Investigating the Origins of Toxic Response in TiO2 Nanoparticle-Treated Cells
Abstract
:1. Introduction
2. Results
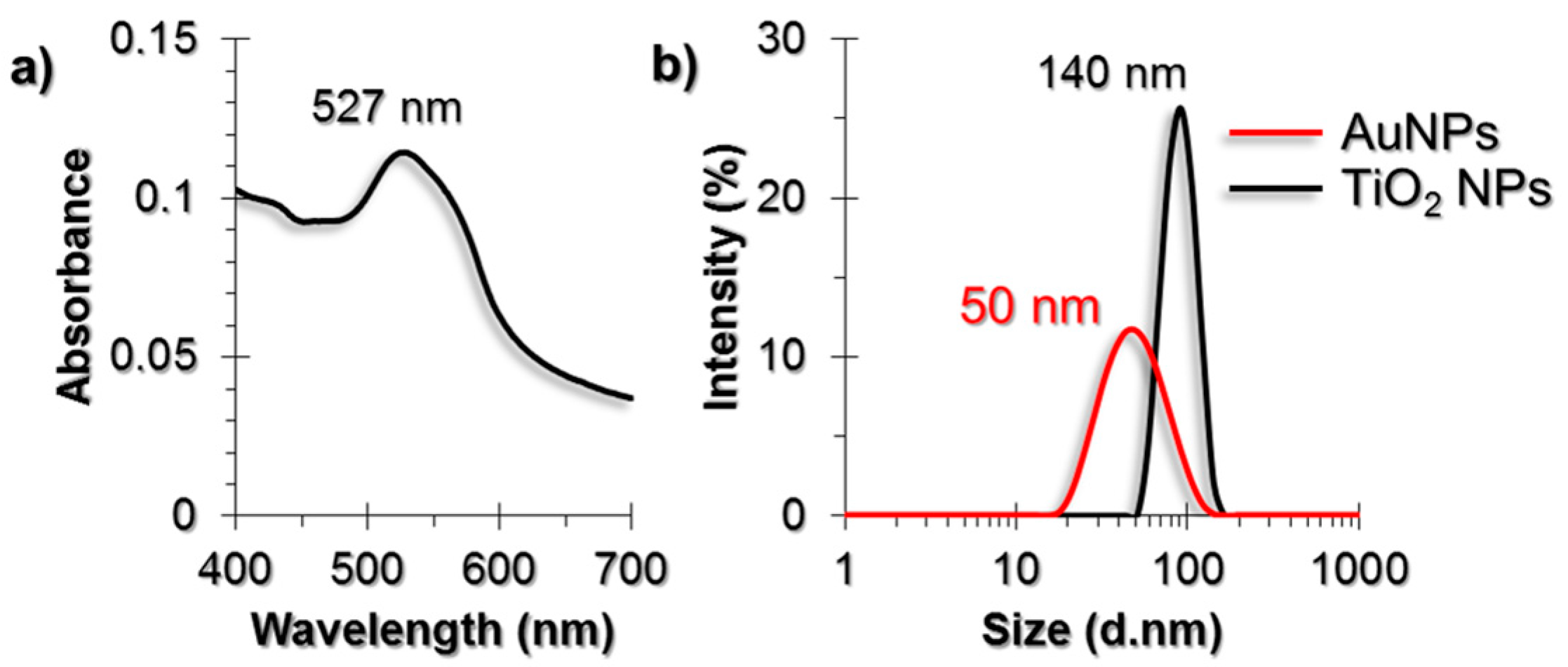
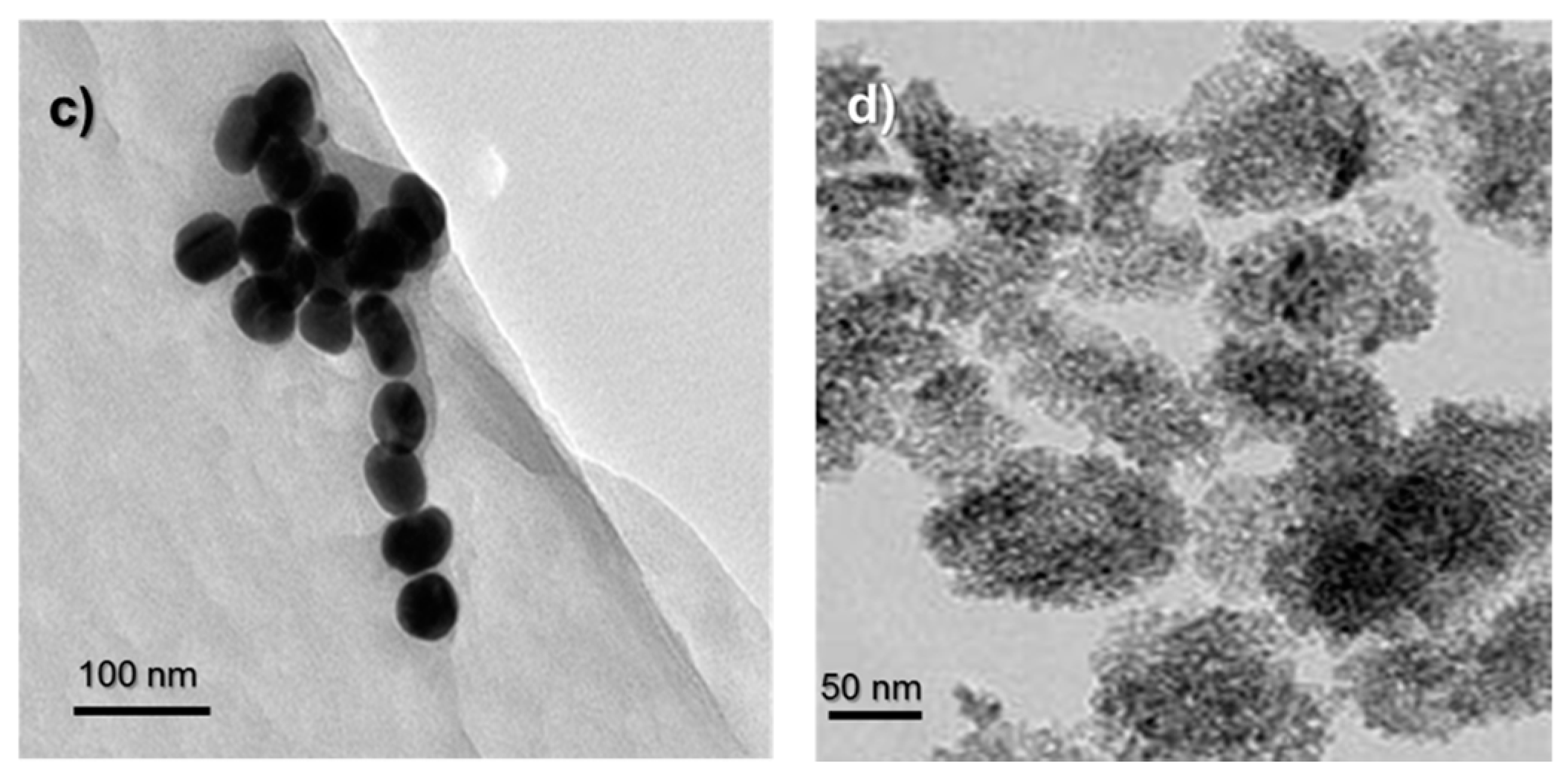
2.1. Characterization of AuNPs and TiO2 NPs
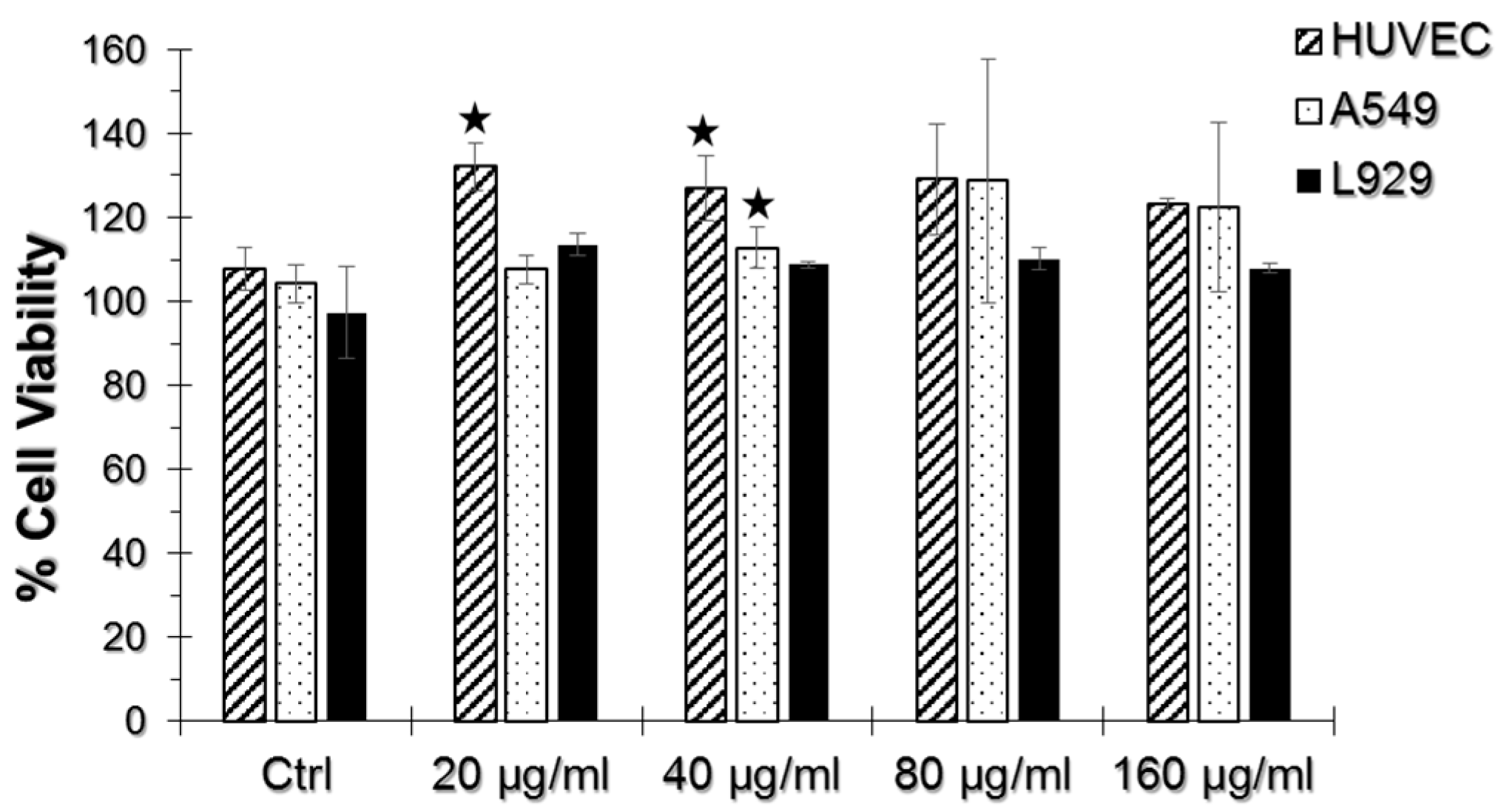
2.2. WST-1 Assay
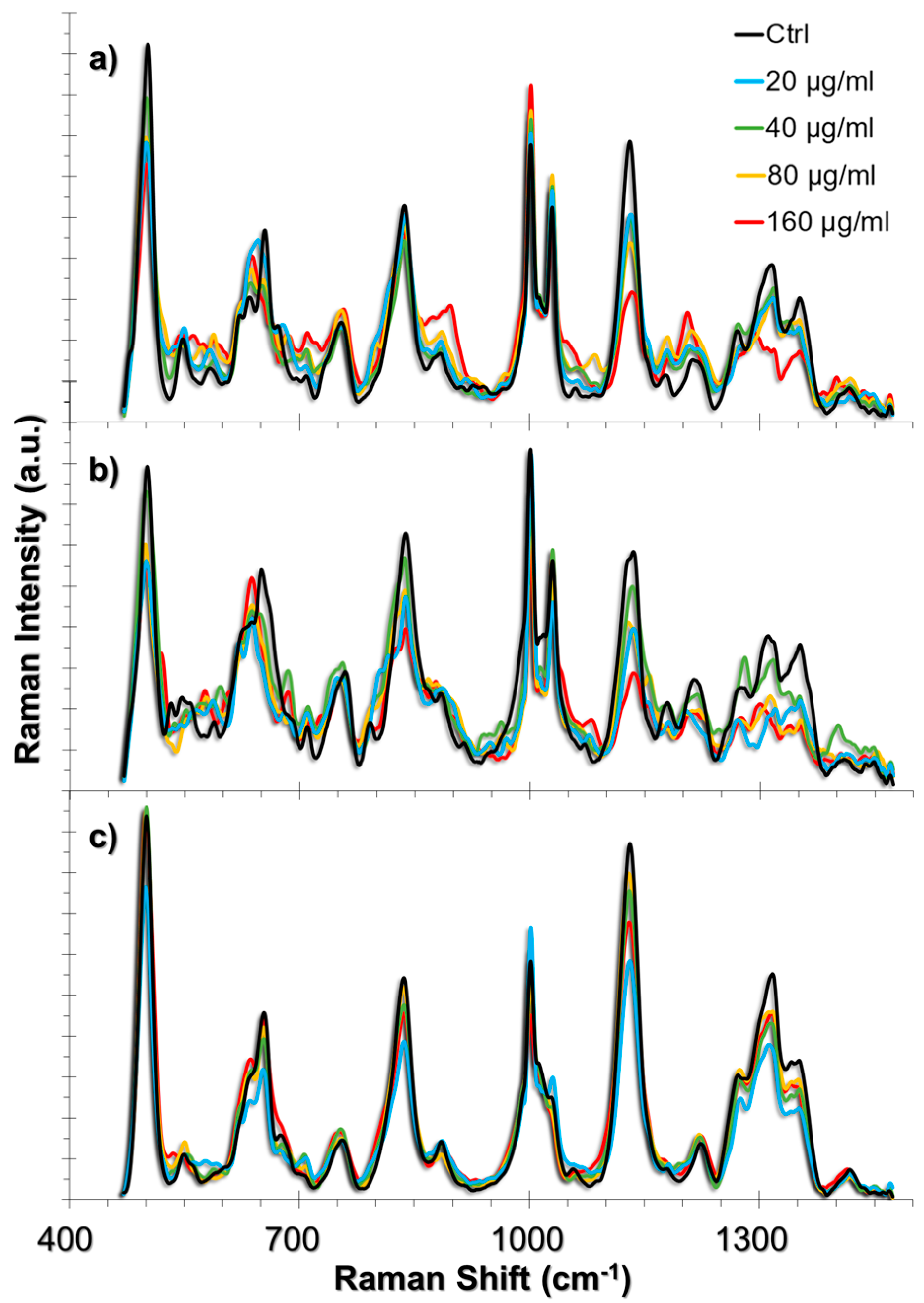
2.3. SERS Spectra of Cells Incubated with TiO2 NPs
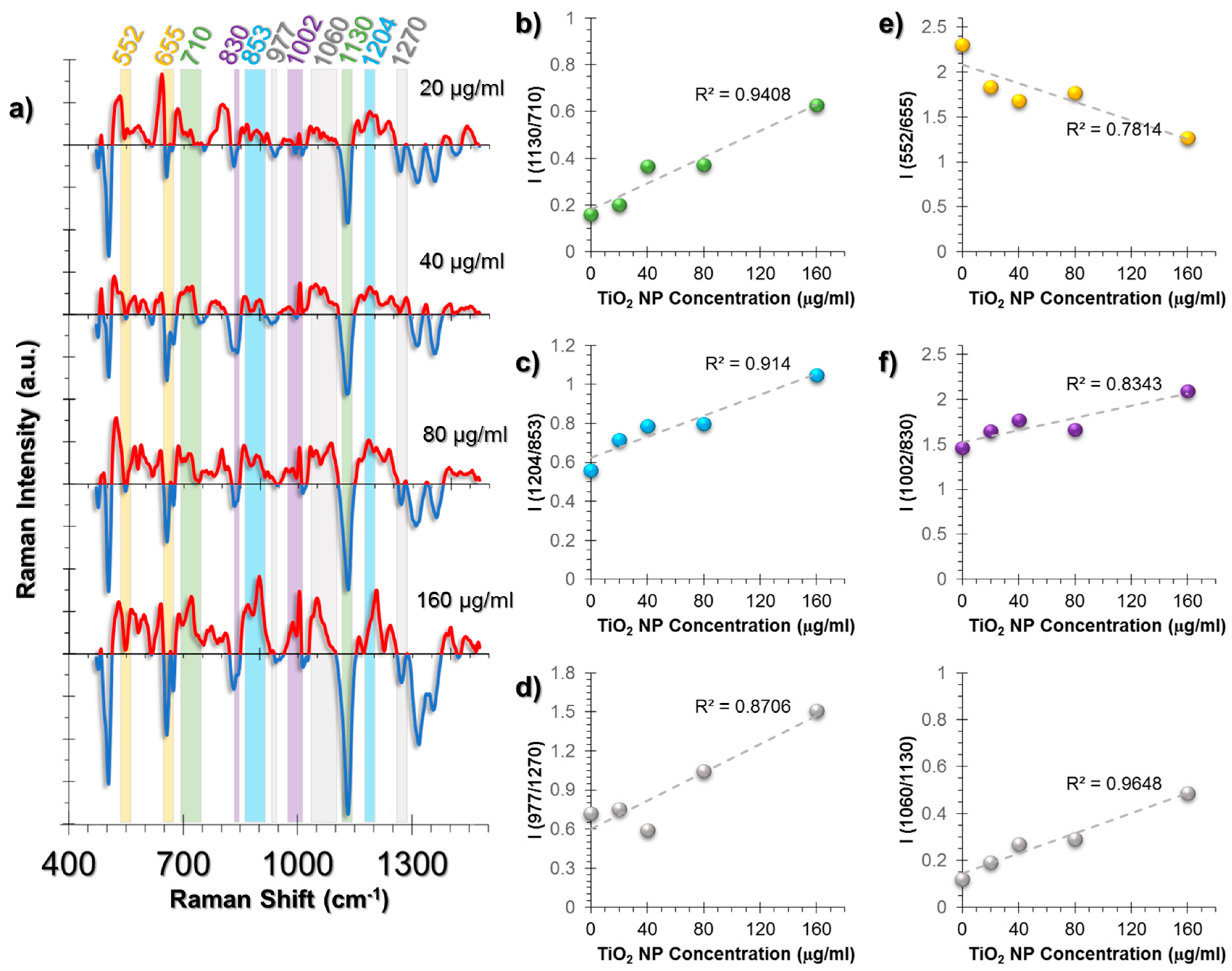
2.3.1. SERS Spectral Patterns of HUVEC Cell Line
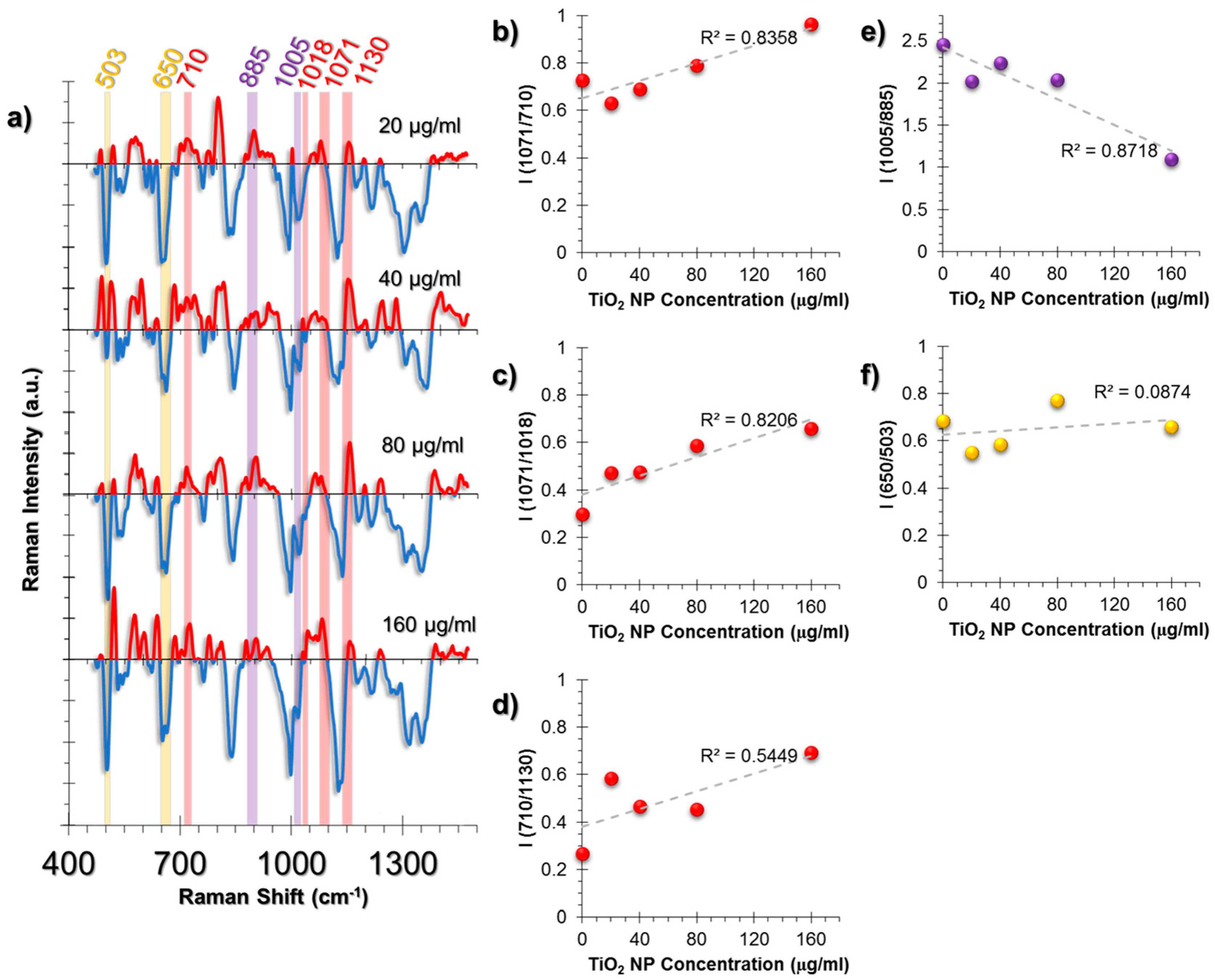
2.3.2. SERS Spectral Patterns of A549 Cell Line
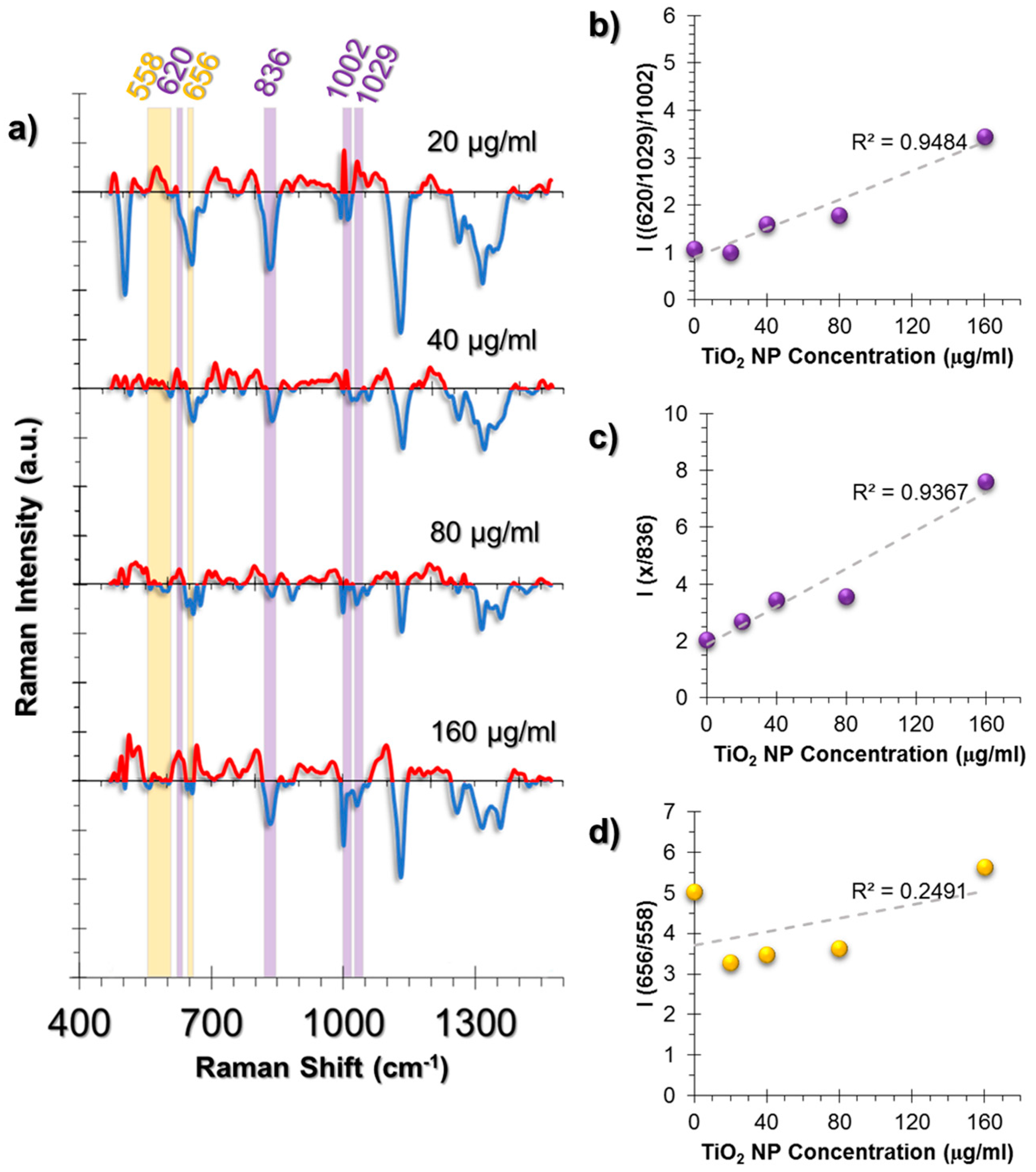
2.3.3. SERS Spectral Patterns of L929 Cell Line
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. AuNP Synthesis, TiO2 NP Dispersion, and Characterization
4.3. Incubation of Cells with AuNPs and TiO2 NPs
4.4. WST-1 Assay
4.5. SERS Experimental Setup
4.6. SERS Data Processing
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hong, F.; Yu, X.; Wu, N.; Zhang, Y.-Q. Progress of In Vivo studies on the systemic toxicities induced by titanium dioxide nanoparticles. Toxicol. Res. 2017, 6, 115–133. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schmuki, P.; von der Mark, K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009, 9, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S.; Yoriya, S.; Grissom, L.; Grimes, C.A.; Popat, K.C. Hemocompatibility of Titania nanotube arrays. J. Biomed. Mater. Res. A 2010, 95A, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. Nanomed. 2013, 8, 3093–3105. [Google Scholar]
