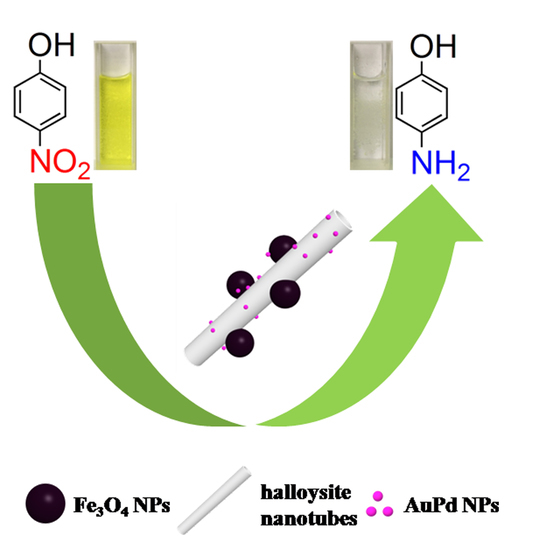
AuPd Bimetallic Nanocrystals Embedded in Magnetic Halloysite Nanotubes: Facile Synthesis and Catalytic Reduction of Nitroaromatic Compounds
Abstract
:1. Introduction
2. Results and Discussion
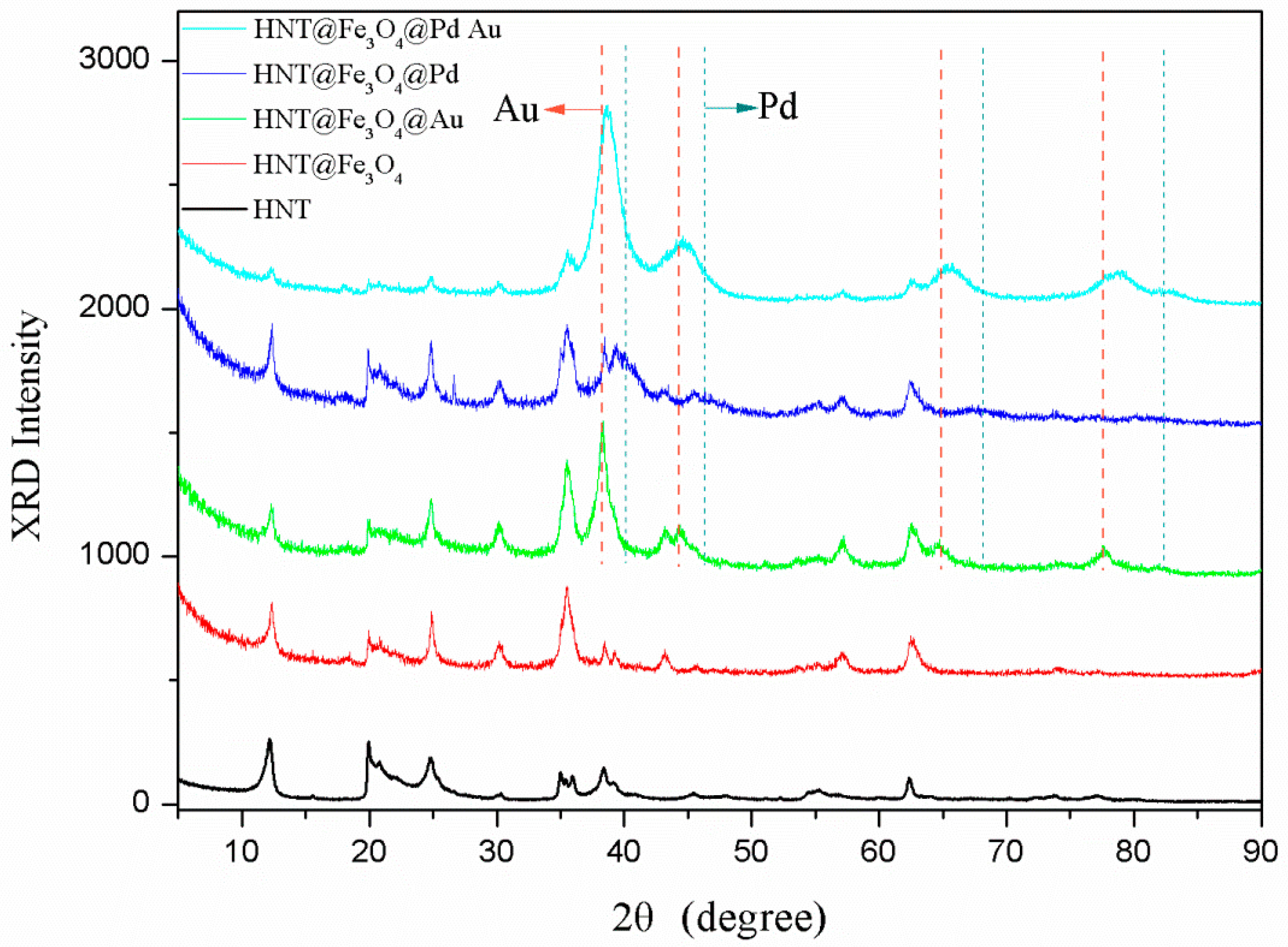
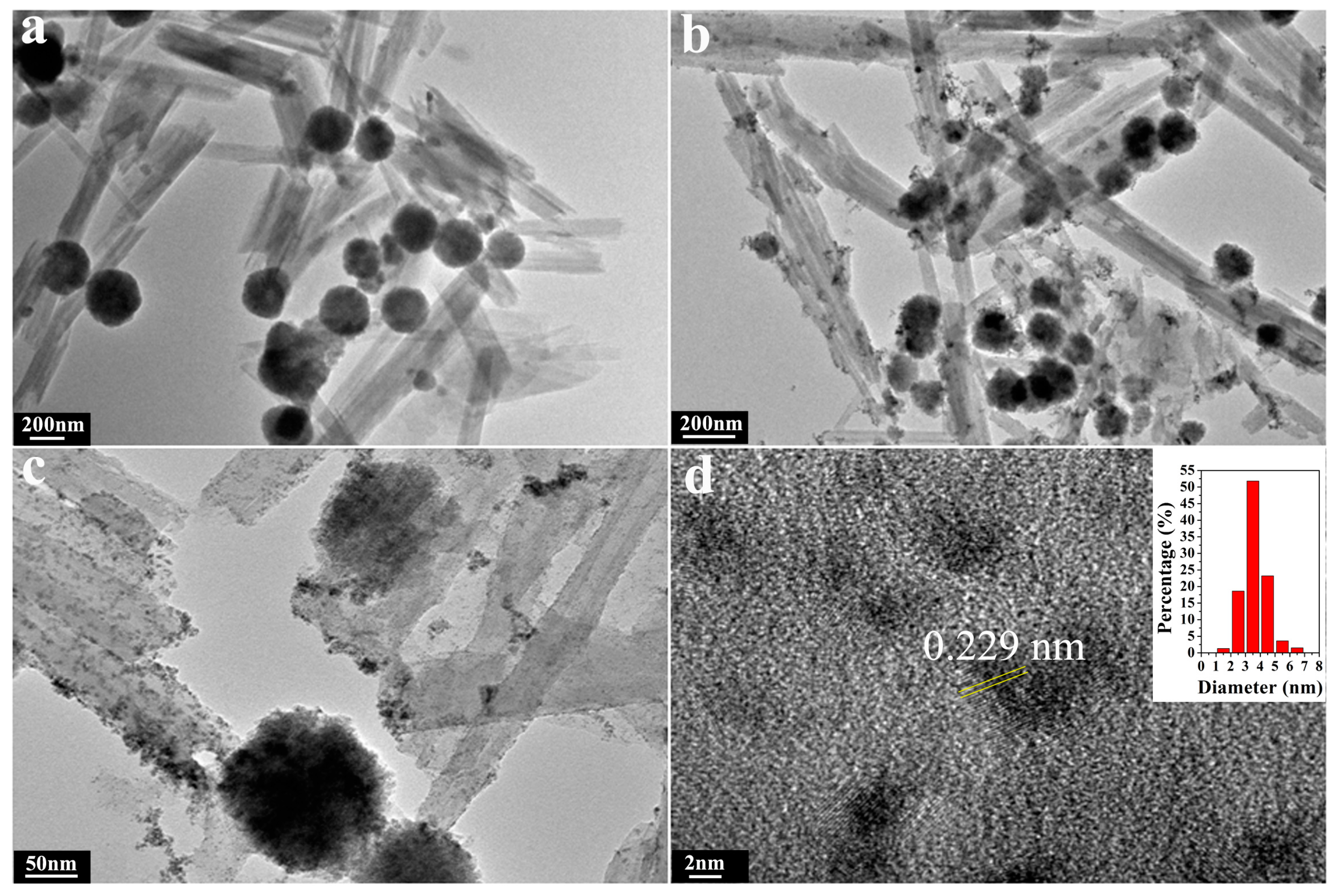
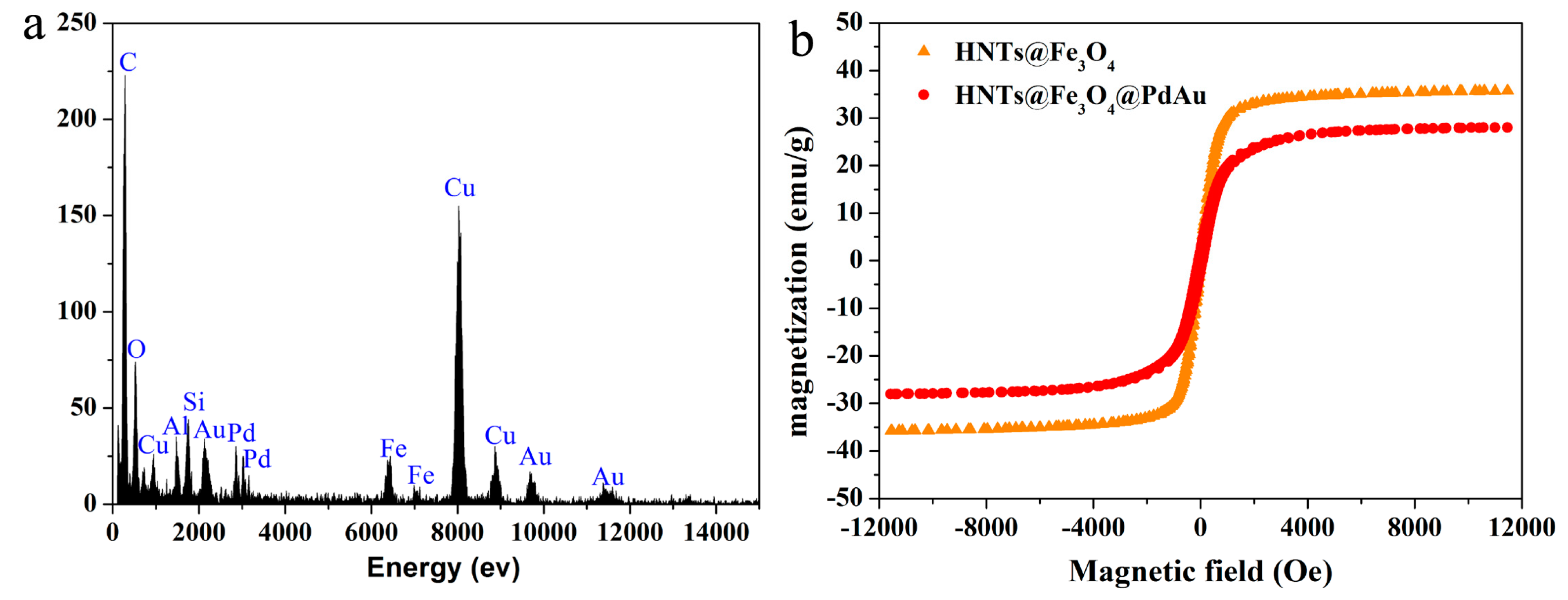
2.1. Structural and Morphology Characterization
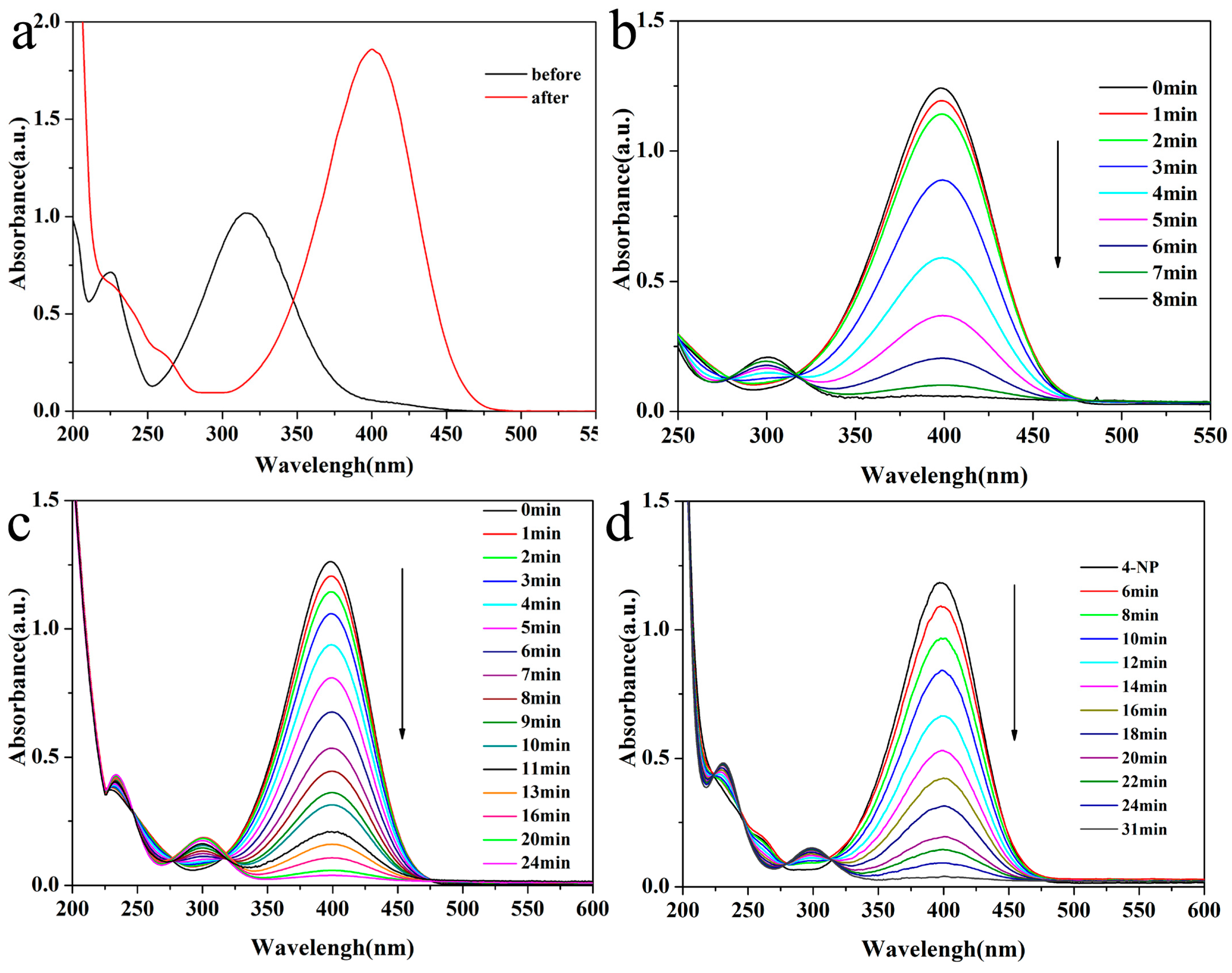
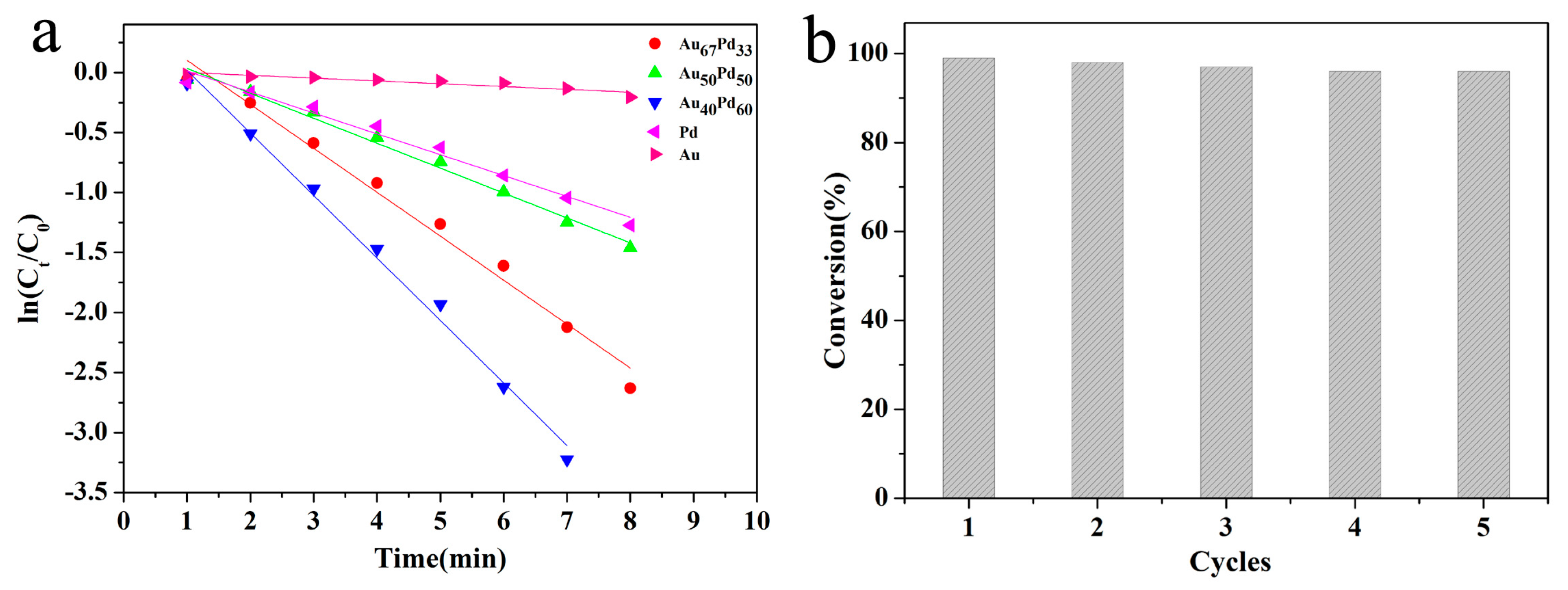
2.2. Catalytic Activities of Bimetallic or Monometallic Au/Pd Modified Nanocatalysts in the Reduction of 4-NP
2.3. Reusability of the HNT@Fe3O4@Au40Pd60
3. Materials and Methods
3.1. Materials
3.2. Preparation of HNT@Fe3O4
3.3. Preparation of HNTs@Fe3O4@AuPd, HNTs@Fe3O4@Au and HNTs@Fe3O4@Pd
3.4. Catalytic Reduction of Nitrobenzene
3.5. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Toyoda, K.; Hamada, I.; Lee, K.; Yanagisawa, S.; Morikawa, Y. Density functional theoretical study of pentacene/noble metal interfaces with van der Waals corrections: Vacuum level shifts and electronic structures. J. Chem. Phys. 2010, 132, 134703. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.J.; Ling, X.Y.; Henzie, J.; Yang, P. Anisotropic etching of silver nanoparticles for plasmonic structures capable of single-particle SERS. J. Am. Chem. Soc. 2009, 132, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Say, Z.; Kivrak, H.; Kaya, M.; Gulcan, M.; Ozensoy, E.; Zahmakiran, M. MnOx-Promoted PdAg Alloy Nanoparticles for the Additive-Free Dehydrogenation of Formic Acid at Room Temperature. ACS Catal. 2015, 5, 6099–6110. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Román-Leshkov, Y. