Synthesis of Multi-Walled Carbon Nanotubes from Plastic Waste Using a Stainless-Steel CVD Reactor as Catalyst
Abstract
:1. Introduction
2. Results and Discussion
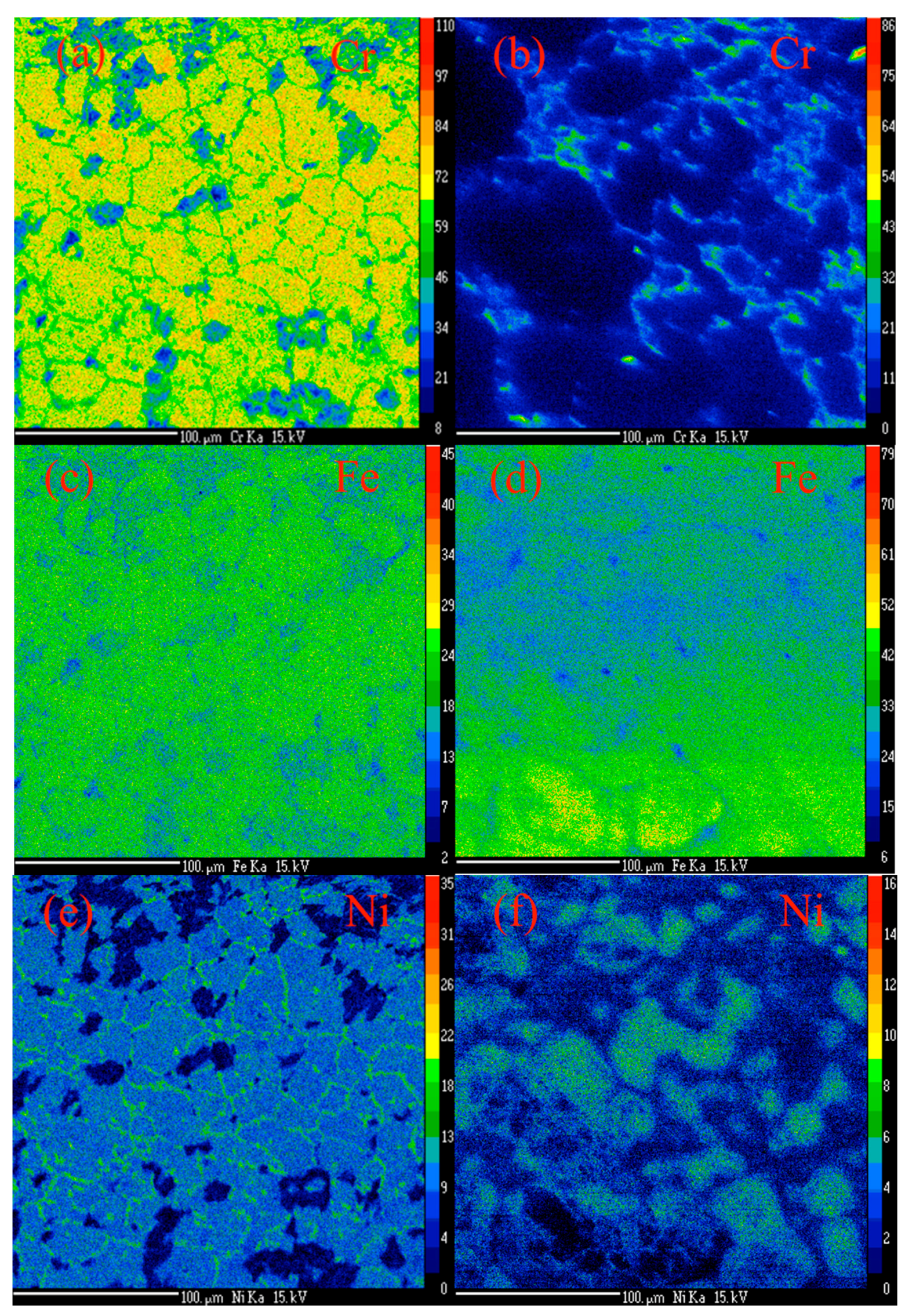
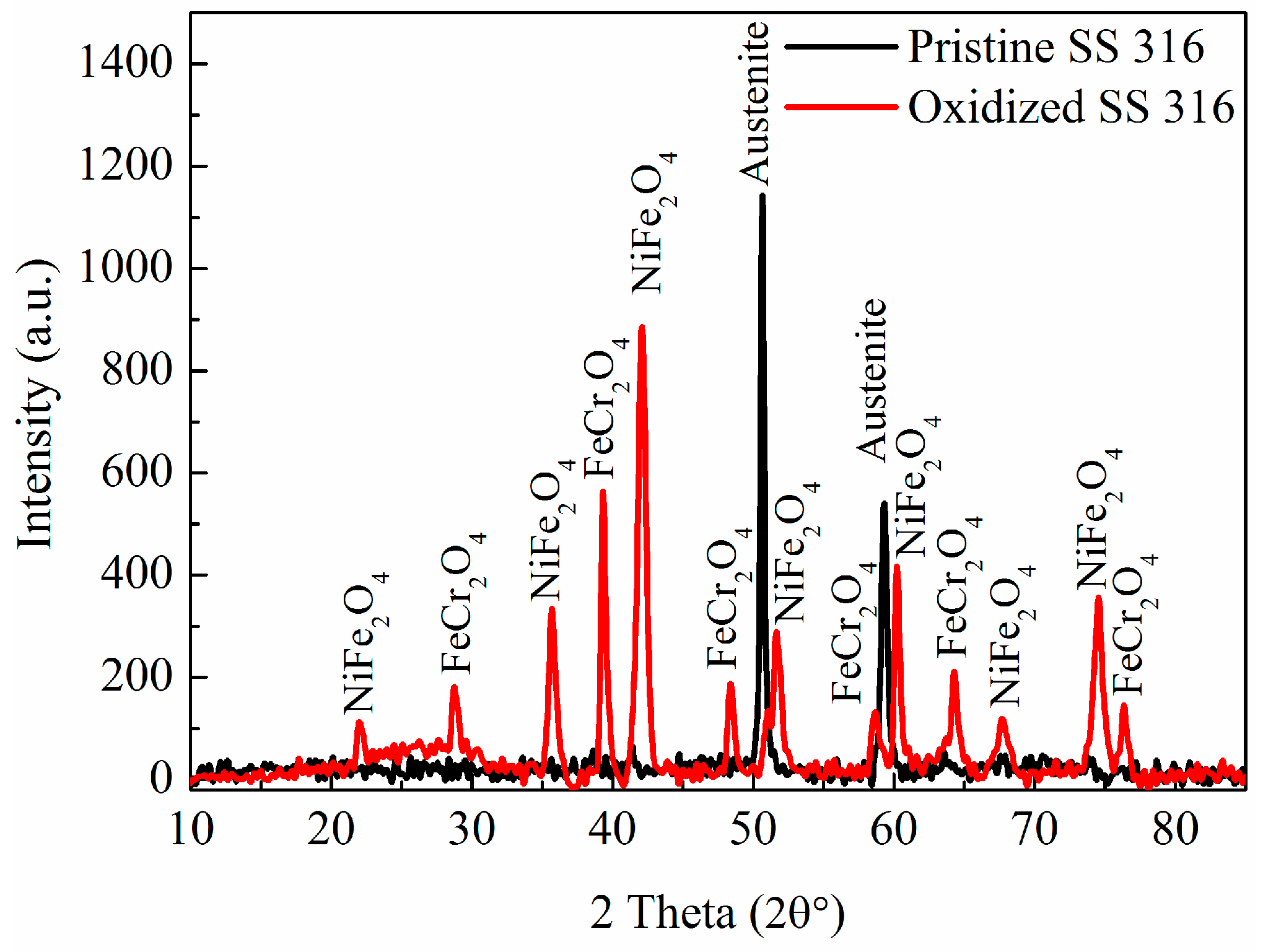
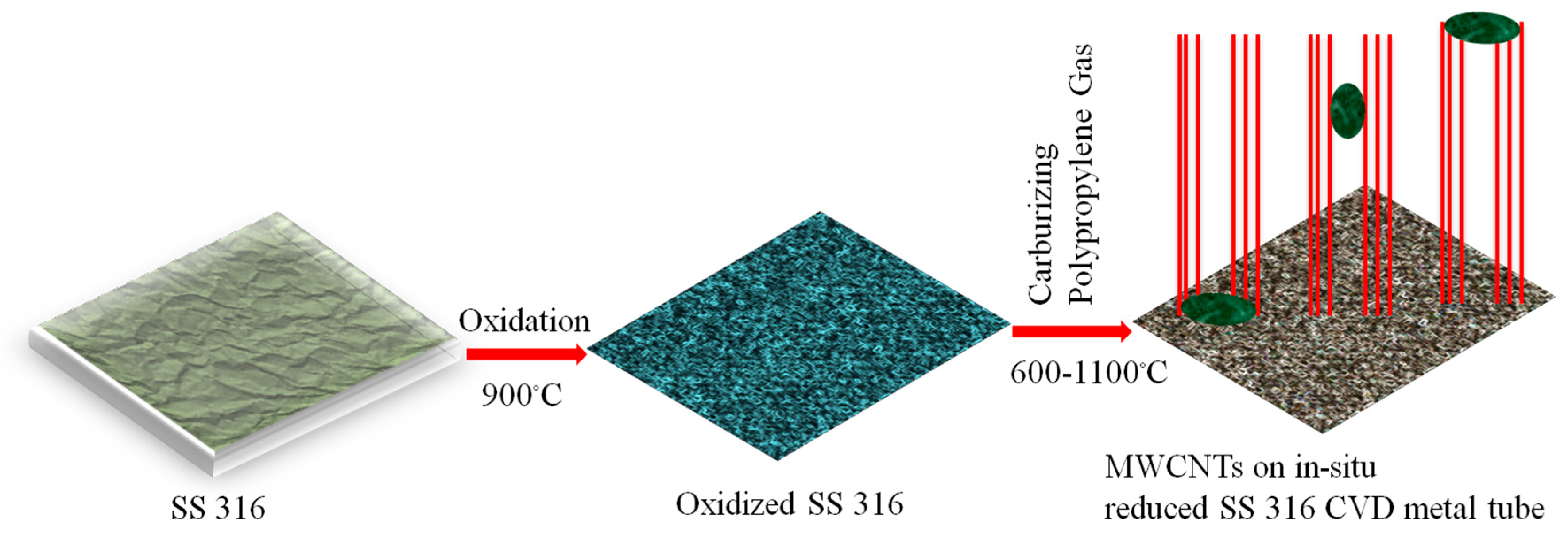
2.1. Synthesis of MWCNTs Using Plastic Waste as a Carbon Feed and an SS 316 Steel Reactor as Catalyst
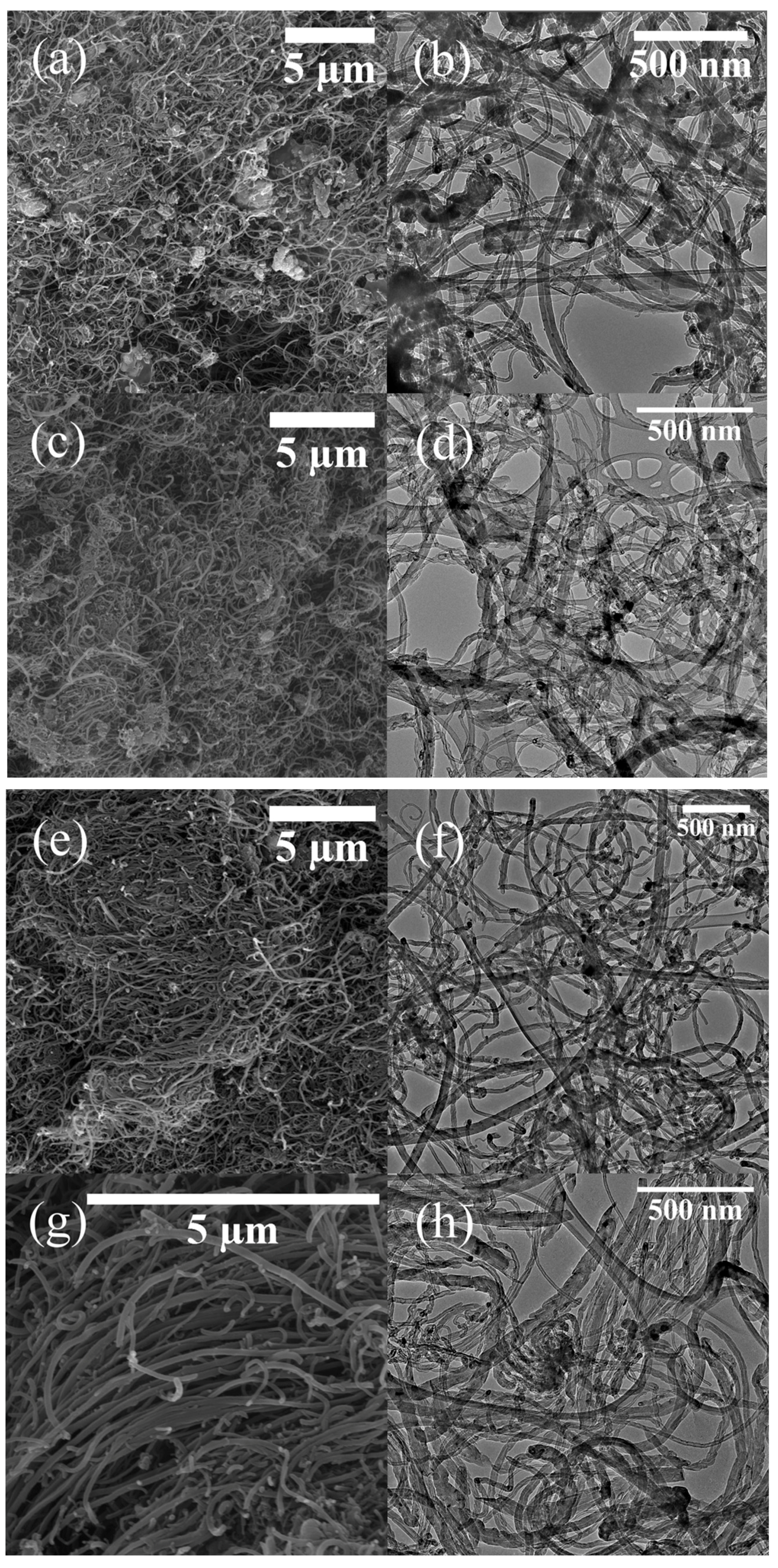
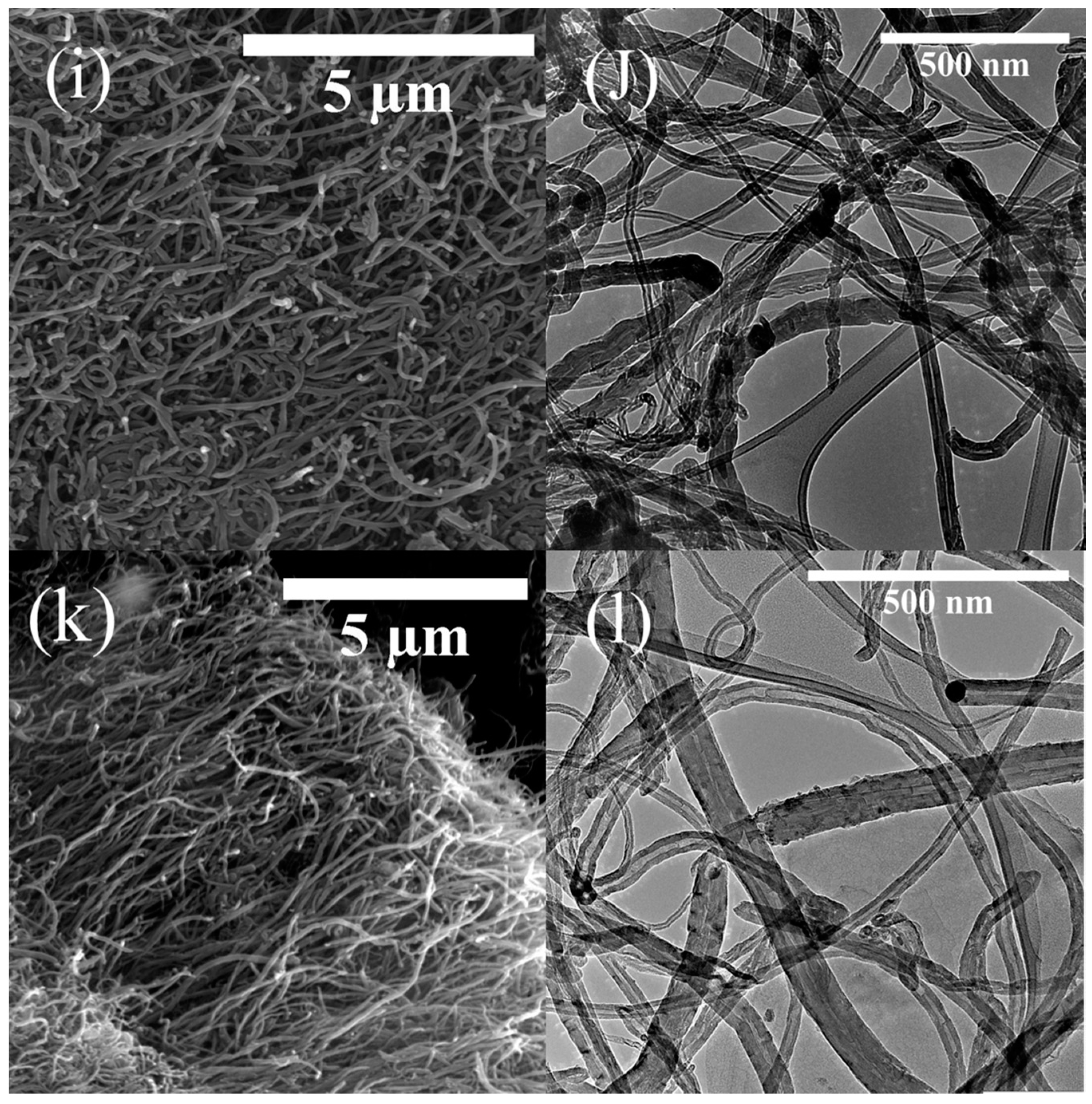
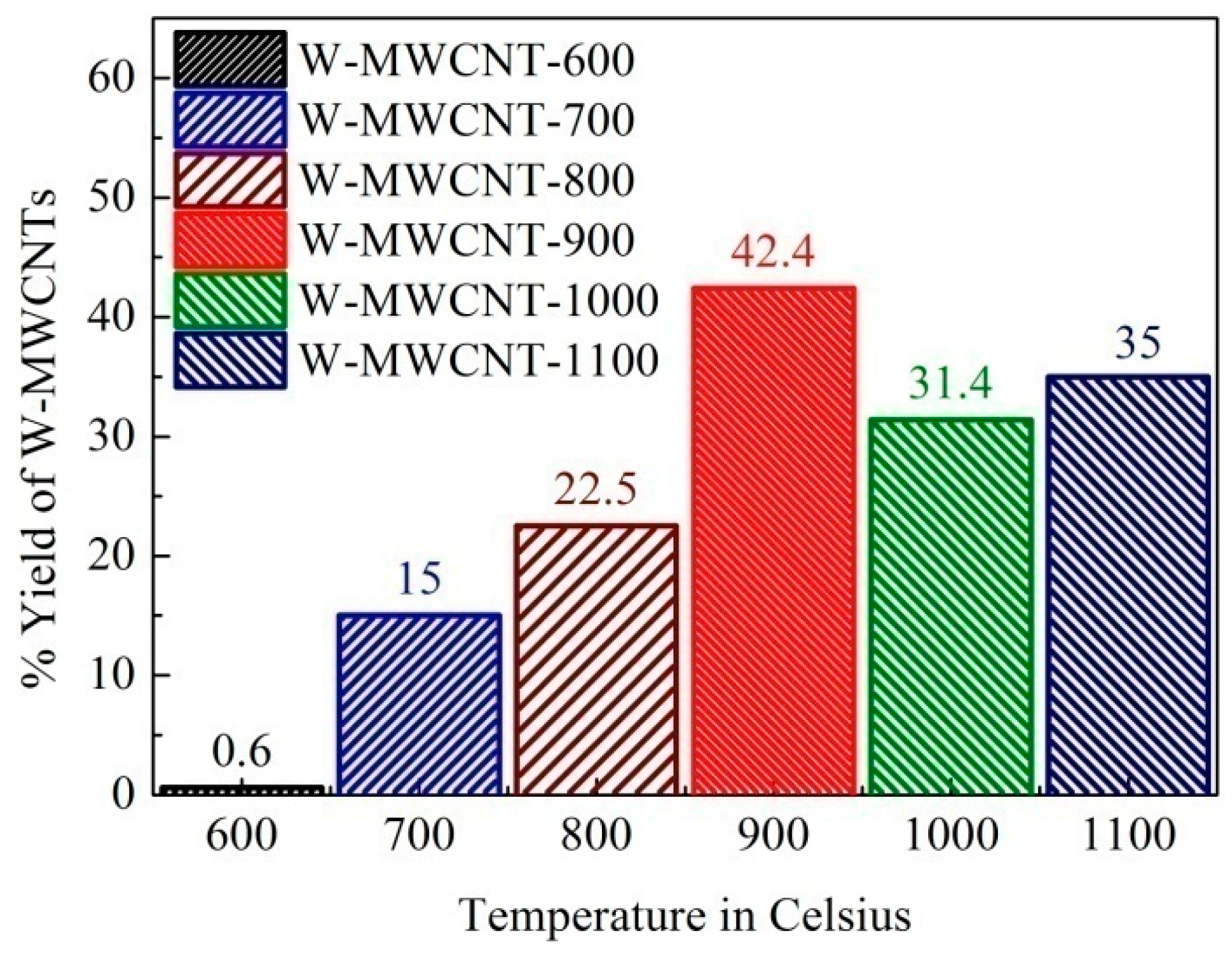
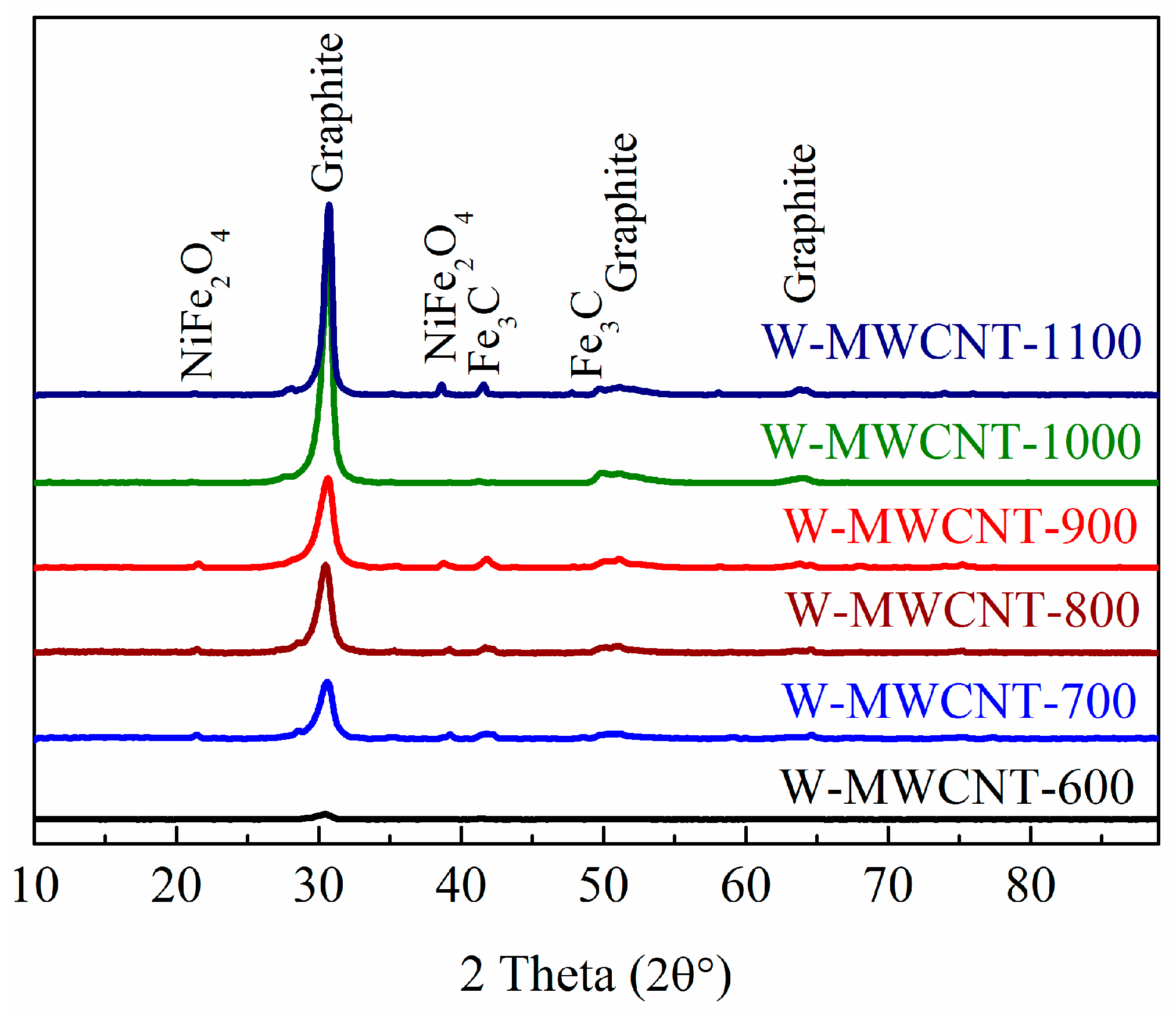
2.2. Morphology, Yield and Crystallinity of the Synthesized MWCNTs
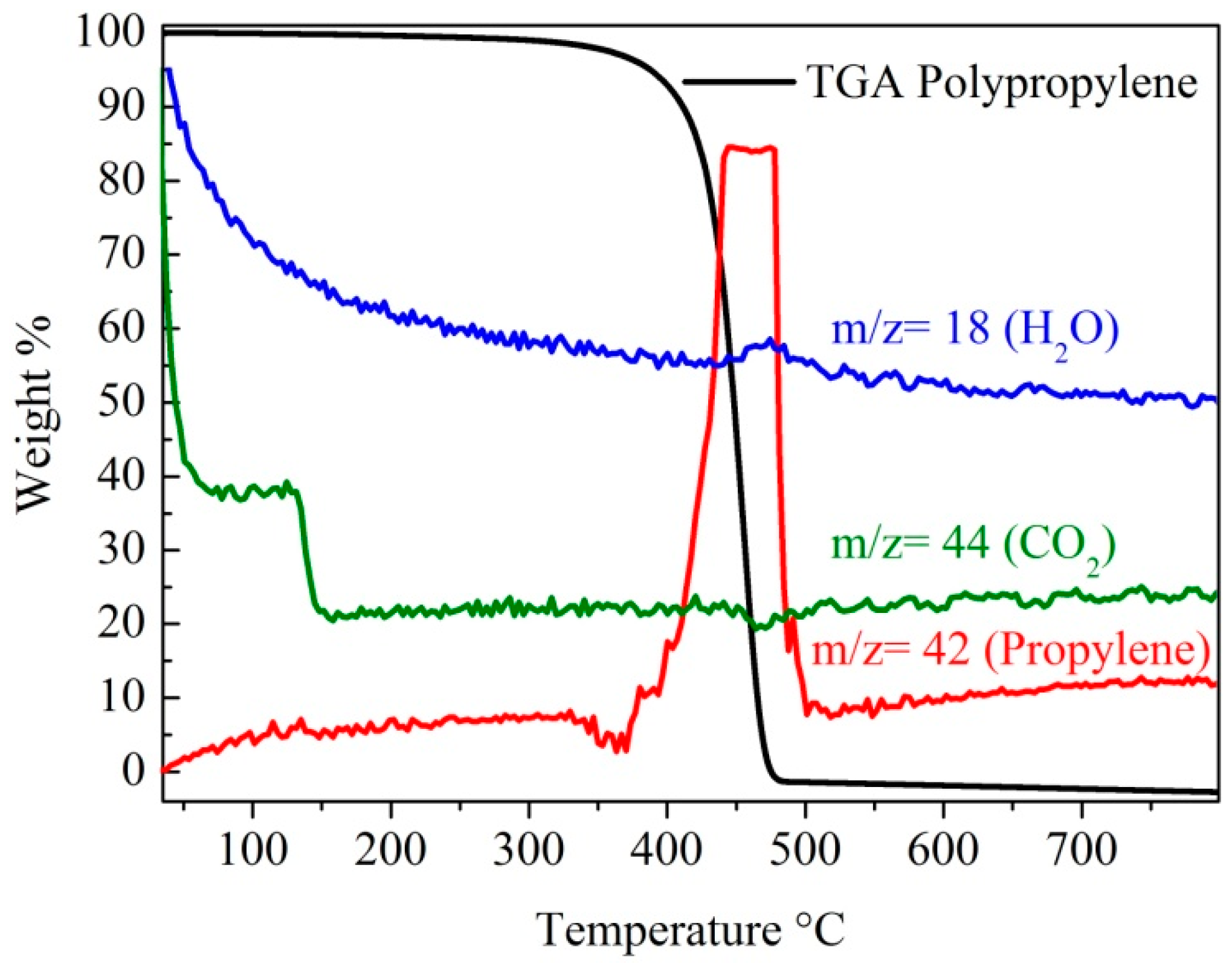
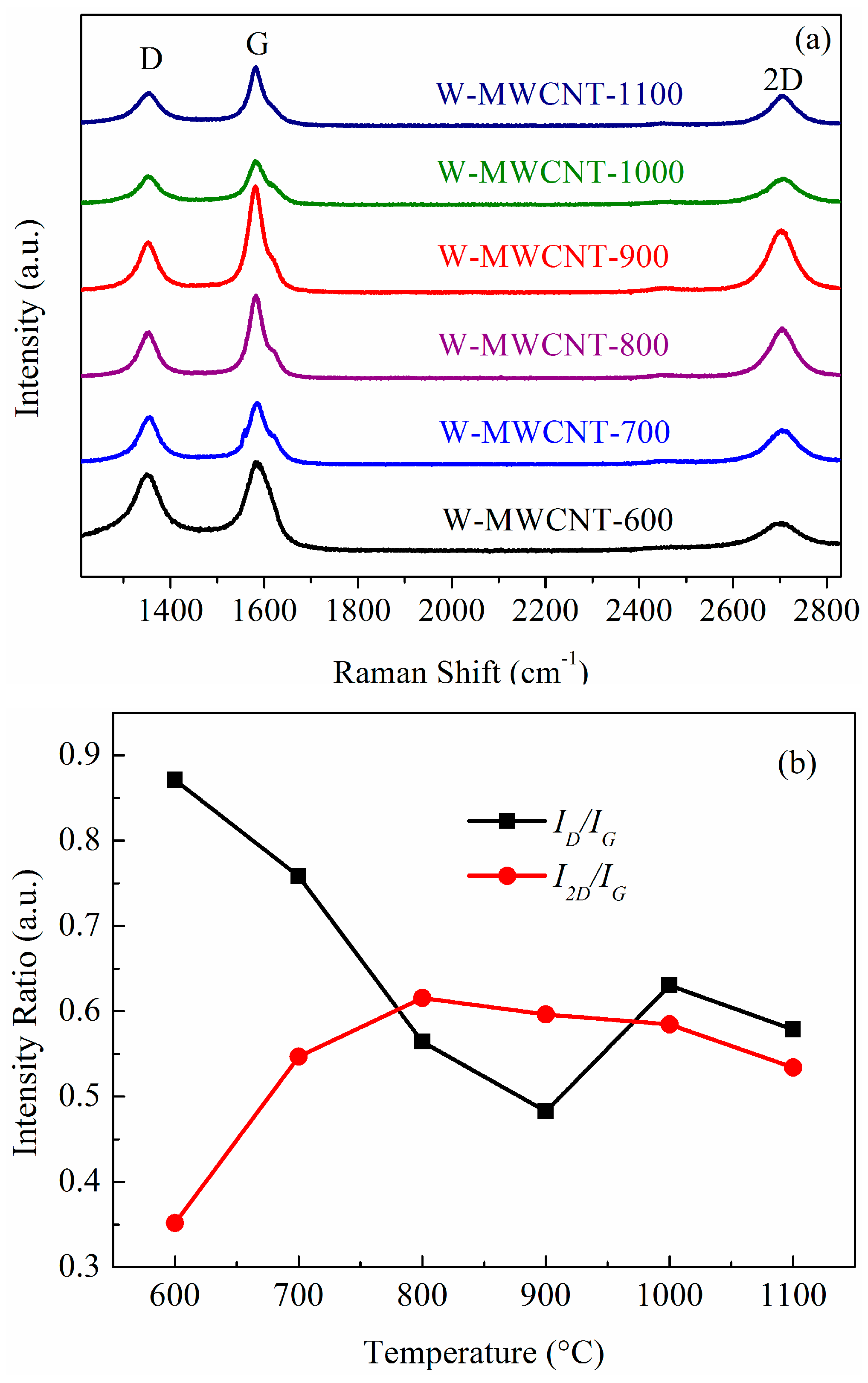
2.3. Raman Spectra and TGA Analysis of the MWCNTs
2.4. Mechanism of MWCNT Synthesis from Plastic Waste
3. Materials and Methods
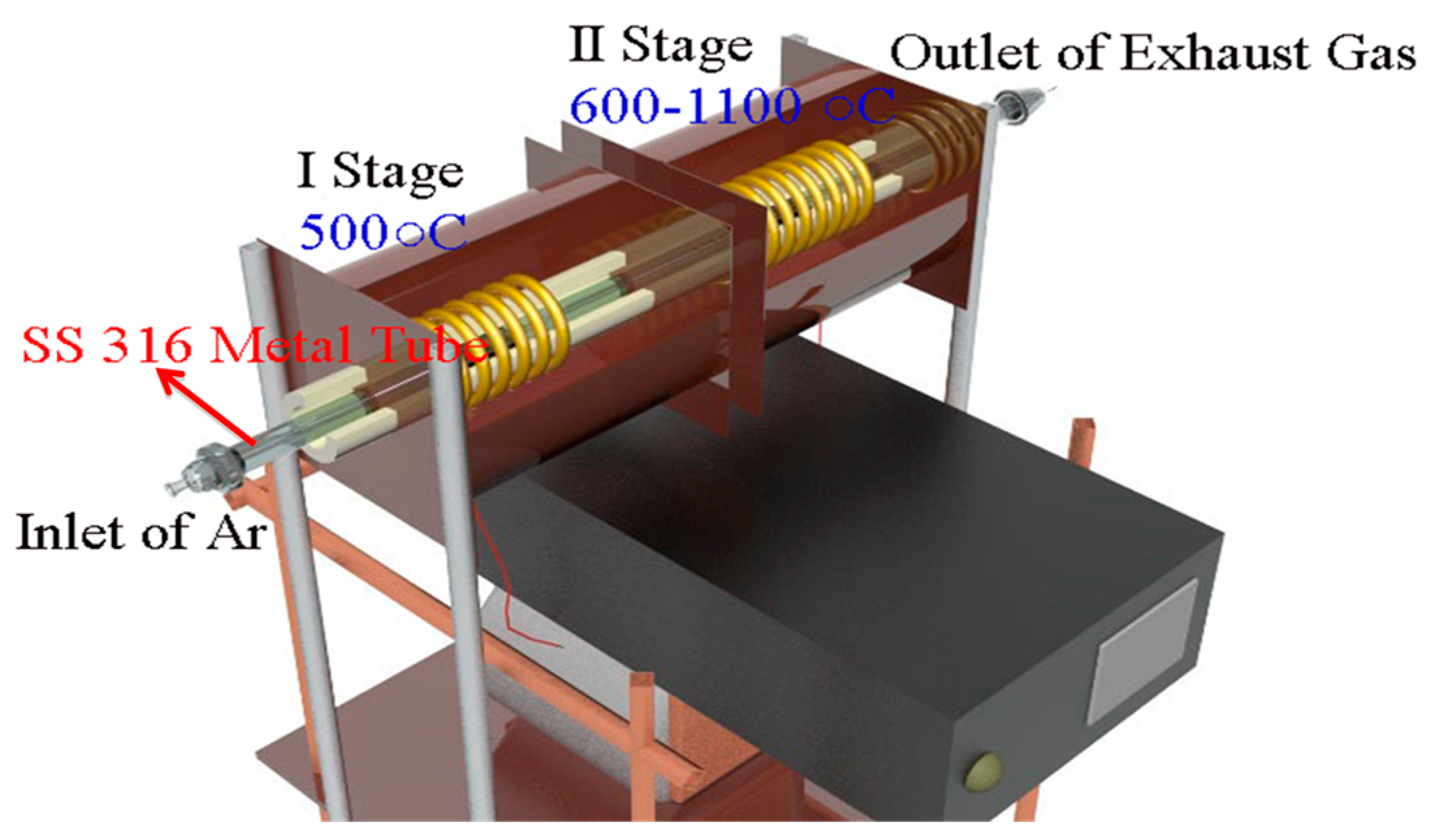
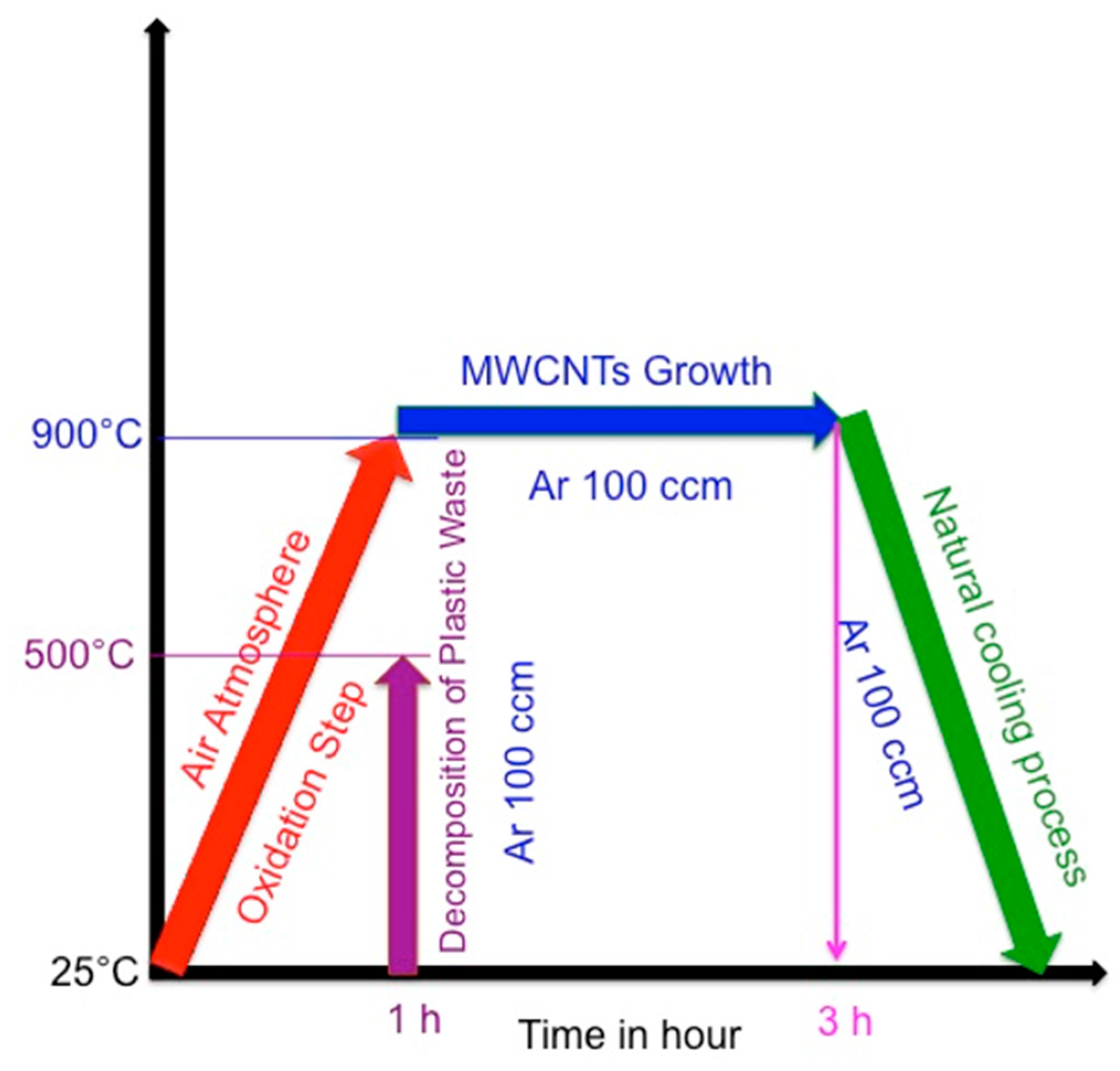
3.1. Synthesis
3.2. Purification of W-MWCNTs
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ni, H.-G.; Lu, S.-Y.; Mo, T.; Zeng, H. Brominated flame retardant emissions from the open burning of five plastic wastes and implications for environmental exposure in china. Environ. Pollut. 