- Song, Y.-Y.; Schmidt-Stein, F.; Bauer, S.; Schmuki, P. Amphiphilic TiO2 nanotube arrays: An actively controllable drug delivery system. J. Am. Chem. Soc. 2009, 131, 4230–4232. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, S.; Oh, W.-K.; Choi, M.; Jang, J. Efficient intracellular delivery of camptothecin by Silica/Titania hollow nanoparticles. Chem. Eur. J. 2012, 18, 4902–4908. [Google Scholar] [CrossRef] [PubMed]
- Thurn, K.T.; Paunesku, T.; Wu, A.; Brown, E.M.B.; Lai, B.; Vogt, S.; Maser, J.; Aslam, M.; Dravid, V.; Bergan, R.; et al. Labeling TiO2 nanoparticles with dyes for optical fluorescence microscopy and determination of TiO2–DNA nanoconjugate stability. Small 2009, 5, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Xu, J.-J.; Chen, H.-Y. Dopamine sensitized nanoporous TiO2 film on electrodes: Photoelectrochemical sensing of NADH under visible irradiation. Biosens. Bioelectron. 2009, 24, 2494–2498. [Google Scholar] [CrossRef] [PubMed]
- Rajh, T.; Dimitrijevic, N.M.; Bissonnette, M.; Koritarov, T.; Konda, V. Titanium dioxide in the service of the biomedical revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar] [CrossRef] [PubMed]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Liu, Y.; Sun, L.; Chen, T.; Zhang, Y.; Zhao, A.; Wang, X.; Cristau, M.; Wang, K.; Jia, W. Metabolic profiling reveals disorder of carbohydrate metabolism in mouse fibroblast cells induced by titanium dioxide nanoparticles. J. Appl. Toxicol. 2013, 33, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-Y.; Zhu, B.-S.; Wang, X.-F.; Lu, Q.-H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-N.; Chang, S.-H.; Park, S.J.; Lim, J.; Lee, J.; Yoon, T.-J.; Kim, J.-S.; Cho, M.-H. Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated autophagic cell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS ONE 2015, 10, e0131208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Howe, J.L.C.; Yu, Z.; Leong, D.T.; Chu, J.J.H.; Loo, J.S.C.; Ng, K.W. Exposure to Titanium Dioxide nanoparticles induces autophagy in primary human keratinocytes. Small 2013, 9, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Loitto, V.; Audinot, J.-N.; Bayat, N.; Gutleb, A.C.; Cristobal, S. Dose-dependent autophagic effect of titanium dioxide nanoparticles in human HaCaT cells at non-cytotoxic levels. J. Nanobiotechnol. 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Gurr, J.-R.; Wang, A.S.S.; Chen, C.-H.; Jan, K.-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jugan, M.-L.; Barillet, S.; Simon-Deckers, A.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology 2012, 6, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Golbamaki, N.; Rasulev, B.; Cassano, A.; Robinson, R.L.M.; Benfenati, E.; Leszczynski, J.; Cronin, M.T.D. Genotoxicity of metal oxide nanomaterials: Review of recent data and discussion of possible mechanisms. Nanoscale 2015, 7, 2154–2198. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman Spectroelectrochemistry. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Aydin, Ö.; Kahraman, M.; Kiliç, E.; Çulha, M. Surface-enhanced Raman scattering of rat tissues. Appl. Spectrosc. 2009, 63, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Culha, M. Surface-enhanced Raman scattering: An emerging label-free detection and identification technique for proteins. Appl. Spectrosc. 2013, 67, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.M.; McLeish, J.A.; Jamieson, L.E.; Jiang, J.; Hopgood, J.R.; McLaughlin, S.; Donaldson, K.; Campbell, C.J. SERS as a tool for In Vitro toxicology. Faraday Discuss. 