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 2016, 352, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Sapurina, I.; Stejskal, J.; Šeděnková, I.; Trchová, M.; Kovářová, J.; Hromádková, J.; Kopecká, J.; Cieslar, M.; El-Nasr, A.A.; Ayad, M.M. Catalytic activity of polypyrrole nanotubes decorated with noble-metal nanoparticles and their conversion to carbonized analogues. Synth. Met. 2016, 214, 14–22. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Q.; Sha, X.; Zhong, P.; Zhang, J.; Yin, Y.; Gao, C. Self-Assembly of Noble Metal Nanoparticles into Sub-100 nm Colloidosomes with Collective Optical and Catalytic Properties. Chem. Sci. 2017, 8, 6103–6110. [Google Scholar] [CrossRef]
- Howes, P.D.; Chandrawati, R.; Stevens, M.M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346, 1247390. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int. J. Pharm. 2015, 496, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.Y.; Du, X.L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Efficient subnanometric gold-catalyzed hydrogen generation via formic acid decomposition under ambient conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, H.; Yasukawa, T.; Kobayashi, S. Aerobic oxidative esterification of alcohols catalyzed by polymer-incarcerated gold nanoclusters under ambient conditions. Green Chem. 2010, 12, 776–778. [Google Scholar] [CrossRef]
- Suvith, V.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Murayama, T.; Haruta, M. Preparation of gold nanoparticles supported on Nb2O5 by deposition precipitation and deposition reduction methods and their catalytic activity for CO oxidation. Chin. J. Catal. 2016, 37, 1694–1701. [Google Scholar] [CrossRef]
- Xiong, Z.; Qin, F.; Huang, P.S.; Nettleship, I.; Lee, J.K. Effect of Synthesis Techniques on Crystallization and Optical Properties of Ag-Cu Bimetallic Nanoparticles. JOM 2016, 68, 1163–1168. [Google Scholar] [CrossRef]