2016, 214, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings—Entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. B 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, G.; Chen, J.; Huang, Y.; An, T.; Zhang, C. Pollution characteristics and health risk assessment of volatile organic compounds emitted from different plastic solid waste recycling workshops. Environ. Int. 2015, 77, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liu, J.; Chen, X.; Jiang, Z.; Wen, X.; Mijowska, E.; Tang, T. Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater. J. Mater. Chem. A 2015, 3, 341–351. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Policy: Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bansal, P.P. Use of different forms of waste plastic in concrete—A review. J. Clean. Prod. 2016, 112, 473–482. [Google Scholar] [CrossRef]
- Nikbin, I.M.; Rahimi, S.; Allahyari, H.; Fallah, F. Feasibility study of waste poly ethylene terephthalate (pet) particles as aggregate replacement for acid erosion of sustainable structural normal and lightweight concrete. J. Clean. Prod. 2016, 126, 108–117. [Google Scholar] [CrossRef]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical properties of carbon nanotube based fibers and their future use in electrical wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Szymanski, L.; Kolacinski, Z.; Wiak, S.; Raniszewski, G.; Pietrzak, L. Synthesis of carbon nanotubes in thermal plasma reactor at atmospheric pressure. Nanomaterials 2017, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, X.; Wang, S.; Tang, Z.; Yang, Q.; Hu, G.-H.; Xiong, C. Flexible carbon nanotube modified separator for high-performance lithium-sulfur batteries. Nanomaterials 2017, 7, 196. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Lu, Y.; Wei, S.; Wujcik, E.; Guo, Z. Multifunctional carbon nanostructures for advanced energy storage applications. Nanomaterials 2015, 5, 755–777. [Google Scholar] [CrossRef] [PubMed]
- Hintsho, N.; Shaikjee, A.; Triphati, P.K.; Masenda, H.; Naidoo, D.; Franklyn, P.; Durbach, S. Effect of nitrogen and hydrogen gases on the synthesis of carbon nanomaterials from coal waste fly ash as a catalyst. J. Nanosci. Nanotechnol. 2016, 16, 4672–4683. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P.; Wu, J.; Yadav, R.M.; Liu, M.; Wright, C.J.; Tiwary, C.S.; Yakobson, B.I.; Lou, J.; Ajayan, P.M.; Zhou, X.-D. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: On the understanding of defects, defect density, and selectivity. Angew. Chem. 2015, 127, 13905–13909. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.K.; Gan, L.; Liu, M. Synthesis of large pore carbon nanoparticles for removal of malachite green. J. Nanosci. Nanotechnol. 2016, 16, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.K.; Rao, N.N.; Chauhan, C.; Pophali, G.R.; Kashyap, S.M.; Lokhande, S.K.; Gan, L. Treatment of refractory nano-filtration reject from a tannery using pd-catalyzed wet air oxidation. J. Hazard. Mater. 2013, 261, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, A.; McKay, G. A review—Synthesis of carbon nanotubes from plastic wastes. Chem. Eng. J. 2012, 195, 377–391. [Google Scholar] [CrossRef]
- Pol, V.G.; Wen, J.; Lau, K.C.; Callear, S.; Bowron, D.T.; Lin, C.-K.; Deshmukh, S.A.; Sankaranarayanan, S.; Curtiss, L.A.; David, W.I.F.; et al. Probing the evolution and morphology of hard carbon spheres. Carbon 2014, 68, 104–111. [Google Scholar] [CrossRef]
- Mishra, N.; Das, G.; Ansaldo, A.; Genovese, A.; Malerba, M.; Povia, M.; Ricci, D.; Di Fabrizio, E.; Di Zitti, E.; Sharon, M.; et al. Pyrolysis of waste polypropylene for the synthesis of carbon nanotubes. J. Anal. Appl. Pyrol. 2012, 94, 91–98. [Google Scholar] [CrossRef]