2016, 187, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, X.; Su, L.; Du, Z.; Shen, A.; Materny, A.; Hu, J. Combined labelled and label-free SERS probes for triplex three-dimensional cellular imaging. Sci. Rep. 2016, 6, 19173. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.D.; Chumanov, G. Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy). Anal. Bioanal. Chem. 2009, 394, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; So, P.T.C.; Dasari, R.R.; Lim, D.-K. High resolution live cell Raman imaging using subcellular organelle-targeting SERS-sensitive gold nanoparticles with highly narrow intra-nanogap. Nano Lett. 2015, 15, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Manoharan, R.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-enhanced Raman scattering (SERS)—A new tool for single molecule detection and identification. Bioimaging 1998, 6, 104–110. [Google Scholar] [CrossRef]
- Lahr, R.H.; Vikesland, P.J. Surface-enhanced Raman spectroscopy (SERS) cellular imaging of intracellulary biosynthesized gold nanoparticles. ACS Sustain. Chem. Eng. 2014, 2, 1599–1608. [Google Scholar] [CrossRef]
- McAughtrie, S.; Lau, K.; Faulds, K.; Graham, D. 3D optical imaging of multiple SERS nanotags in cells. Chem. Sci. 2013, 4, 3566–3572. [Google Scholar] [CrossRef]
- Willets, K.A. Surface-enhanced Raman scattering (SERS) for probing internal cellular structure and dynamics. Anal. Bioanal. Chem. 2009, 394, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.V.; Medarova, Z. In vivo and ex vivo applications of gold nanoparticles for biomedical SERS imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 232–241. [Google Scholar] [PubMed]