- Tuteja, J.; Nishimura, S.; Ebitani, K. Change in reactivity of differently capped AuPd bimetallic nanoparticle catalysts for selective oxidation of aliphatic diols to hydroxycarboxylic acids in basic aqueous solution. Catal. Today 2016, 265, 231–239. [Google Scholar] [CrossRef]
- Wu, W.; Lei, M.; Yang, S.; Zhou, L.; Liu, L.; Xiao, X.; Jiang, C.; Roy, V.A. A one-pot route to the synthesis of alloyed Cu/Ag bimetallic nanoparticles with different mass ratios for catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 3450–3455. [Google Scholar] [CrossRef]
- Xiang, J.; Li, P.; Chong, H.; Feng, L.; Fu, F.; Wang, Z.; Zhang, S.; Zhu, M. Bimetallic Pd-Ni core-shell nanoparticles as effective catalysts for the Suzuki reaction. Nano Res. 2014, 7, 1337–1343. [Google Scholar] [CrossRef]
- Yan, J.M.; Wang, Z.L.; Gu, L.; Li, S.J.; Wang, H.L.; Zheng, W.T.; Jiang, Q. AuPd-MnOx/MOF-graphene: An efficient catalyst for hydrogen production from formic acid at room temperature. Adv. Energy Mater. 2015, 5, 4502–4505. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, W.; Cheng, S.; Guo, S.; Shang, N.; Gao, S.; Feng, C.; Wang, C.; Wang, Z. Pd9Ag1-N-doped-MOF-C: An efficient catalyst for catalytic transfer hydrogenation of nitro-compounds. Catal. Commun. 2017, 95, 50–53. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Pitchumani, K. Palladium nanoparticles supported on triazine functionalised mesoporous covalent organic polymers as efficient catalysts for Mizoroki–Heck cross coupling reaction. Green Chem. 2014, 16, 4223–4233. [Google Scholar] [CrossRef]
