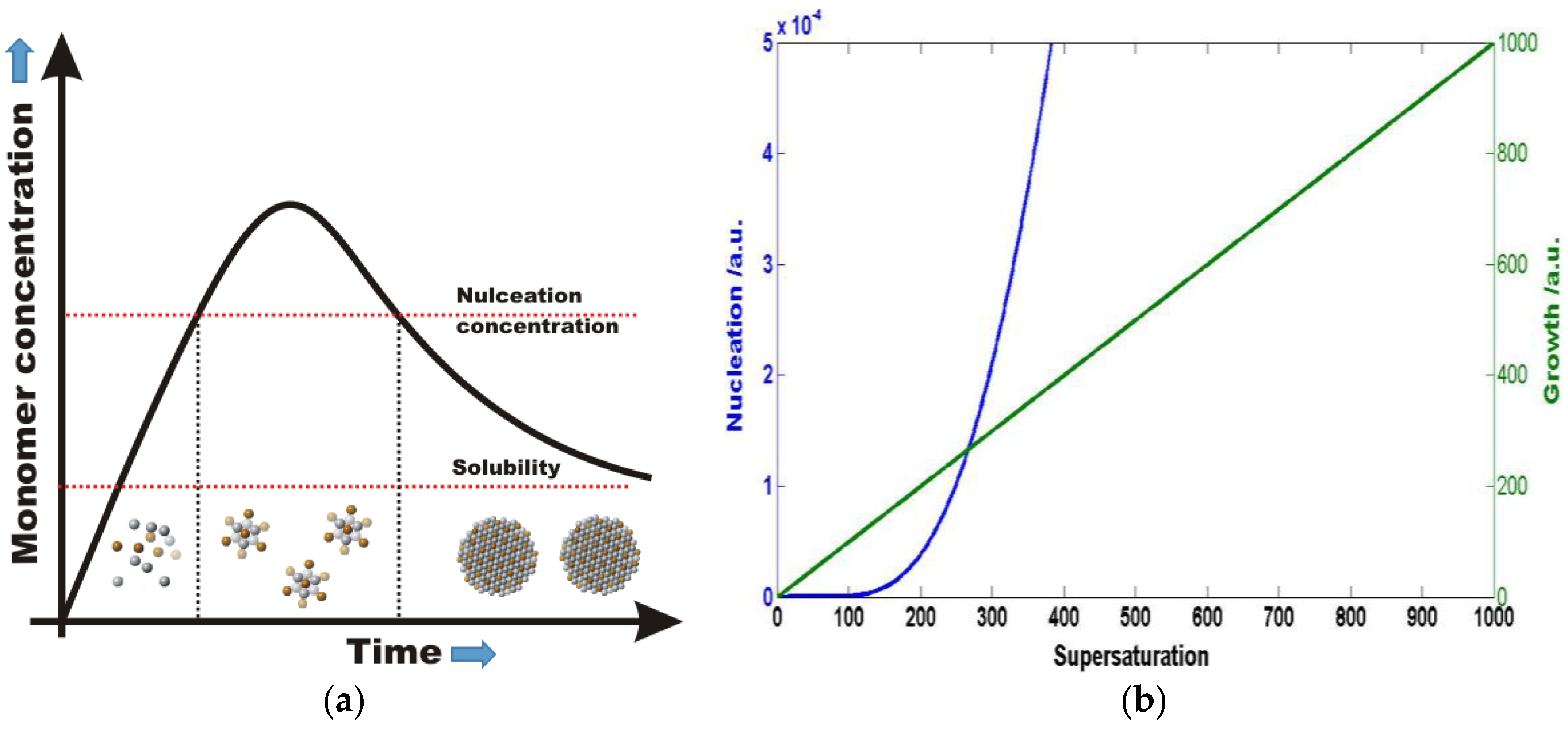
:1. Introduction
2. Microwave-Assisted NP Synthesis
3. Synthesis of Ultra-Small NPs Using Magnetic Heating
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HI | Hot Injection Method |
| QD | quantum dot |
| NP | nanoparticle |
| FO | fiber optic |
| IR | infrared |
References
- Banin, U.; Cao, Y.; Katz, D.; Millo, O. Identification of atomic-like electronic states in indium arsenide nanocrystal quantum dots. Nature 1999, 400, 542–544. [Google Scholar]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Loss, D.; DiVincenzo, D.P. Quantum computation with quantum dots. Phys. Rev. A 1998, 57, 120–126. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Klimov, V.I.; Mikhailovsky, A.A.; Xu, S.; Malko, A.; Hollingsworth, J.A.; Leatherdale, C.A.; Eisler, H.-J.; Bawendi, M.G. Optical Gain and Stimulated Emission in Nanocrystal Quantum Dots. Science 2000, 290, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Robel, I.; Subramanian, V.; Kuno, M.; Kamat, P.V. Quantum Dot Solar Cells. Harvesting Light Energy with CdSe Nanocrystals Molecularly Linked to Mesoscopic TiO2 Films. J. Am. Chem. Soc. 2006, 128, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Vossmeyer, T.; Katsikas, L.; Giersig, M.; Popovic, I.G.; Diesner, K.; Chemseddine, A.; Eychmueller, A.; Weller, H. CdS Nanoclusters: Synthesis, Characterization, Size Dependent Oscillator Strength, Temperature Shift of the Excitonic Transition Energy, and Reversible Absorbance Shift. J. Phys. Chem. 1994, 98, 7665–7673. [Google Scholar] [CrossRef]
- Nozik, A.J. Quantum dot solar cells. Phys. E 2002, 14, 115–120. [Google Scholar] [CrossRef]
- van Embden, J.; Chesman, A.S.R.; Jasieniak, J.J. The Heat-Up Synthesis of Colloidal Nanocrystals. Chem. Mater. 2015, 27, 2246–2285. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. Crystallization of nanomaterials. Curr. Opin. Chem. Eng. 2012, 1, 108–116. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Rogach, A.L.; Haase, M.; Weller, H. Evolution of an Ensemble of Nanoparticles in a Colloidal Solution: Theoretical Study. J. Phys. Chem. B 2001, 105, 12278–12285. [Google Scholar] [CrossRef]
- Razgoniaeva, N.; Acharya, A.; Sharma, N.; Adhikari, P.; Shaughnessy, M.; Moroz, P.; Khon, D.; Zamkov, M. Measuring the Time-Dependent Monomer Concentration during the Hot-Injection Synthesis of Colloidal Nanocrystals. Chem. Mater. 2015, 27, 6102–6108. [Google Scholar] [CrossRef]