- Kuku, G.; Saricam, M.; Akhatova, F.; Danilushkina, A.; Fakhrullin, R.F.; Culha, M. Surface-enhanced Raman scattering to evaluate nanomaterial cytotoxicity on living cells. Anal. Chem. 2016, 88, 9813–9820. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Auchinvole, C.; Fisher, K.; Campbell, C.J. Quantitative measurement of redox potential in hypoxic cells using SERS nanosensors. Nanoscale 2014, 6, 12104–12110. [Google Scholar] [CrossRef] [PubMed]
- Panikkanvalappil, S.R.; James, M.; Hira, S.M.; Mobley, J.; Jilling, T.; Ambalavanan, N.; El-Sayed, M.A. Hyperoxia induces intracellular acidification in neonatal mouse lung fibroblasts: Real-time investigation using plasmonically enhanced Raman spectroscopy. J. Am. Chem. Soc. 2016, 138, 3779–3788. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Austin, L.A.; El-Sayed, M.A. Observing real-time molecular event dynamics of apoptosis in living cancer cells using nuclear-targeted plasmonically enhanced Raman nanoprobes. ACS Nano 2014, 8, 4883–4892. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Afifi, M.M.; Austin, L.A.; El-Sayed, M.A. Exploiting the nanoparticle plasmon effect: Observing drug delivery dynamics in single cells via Raman/fluorescence imaging spectroscopy. ACS Nano 2013, 7, 7420–7427. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Austin, L.A.; El-Sayed, M.A. Real-time molecular imaging throughout the entire cell cycle by targeted plasmonic-enhanced rayleigh/Raman spectroscopy. Nano Lett. 2012, 12, 5369–5375. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.A.; Kang, B.; Yen, C.-W.; El-Sayed, M.A. Plasmonic imaging of human oral cancer cell communities during programmed cell death by nuclear-targeting silver nanoparticles. J. Am. Chem. Soc. 2011, 133, 17594–17597. [Google Scholar] [CrossRef] [PubMed]