- Shang, L.; Bian, T.; Zhang, B.; Zhang, D.; Wu, L.Z.; Tung, C.H.; Yin, Y.; Zhang, T. Graphene-supported ultrafine metal nanoparticles encapsulated by mesoporous silica: Robust catalysts for oxidation and reduction reactions. Angew. Chem. 2014, 126, 254–258. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Óvári, L.; Berkó, A.; Majzik, Z.; Kiss, J. Formation of Rh-Au core-shell nanoparticles on TiO2(110) surface studied by STM and LEIS. Langmuir 2010, 2, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Óvári, L.; Bugyi, L.; Majzik, Z.; Berkó, A.; Kiss, J. Surface structure and composition of Au-Rh bimetallic nanoclusters on TiO2(110): A LEIS and STM study. J. Phys. Chem. C 2008, 112, 18011–18016. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Cavallaro, G.; Colletti, C.G.; Milioto, S.; Noto, R.; Parisi, F.; Lazzara, G. Palladium supported on Halloysite-triazolium salts as catalyst for ligand free Suzuki cross-coupling in water under microwave irradiation. J. Mol. Catal. A Chem. 2015, 408, 12–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, A.; Yang, H.; Ouyang, J. Applications and interfaces of halloysite nanocomposites. Appl. Clay. Sci. 2016, 119, 8–17. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite nanotubes: Controlled access and release by smart gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Hydrophobically modified halloysite nanotubes as reverse micelles for water-in-oil emulsion. Langmuir 2015, 31, 7472–7478. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.M.; Fakhrullin, R.F. Toxicity of halloysite clay nanotubes In Vivo: A Caenorhabditis elegans study. Environ. Sci. Nano 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of toxicity of nanoclays and graphene oxide In Vivo: A Paramecium caudatum study. Environ. Sci. Nano 2016, 3, 442–452. [Google Scholar] [CrossRef]