- Pol, V.G.; Thiyagarajan, P. Remediating plastic waste into carbon nanotubes. J. Environ. Monitor. 2010, 12, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Pol, V.G.; Thackeray, M.M. Spherical carbon particles and carbon nanotubes prepared by autogenic reactions: Evaluation as anodes in lithium electrochemical cells. Energy Environ. Sci. 2011, 4, 1904–1912. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Wang, L.; Williams, P.T.; Huang, J. Sustainable processing of waste plastics to produce high yield hydrogen-rich synthesis gas and high quality carbon nanotubes. RSC Adv. 2012, 2, 4045–4047. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Somani, R.S.; Panda, A.B.; Bajaj, H.C. Utilization of plastic wastes for synthesis of carbon microspheres and their use as a template for nanocrystalline copper(ii) oxide hollow spheres. ACS Sustain. Chem. Eng. 2013, 1, 1390–1397. [Google Scholar] [CrossRef]
- Shen, Y.; Gong, W.; Zheng, B.; Gao, L. Ni–Al bimetallic catalysts for preparation of multiwalled carbon nanotubes from polypropylene: Influence of the ratio of Ni/Al. Appl. Catal. B 2016, 181, 769–778. [Google Scholar] [CrossRef]
- MacKenzie, K.J.; Dunens, O.M.; Harris, A.T. An updated review of synthesis parameters and growth mechanisms for carbon nanotubes in fluidized beds. Ind. Eng. Chem. Res. 2010, 49, 5323–5338. [Google Scholar] [CrossRef]
- Paradise, M.; Goswami, T. Carbon nanotubes—Production and industrial applications. Mater. Des. 2007, 28, 1477–1489. [Google Scholar] [CrossRef]
- Karwa, M.; Iqbal, Z.; Mitra, S. Scaled-up self-assembly of carbon nanotubes inside long stainless steel tubing. Carbon 2006, 44, 1235–1242. [Google Scholar] [CrossRef]
- Zeng, Z.; Natesan, K. Relationship between the growth of carbon nanofilaments and metal dusting corrosion. Chem. Mater. 2005, 17, 3794–3801. [Google Scholar] [CrossRef]
- Pattinson, S.W.; Viswanath, B.; Zakharov, D.N.; Li, J.; Stach, E.A.; Hart, A.J. Mechanism and enhanced yield of carbon nanotube growth on stainless steel by oxygen-induced surface reconstruction. Chem. Mater. 2015, 27, 932–937. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Hall, L.J. Carbon nanotube synthesis upon stainless steel meshes. Carbon 2003, 41, 659–672. [Google Scholar] [CrossRef]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger, L.; Meunier, J.-L. A simple thermal cvd method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2009, 47, 313–318. [Google Scholar] [CrossRef]
- Chang, J.-K.; Tsai, H.-Y.; Tsai, W.-T. A metal dusting process for preparing nano-sized carbon materials and the effects of acid post-treatment on their hydrogen storage performance. Int. J. Hydrogen Energy 2008, 33, 6734–6742. [Google Scholar] [CrossRef]
- Young, D.J.; Zhang, J.; Geers, C.; Schütze, M. Recent advances in understanding metal dusting: A review. Mater. Corros. 2011, 62, 7–28. [Google Scholar] [CrossRef]
- Zhuo, C.; Wang, X.; Nowak, W.; Levendis, Y.A. Oxidative heat treatment of 316l stainless steel for effective catalytic growth of carbon nanotubes. Appl. Surf. Sci. 2014, 313, 227–236. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Proc. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, E.N.; Coville, N.J. Nitrogen doped carbon nanotubes from organometallic compounds: A review. Materials 2010, 3, 2141. [Google Scholar] [CrossRef]
- Sandvik Materials. Technology, SS 316. Available online: http://smt.sandvik.com/en/materials-center/material-datasheets/bar-and-hollow-bar/hollow-bar/sanmac-316316l/ (accessed on 30 August 2016).