- Panikkanvalappil, S.R.; Hira, S.M.; Mahmoud, M.A.; El-Sayed, M.A. Unraveling the biomolecular snapshots of mitosis in healthy and cancer cells using plasmonically-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2014, 136, 15961–15968. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Bajpai, S.; Chaturvedi, P.; Wirtz, D.; Semenza, G.L. Hypoxia-inducible Factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 Expression in Fibroblasts. J. Biol. Chem. 2013, 288, 10819–10829. [Google Scholar] [CrossRef] [PubMed]
- Niecknig, H.; Tug, S.; Reyes, B.D.; Kirsch, M.; Fandrey, J.; Berchner-Pfannschmidt, U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic. Res. 2012, 46, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Engelholm, L.H.; List, K.; Netzel-Arnett, S.; Cukierman, E.; Mitola, D.J.; Aaronson, H.; Kjøller, L.; Larsen, J.K.; Yamada, K.M.; Strickland, D.K.; et al. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J. Cell Biol. 2003, 160, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Kjøller, L.; Engelholm, L.H.; Høyer-Hansen, M.; Danø, K.; Bugge, T.H.; Behrendt, N. uPARAP/Endo180 directs lysosomal delivery and degradation of collagen IV. Exp. Cell Res. 2004, 293, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Na, H.-J.; Ding, Y.; Wang, Z.; Lee, S.J.; Choi, M.E. Autophagy promotes intracellular degradation of Type I collagen induced by transforming growth factor (TGF)-β1. J. Biol. Chem. 2012, 287, 11677–11688. [Google Scholar] [CrossRef] [PubMed]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- Vitagliano, L.; Berisio, R.; Mazzarella, L.; Zagari, A. Structural bases of collagen stabilization induced by proline hydroxylation. Biopolymers 2001, 58, 459–464. [Google Scholar] [CrossRef]
- Brodsky, B.; Persikov, A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [PubMed]