- Machado, G.S.; de Freitas Castro, K.A.D.; Wypych, F.; Nakagaki, S. Immobilization of metalloporphyrins into nanotubes of natural halloysite toward selective catalysts for oxidation reactions. J. Mol. Catal. Chem. 2008, 283, 99–107. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, G.; Ding, Y.; Wang, Y.; Sun, X.; Wang, X.; Chen, W. Photocatalytic activity of heterostructures based on TiO2 and halloysite nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 4154–4158. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Massaro, M.; Milioto, S.; Noto, R.; Parisi, F.; Riela, S. Biocompatible poly(N-isopropylacrylamide)-halloysite nanotubes for thermoresponsive curcumin release. J. Phys. Chem. C 2015, 119, 8944–8951. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, M.A.; Gentile, P.; Ferreira, A.M.; Cometa, S.; De Giglio, E. Insight into halloysite nanotubes-loaded gellan gum hydrogels for soft tissue engineering applications. Carbohydr. Polym. 2017, 163, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Lvov, Y.M. Halloysite clay nanotubes for tissue engineering. Nanomedicine 2016, 11, 2243–2246. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.Y.; Liu, Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426–8430. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Alizadeh, M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red. J. Alloys. Compd. 2016, 680, 309–314. [Google Scholar] [CrossRef]
- Giribabu, K.; Suresh, R.; Manigandan, R.; Munusamy, S.; Kumar, S.P.; Muthamizh, S.; Narayanan, V. Nanomolar determination of 4-nitrophenol based on a poly (methylene blue)-modified glassy carbon electrode. Analyst 2013, 138, 5811–5818. [Google Scholar] [CrossRef] [PubMed]
- Urkude, K.; Thakare, S.R.; Gawande, S. An energy efficient photocatalytic reduction of 4-nitrophenol. J. Environ. Chem. Eng. 2014, 2, 759–764. [Google Scholar] [CrossRef]