- Peng, X.; Wickham, J.; Alivisatos, A.P. Kinetics of II-VI and III-V Colloidal Semiconductor Nanocrystal Growth: “Focusing” of Size Distributions. J. Am. Chem. Soc. 1998, 120, 5343–5344. [Google Scholar] [CrossRef]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of Monodisperse Iron Oxide Nanocrystal Formation by “Heating-Up” Process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef] [PubMed]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis—A critical technology overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Damm, M.; Glasnov, T.N.; Kappe, C.O. Translating High-Temperature Microwave Chemistry to Scalable Continuous Flow Processes. Org. Process Res. Dev. 2010, 14, 215–224. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-Assisted Green Synthesis of Silver Nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Chia, L.H.L.; Boey, F.Y.C. Thermal and non-thermal interaction of microwave radiation with materials. J. Mater. Sci. 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- Collins, M.J. Future trends in microwave synthesis. Future Med. Chem. 2010, 2, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Schanche, J.-S. Microwave synthesis solutions from personal chemistry. Mol. Divers. 2003, 7, 291–298. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar] [CrossRef]
- Jhung, S.H.; Jin, T.; Hwang, Y.K.; Chang, J.-S. Microwave Effect in the Fast Synthesis of Microporous Materials: Which Stage Between Nucleation and Crystal Growth is Accelerated by Microwave Irradiation? Chem. Eur. J. 2007, 13, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- Washington, A.L.; Strouse, G.F. Selective Microwave Absorption by Trioctyl Phosphine Selenide: Does It Play a Role in Producing Multiple Sized Quantum Dots in a Single Reaction? Chem. Mater. 2009, 21, 2770–2776. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Kubokawa, M.; Tsuji, T. Microwave-Assisted Synthesis of Metallic Nanostructures in Solution. Chem. Eur. J. 2005, 11, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.J.; Mahesh, K.; Kumar, S. A strategic approach for preparation of oxide nanomaterials. Bull. Mater. Sci. 2005, 28, 19–24. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C. Continuous Aspect-Ratio Tuning and Fine Shape Control of Monodisperse α-Fe2O3 Nanocrystals by a Programmed Microwave–Hydrothermal Method. Adv. Funct. Mater. 2008, 18, 880–887. [Google Scholar] [CrossRef]
- Shen, P.K.; Yin, S.; Li, Z.; Chen, C. Preparation and performance of nanosized tungsten carbides for electrocatalysis. Electrochimica Acta 2010, 55, 7969–7974. [Google Scholar] [CrossRef]
- Seol, S.K.; Kim, D.; Jung, S.; Hwu, Y. Microwave synthesis of gold nanoparticles: Effect of applied microwave power and solution pH. Mater. Chem. Phys. 2011, 131, 331–335. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Xiong, X.; Qin, Y. Impact of microwave power on the preparation of silver nanowires via a microwave-assisted method. RSC Adv. 2013, 3, 8431–8436. [Google Scholar] [CrossRef]