- Trelstad, R.L.; Lawley, K.R.; Holmes, L.B. Nonenzymatic hydroxylations of proline and lysine by reduced oxygen derivatives. Nature 1981, 289, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Gostner, J.M.; Becker, K.; Ueberall, F.; Fuchs, D. Immune activation and inflammation increase the plasma phenylalanine-to-tyrosine ratio. Pteridines 2013, 24, 27–31. [Google Scholar] [CrossRef]
- Vankoningsloo, S.; Piens, M.; Lecocq, C.; Gilson, A.; Pauw, A.D.; Renard, P.; Demazy, C.; Houbion, A.; Raes, M.; Arnould, T. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: Role of fatty acid β-oxidation and glucose. J. Lipid Res. 2005, 46, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Berniakovich, I.; Trinei, M.; Stendardo, M.; Migliaccio, E.; Minucci, S.; Bernardi, P.; Pelicci, P.G.; Giorgio, M. p66Shc-generated oxidative signal promotes fat accumulation. J. Biol. Chem. 2008, 283, 34283–34293. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Zhang, J.; Choi, A.M.K.; Kim, H.P. Mitochondrial dysfunction induces formation of lipid droplets as a Generalized Response to Stress. Oxid. Med. Cell. Longev. 2013, 2013, e327167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Choudhury, D.; Liu, C.; Billet, S.; Hu, T.; Bhowmick, N.A. Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases. Cell Death Discov. 2015, 1, 15046. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Peterson, T.E.; Holmuhamedov, E.L.; Terzic, A.; Caplice, N.M.; Oberley, L.W.; Katusic, Z.S. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Popescu, T.; Lupu, A.R.; Diamandescu, L.; Tarabasanu-Mihaila, D.; Teodorescu, V.S.; Raditoiu, V.; Purcar, V.; Vlaicu, A.M. Effects of TiO2 nanoparticles on the NO2− levels in cell culture media analysed by Griess colorimetric methods. J. Nanopart. Res. 2013, 15, 1449. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