- Silambarasan, S.; Vangnai, A.S. Biodegradation of 4-nitroaniline by plant-growth promoting Acinetobacter sp. AVLB2 and toxicological analysis of its biodegradation metabolites. J. Hazard. Mater. 2016, 302, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M.; Gayatri, S.L. Batch adsorption of 4-nitrophenol by acid activated jute stick char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Murugaesan, P.; Aravind, P.; Guruswamy, M.N.; Kandasamy, S. Performance of three different anodes in electrochemical degradation of 4-para-nitrophenol. Environ. Technol. 2015, 36, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Mandlimath, T.R.; Gopal, B. Catalytic activity of first row transition metal oxides in the conversion of p-nitrophenol to p-aminophenol. J. Mol. Catal. A Chem. 2011, 350, 9–15. [Google Scholar] [CrossRef]
- Lara, L.R.; Zottis, A.D.; Elias, W.C.; Faggion, D.; de Campos, C.E.M.; Acuña, J.J.S.; Domingos, J.B. The catalytic evaluation of in situ grown Pd nanoparticles on the surface of Fe3O4@dextran particles in the p-nitrophenol reduction reaction. RSC Adv. 2015, 5, 8289–8296. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. One-pot protocol for Au-based hybrid magnetic nanostructures via a noble-metal-induced reduction process. J. Am. Chem. Soc. 2010, 132, 6280–6281. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; McLellan, J.M.; Yin, Y.; Xia, Y. Synthesis of palladium icosahedra with twinned structure by blocking oxidative etching with citric acid or citrate ions. Angew. Chem. 2007, 119, 804–808. [Google Scholar] [CrossRef]
- Jiang, H.L.; Akita, T.; Ishida, T.; Haruta, M.; Xu, Q. Synergistic catalysis of Au@Ag core—Shell nanoparticles stabilized on metal—Organic framework. J. Am. Chem. Soc. 2011, 133, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cai, Z.; Chen, X.; Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: Their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J. Mater. Chem. A 2014, 2, 5668–5674. [Google Scholar] [CrossRef]