- Kappe, C.O. Unraveling the Mysteries of Microwave Chemistry Using Silicon Carbide Reactor Technology. Acc. Chem. Res. 2013, 46, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Ashley, B.; Lovingood, D.D.; Chiu, Y.-C.; Gao, H.; Owens, J.; Strouse, G.F. Specific effects in microwave chemistry explored through reactor vessel design, theory, and spectroscopy. Phys. Chem. Chem. Phys. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Hyeon, T. Colloidal Chemical Synthesis and Formation Kinetics of Uniformly Sized Nanocrystals of Metals, Oxides, and Chalcogenides. Acc. Chem. Res. 2008, 41, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Washington, A.L., II; Strouse, G.F. Microwave Synthesis of CdSe and CdTe Nanocrystals in Nonabsorbing Alkanes. J. Am. Chem. Soc. 2008, 130, 8916–8922. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. How to measure reaction temperature in microwave-heated transformations. Chem. Soc. Rev. 2013, 42, 4977–4990. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Kingman, S.; Irvine, D.; Licence, P.; Smith, A.; Dimitrakis, G.; Obermayer, D.; Kappe, C.O. Understanding microwave heating effects in single mode type cavities—Theory and experiment. Phys. Chem. Chem. Phys. 2010, 12, 4750–4758. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, D.; Damm, M.; Kappe, C.O. Design and evaluation of improved magnetic stir bars for single-mode microwave reactors. Org. Biomol. Chem. 2013, 11, 4949–4956. [Google Scholar] [CrossRef] [PubMed]
- Conner, W.C.; Tompsett, G.A. How Could and Do Microwaves Influence Chemistry at Interfaces? J. Phys. Chem. B 2008, 112, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, I.; Daniels, S.; Pickett, N. Preparation of nanoparticle materials. U.S. Patent 7,588,828, 15 September 2009. [Google Scholar]
- Wang, X.; Qu, L.; Zhang, J.; Peng, X.; Xiao, M. Surface-Related Emission in Highly Luminescent CdSe Quantum Dots. Nano Lett. 2003, 3, 1103–1106. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Chae, W.-S.; Im, S.-J.; Kim, Y.-R. Mild synthesis of ultra-small CdSe quantum dots in ethylenediamine solution. Mater. Lett. 2005, 59, 1430–1433. [Google Scholar] [CrossRef]
- Bowers, M.J.; McBride, J.R.; Rosenthal, S.J. White-Light Emission from Magic-Sized Cadmium Selenide Nanocrystals. J. Am. Chem. Soc. 2005, 127, 15378–15379. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikan, V.; McLaurin, E.J. Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating. Nanomaterials 2016, 6, 85. https://doi.org/10.3390/nano6050085
Chikan V, McLaurin EJ. Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating. Nanomaterials. 2016; 6(5):85. https://doi.org/10.3390/nano6050085
Chicago/Turabian StyleChikan, Viktor, and Emily J. McLaurin. 2016. "Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating" Nanomaterials 6, no. 5: 85. https://doi.org/10.3390/nano6050085