| Peak (cm−1) | Peak Assignment |
|---|---|
| 480 | Glycogen |
| 500–560 | S-S bond vibrations |
| 620 | C-C twist aromatic ring |
| 640 | C-S stretching and C-C twisting of proteins |
| 655 | C-S stretching mode of cystine (collagen type I) |
| 710 | Cholesterol, C-N (membrane phospholipid head) |
| 830 | Tyrosine |
| 853 | Proline |
| 885 | Tyrosine |
| 977 | C-C stretching β-sheet (proteins) |
| 1003 | vs(C-C), symmetric ring breathing, Phenylalanine |
| 1018 | Glycogen |
| 1030 | Phenylalanine and changes in collagen |
| 1060 | Triglycerides, Lipids, Ceramide, Proline |
| 1130 | Phospholipid structural changes trans-gauche |
| 1204 | Hydroxyproline |
| 1216 | Amide III (β-sheet) |
| 1270 | Amide III, α-helix, collagen |
| 1317 | Twisting or bending mode of lipid/collagen |
| 1352 | Nucleic acid mode, Collagen content changes |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuku, G.; Culha, M. Investigating the Origins of Toxic Response in TiO2 Nanoparticle-Treated Cells. Nanomaterials 2017, 7, 83. https://doi.org/10.3390/nano7040083
Kuku G, Culha M. Investigating the Origins of Toxic Response in TiO2 Nanoparticle-Treated Cells. Nanomaterials. 2017; 7(4):83. https://doi.org/10.3390/nano7040083
Chicago/Turabian StyleKuku, Gamze, and Mustafa Culha. 2017. "Investigating the Origins of Toxic Response in TiO2 Nanoparticle-Treated Cells" Nanomaterials 7, no. 4: 83. https://doi.org/10.3390/nano7040083
APA StyleKuku, G., & Culha, M. (2017). Investigating the Origins of Toxic Response in TiO2 Nanoparticle-Treated Cells. Nanomaterials, 7(4), 83. https://doi.org/10.3390/nano7040083