- Jiang, F.; Li, R.; Cai, J.; Xu, W.; Cao, A.; Chen, D.; Zhang, X.; Wang, C.; Shu, C. Ultrasmall Pd/Au bimetallic nanocrystals embedded in hydrogen-bonded supramolecular structures: Facile synthesis and catalytic activities in the reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 19433–19438. [Google Scholar] [CrossRef]
- Zhu, H.Z.; Lu, Y.M.; Fan, F.J.; Yu, S.H. Selective hydrogenation of nitroaromatics by ceria nanorods. Nanoscale 2013, 5, 7219–7223. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Liu, R.H.; Sun, Q.J.; Chang, J.B.; Gao, X.; Liu, Y.; Lee, S.T.; Kang, Z.H.; Wang, S.D. Controlled synthesis and synergistic effects of graphene-supported PdAu bimetallic nanoparticles with tunable catalytic properties. Nanoscale 2015, 7, 6356–6362. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Yue, H.; Jiang, J. Environmentally friendly light-driven synthesis of Ag nanoparticles in situ grown on magnetically separable biohydrogels as highly active and recyclable catalysts for 4-nitrophenol reduction. J. Mater. Chem. 2012, 22, 23447–23453. [Google Scholar] [CrossRef]
- Lv, J.J.; Wang, A.J.; Ma, X.; Xiang, R.Y.; Chen, J.R.; Feng, J.J. One-pot synthesis of porous Pt–Au nanodendrites supported on reduced graphene oxide nanosheets toward catalytic reduction of 4-nitrophenol. J. Mater. Chem. A. 2015, 3, 290–296. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Chen, B.; Ji, N.; Chen, F.; Zhang, Y.; Zhang, Z. Nanocomposites of size-controlled gold nanoparticles and graphene oxide: Formation and applications in SERS and catalysis. Nanoscale 2010, 2, 2733–2738. [Google Scholar] [CrossRef] [PubMed]