- Nessim, G.D. Properties, synthesis, and growth mechanisms of carbon nanotubes with special focus on thermal chemical vapor deposition. Nanoscale 2010, 2, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Olaseinde, O.A.; Merwe, J.V.D.; Cornish, L. Characterization and corrosion behaviour of selected duplex stainless steels in acidic and acidic-chloride solution. Adv. Chem. Eng. Sci. 2014, 4, 89–93. [Google Scholar] [CrossRef]
- Iqubal, M.; Sharma, R.; Jheeta, S.; Kamaluddin. Thermal condensation of glycine and alanine on metal ferrite surface: Primitive peptide bond formation scenario. Life 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Fu, X.-Z.; Zhou, G.-H.; Luo, J.-L.; Chuang, K.T.; Sanger, A.R. FeCr2O4 nanoparticles as anode catalyst for ethane proton conducting fuel cell reactors to coproduce ethylene and electricity. Adv. Phys. Chem. 2011, 2011, 407480. [Google Scholar] [CrossRef]
- Ran, M.-F.; Chu, W.; Wen, J.; Li, Y.-F. Promoting effects of chromium on Ni/MgO catalysts for CNTs synthesis by chemical vapor deposition method. Chem. J. Chin. Univ. 2009, 30, 231. [Google Scholar]
- Huang, X.; Liang, W.; Zhang, S. Radial corrugations of multi-walled carbon nanotubes driven by inter-wall nonbonding interactions. Nanoscale Res. Lett. 2010, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Basavaraja, C.; Park, J.Y.; Huh, D.S. Solid-state synthesis and dielectric properties of nanocomposites containing poly (3,4-ethylenedioxythiophene)/graphene nanoplatelets. Int. J. Eng. Appl. Sci. 2015, 2, 1–7. [Google Scholar]
- Qiu, J.; Li, Q.; Wang, Z.; Sun, Y.; Zhang, H. Cvd synthesis of coal-gas-derived carbon nanotubes and nanocapsules containing magnetic iron carbide and oxide. Carbon 2006, 44, 2565–2568. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Nagaiah, T.C.; Xia, W.; Wang, Y.; Dommele, S.V.; Bitter, J.H.; Santa, M.; Grundmeier, G.; Bron, M.; Schuhmann, W.; et al. Electrocatalytic activity and stability of nitrogen-containing carbon nanotubes in the oxygen reduction reaction. J. Phys. Chem. C 2009, 113, 14302–14310. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Pinault, M.; Coste-Leconte, S.; Porterat, D.; Bendiab, N.; Reynaud, C.; Mayne-L’Hermite, M. Dynamics of catalyst particle formation and multi-walled carbon nanotube growth in aerosol-assisted catalytic chemical vapor deposition. Carbon 2010, 48, 3807–3816. [Google Scholar] [CrossRef]
- Popovska, N.; Danova, K.; Jipa, I.; Zenneck, U. Catalytic growth of carbon nanotubes on zeolite supported iron, ruthenium and iron/ruthenium nanoparticles by chemical vapor deposition in a fluidized bed reactor. Powder Technol. 2011, 207, 17–25. [Google Scholar] [CrossRef]
- Bom, D.; Andrews, R.; Jacques, D.; Anthony, J.; Chen, B.; Meier, M.S.; Selegue, J.P. Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: Evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett. 2002, 2, 615–619. [Google Scholar] [CrossRef]
- Mahajan, A.; Kingon, A.; Kukovecz, Á.; Konya, Z.; Vilarinho, P.M. Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mater. Lett. 2013, 90, 165–168. [Google Scholar] [CrossRef]
- Ramirez, A.; Royo, C.; Latorre, N.; Mallada, R.; Tiggelaar, R.M.; Monzón, A. Unraveling the growth of vertically aligned multi-walled carbon nanotubes by chemical vapor deposition. Mater. Res. Exp. 2014, 1, 045604. [Google Scholar] [CrossRef]
- Tessonnier, J.-P.; Su, D.S. Recent progress on the growth mechanism of carbon nanotubes: A review. ChemSusChem 2011, 4, 824–847. [Google Scholar] [CrossRef] [PubMed]




| Elements | wt % |
|---|---|
| Carbon | 0.08 |
| Manganese | 2.00 |
| Phosphorus | 0.045 |
| Sulfur | 0.030 |
| Silicon | 0.75 |
| Chromium | 16.00–18.00 |
| Nickel | 10.00–14.00 |
| Molybdenum | 2.00–3.00 |
| Nitrogen | 0.10 |
| Iron | Balance |
| Sample Name | Inner Diameter (nm) | Outer Diameter (nm) |
|---|---|---|
| W-MWCNT-600 | 8 | 28 |
| W-MWCNT-700 | 9 | 30 |
| W-MWCNT-800 | 9 | 33 |
| W-MWCNT-900 | 10 | 35 |
| W-MWCNT-1000 | 9 | 28 |
| W-MWCNT-1100 | 8 | 32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, P.K.; Durbach, S.; Coville, N.J. Synthesis of Multi-Walled Carbon Nanotubes from Plastic Waste Using a Stainless-Steel CVD Reactor as Catalyst. Nanomaterials 2017, 7, 284. https://doi.org/10.3390/nano7100284
Tripathi PK, Durbach S, Coville NJ. Synthesis of Multi-Walled Carbon Nanotubes from Plastic Waste Using a Stainless-Steel CVD Reactor as Catalyst. Nanomaterials. 2017; 7(10):284. https://doi.org/10.3390/nano7100284
Chicago/Turabian StyleTripathi, Pranav K., Shane Durbach, and Neil J. Coville. 2017. "Synthesis of Multi-Walled Carbon Nanotubes from Plastic Waste Using a Stainless-Steel CVD Reactor as Catalyst" Nanomaterials 7, no. 10: 284. https://doi.org/10.3390/nano7100284