- Veisi, H.; Gholami, J.; Ueda, H.; Mohammadi, P.; Noroozi, M. Magnetically palladium catalyst stabilized by diaminoglyoxime-functionalized magnetic Fe3O4 nanoparticles as active and reusable catalyst for Suzuki coupling reactions. J. Mol. Catal. A Chem. 2015, 396, 216–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Chen, D.; Li, F.; Xue, T.; Ahmed, S.F. Ternary Fe3O4@PANI@Au nanocomposites as a magnetic catalyst for degradation of organic dyes. Sci. China Tech. Sci. 2017, 60, 749–757. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, D.; Wu, D.; Ma, X. Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chem. Eng. J. 2011, 168, 959–963. [Google Scholar] [CrossRef]

| Entry | Compound | Time/min | Conversion/% |
|---|---|---|---|
| 1 | p-Nitroaniline | 8 | 99 |
| 2 | m-Nitroaniline | 6 | 99 |
| 3 | o-Nitroaniline | 5 | 99 |
| 4 | 2,4-Nitroaniline | 7 | 99 |
| 5 | m-Nitrotoluene | 68 | 81 |
| 6 | o-Nitrotoluene | 76 | 75 |
| 7 | 2,4-Dinitrotoluene | 82 | 79 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Zhou, T.; Xu, J.; Li, F.; Xu, Z.; Zhang, B.; Guo, S.; Shen, X.; Zhang, W. AuPd Bimetallic Nanocrystals Embedded in Magnetic Halloysite Nanotubes: Facile Synthesis and Catalytic Reduction of Nitroaromatic Compounds. Nanomaterials 2017, 7, 333. https://doi.org/10.3390/nano7100333
Jia L, Zhou T, Xu J, Li F, Xu Z, Zhang B, Guo S, Shen X, Zhang W. AuPd Bimetallic Nanocrystals Embedded in Magnetic Halloysite Nanotubes: Facile Synthesis and Catalytic Reduction of Nitroaromatic Compounds. Nanomaterials. 2017; 7(10):333. https://doi.org/10.3390/nano7100333
Chicago/Turabian StyleJia, Lei, Tao Zhou, Jun Xu, Fenghai Li, Zhouqing Xu, Beibei Zhang, Shengli Guo, Xiaoke Shen, and Wensheng Zhang. 2017. "AuPd Bimetallic Nanocrystals Embedded in Magnetic Halloysite Nanotubes: Facile Synthesis and Catalytic Reduction of Nitroaromatic Compounds" Nanomaterials 7, no. 10: 333. https://doi.org/10.3390/nano7100333